Catalytic Effect of Functional and Fe Composite Biochars on Biofuel and Biochemical Derived from the Pyrolysis of Green Marine Biomass
Abstract
:1. Introduction
- Synthesizing functional biochar and a 3D interconnected algal biochar constituting iron nanoparticles
- Investigating the catalytic behavior of functional and iron composite catalysts in pyrolysis of green macroalgae collected from Caspian Sea Coast, Iran
- Comparative study of biochar based catalysts with other conventional catalysts from the valuable chemical production point of view.
2. Material and Methods
2.1. Experimental Setup and Procedure
2.2. Characterization of Biochar-Based Catalysts and Bio-Products
3. Results and Dissuasion
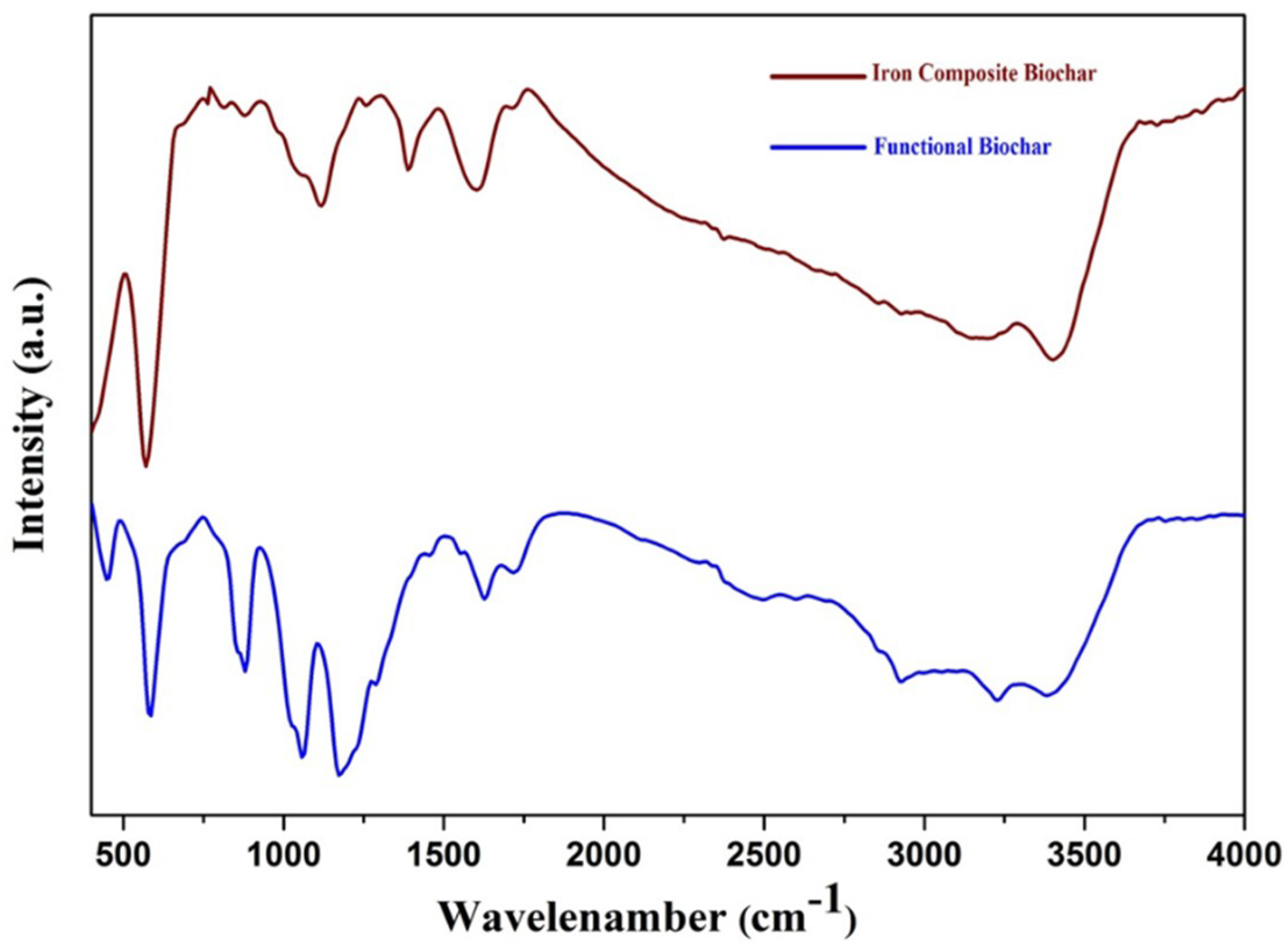
3.1. FT-IR Analyses
3.2. X-ray Diffraction (XRD) Analysis
3.3. Raman Spectroscopy Analysis
3.4. FESEM Images
3.5. Gas Composition Analyses
3.6. Chemical Composition of Bio-Oil
4. Comparison with Previous Results
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Thines, K.R.; Abdullah, E.C.; Mubarak, N.M.; Ruthiraan, M. Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for waste water and polymer application: A review. Renew. Sustain. Energy Rev. 2017, 67, 257–276. [Google Scholar] [CrossRef]
- Mumme, J.; Srocke, F.; Heeg, K.; Werner, M. Use of biochars in anaerobic digestion. Bioresour. Technol. 2014, 164, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Hwang, M.J.; Jeong, T.U.; Ahn, K.H. A novel approach for preparation of modified-biochar derived from marine macroalgae: Dual purpose electro-modification for improvement of surface area and metal impregnation. Bioresour. Technol. 2015, 191, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Pourhosseini, S.E.M.; Norouzi, O.; Naderi, H.R. Study of micro/macro ordered porous carbon with olive-shaped structure derived from Cladophora glomerata macroalgae as efficient working electrodes of supercapacitors. Biomass Bioenergy 2017, 107, 287–298. [Google Scholar] [CrossRef]
- Salimi, P.; Javadian, S.; Norouzi, O.; Gharibi, H. Turning an environmental problem into an opportunity: Potential use of biochar derived from a harmful marine biomass named Cladophora glomerata as anode electrode for Li-ion batteries. Environ. Sci. Pollut. Res. 2017, 24, 27974–27984. [Google Scholar] [CrossRef] [PubMed]
- Pourhosseini, S.E.M.; Norouzi, O.; Salimi, P.; Naderi, H.R. Synthesis of a Novel Interconnected 3D Pore Network Algal Biochar Constituting Iron Nanoparticles Derived from a Harmful Marine Biomass as High-Performance Asymmetric Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2018, 6, 4746–4758. [Google Scholar] [CrossRef]
- Osman, A.I. Enhanced catalytic activity of Ni on -Al 2 O 3 and ZSM-5 on addition of ceria zirconia for the partial oxidation of methane. Appl. Catal. B Environ. 2017, 212, 68–79. [Google Scholar] [CrossRef]
- Norouzi, O.; Jafarian, S.; Safari, F.; Tavasoli, A.; Nejati, B. Promotion of hydrogen-rich gas and phenolic-rich bio-oil production from green macroalgae Cladophora glomerata via pyrolysis over its bio-char. Bioresour. Technol. 2016, 219, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Safari, F.; Norouzi, O.; Tavasoli, A. Hydrothermal gasification of Cladophora glomerata macroalgae over its hydrochar as a catalyst for hydrogen-rich gas production. Bioresour. Technol. 2016, 222, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Safari, F.; Javani, N.; Yumurtaci, Z. Hydrogen production via supercritical water gasification of almond shell over algal and agricultural hydrochars as catalysts. Int. J. Hydrogen Energy 2017, 43, 1–10. [Google Scholar] [CrossRef]
- Jafarian, S.; Tavasoli, A. A comparative study on the quality of bioproducts derived from catalytic pyrolysis of green microalgae Spirulina (Arthrospira) plantensis over transition metals supported on HMS-ZSM5 composite. Int. J. Hydrogen Energy 2018, 43, 19902–19917. [Google Scholar] [CrossRef]
- Yin, C.Y.; Aroua, M.K.; Daud, W. Review of modifications of activated carbon for enhancing contaminant uptakes from aqueous solutions. Sep. Purif. Technol. 2007, 52, 403–415. [Google Scholar] [CrossRef]
- Sabegh, M.Y.; Norouzi, O.; Jafarian, S.; Khosh, A.G.; Tavasoli, A. Pyrolysis of marine biomass to produce bio-oil and its upgrading using a novel multi-metal catalyst prepared from the spent car catalytic converter. Bioresour. Technol. 2018, 249, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.; Dalai, A.K.; Gökalp, I.; Kozinski, J.A. Valorization of horse manure through catalytic supercritical water gasification. Waste Manag. 2016, 52, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Wang, D.; Qian, G.; Walmsley, J.C.; Holmen, A.; Chen, D.; Zhou, X.G. Fabrication of K-promoted iron/carbon nanotubes composite catalysts for the Fischer-Tropsch synthesis of lower olefins. J. Energy Chem. 2016, 25, 311–317. [Google Scholar] [CrossRef]
- Taghavi, S.; Norouzi, O.; Tavasoli, A.; Di Maria, F.; Signoretto, M.; Menegazzo, F.; Di Michele, A. Catalytic conversion of Venice lagoon brown marine algae for producing hydrogen-rich gas and valuable biochemical using algal biochar and Ni/ SBA-15 catalyst. Int. J. Hydrogen Energy 2018, 43, 19918–19929. [Google Scholar] [CrossRef]
- Gao, Y.H.; Niu, H.L.; Chen, Q.W. Preparation and characterization of BaCO3 single-crystalline nanowires by a solvothermal process. Chin J. Inorg. Chem. 2003, 19, 37–40. [Google Scholar]
- Liu, W.; Tian, K.; Jiang, H.; Yu, H. Facile synthesis of highly efficient and recyclable magnetic solid acid from biomass waste. Sci. Rep. 2013, 3, 2419. [Google Scholar] [CrossRef] [PubMed]
- Ru, H.; Bai, N.; Xiang, K.; Zhou, W.; Chen, H.; Zhao, X.S. Porous carbons derived from microalgae with enhanced electrochemical performance for lithium-ion batteries. Electrochim. Acta 2016, 194, 10–16. [Google Scholar] [CrossRef]
- Azargohar, R.; Nanda, S.; Kozinski, J.A.; Dalai, A.K.; Sutarto, R. Effects of temperature on the physicochemical characteristics of fast pyrolysis bio-chars derived from Canadian waste biomass. Fuel 2014, 125, 90–100. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, L.; Wang, X.; Holm, N.; Rajagopalan, K.; Chen, F.; Ma, S.G. Highly ordered macroporous woody biochar with ultra-high carbon content as supercapacitor electrodes. Electrochim. Acta 2013, 113, 481–489. [Google Scholar] [CrossRef]
- Norouzi, O.; Tavasoli, A.; Jafarian, S.; Esmailpour, S. Catalytic upgrading of bio-products derived from pyrolysis of red macroalgae Gracilaria gracilis with a promising novel micro/mesoporous catalyst. Bioresour. Technol. 2017, 243, 1–8. [Google Scholar] [CrossRef] [PubMed]







| Macroalgae Samples | C. Glomerata [8] | G. Gracilis [22] | C. Glomerata [13] | C. Glomerata | C. Glomerata |
|---|---|---|---|---|---|
| Maximum Yield of Bio-Oil (wt%) | 39 | 42 | 29 | 43 | 45 |
| Catalyst | Biochar | ZH-20 composite | Multi-metal catalyst | Iron composite biochar | Functional biochar |
| Acids Selectivity (%) | 28.55 | 22.45 | 20.12 | 11.47 | 21.20 |
| Phenols Selectivity (%) | 8.50 | 6.28 | 2.39 | 11.80 | 31.54 |
| H2 Yield (mmol/g Feedstock) | 8.85 | 7.58 | 1.23 | 7.99 | 4.81 |
| CO2 Yield (mmol/g Feedstock) | 11.25 | 11.48 | 2.94 | 6.30 | 3.40 |
| CO Yield (mmol/g Feedstock) | 2.82 | 2.97 | 1.25 | 5.02 | 1.63 |
| CH4 Yield (mmol/g Feedstock) | 0.80 | 3.58 | 0.74 | 1.84 | 3.33 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norouzi, O.; Di Maria, F. Catalytic Effect of Functional and Fe Composite Biochars on Biofuel and Biochemical Derived from the Pyrolysis of Green Marine Biomass. Fermentation 2018, 4, 96. https://doi.org/10.3390/fermentation4040096
Norouzi O, Di Maria F. Catalytic Effect of Functional and Fe Composite Biochars on Biofuel and Biochemical Derived from the Pyrolysis of Green Marine Biomass. Fermentation. 2018; 4(4):96. https://doi.org/10.3390/fermentation4040096
Chicago/Turabian StyleNorouzi, Omid, and Francesco Di Maria. 2018. "Catalytic Effect of Functional and Fe Composite Biochars on Biofuel and Biochemical Derived from the Pyrolysis of Green Marine Biomass" Fermentation 4, no. 4: 96. https://doi.org/10.3390/fermentation4040096