Two-Stage Aeration Fermentation Strategy to Improve Bioethanol Production by Scheffersomyces stipitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. HSSL Supply and Pre-Treatment
2.2. Microorganism
2.3. Culture Media
2.4. Pre-Inocula and Inocula
2.5. Assays
2.6. Analytical Methods
2.7. Determination of Volumetric Oxygen Transfer Coefficient (KLa)
2.8. Calculations
3. Results and Discussion
3.1. Single-Stage Aeration Experiments
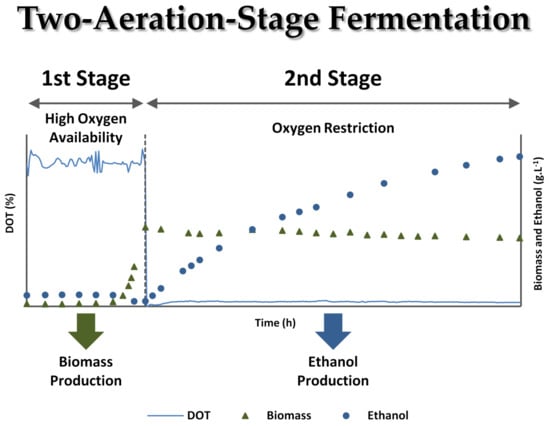
3.2. Two-Stage Aeration Experiments in Synthetic Medium
3.3. Two-Stage Aeration Experiment with 60% of HSSL
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IEA. Key World Energy Statistics; OECD IEA: Paris, France, 2018. [Google Scholar]
- Escobar, J.C.; Lora, E.S.; Venturini, O.J.; Yáñez, E.E.; Castillo, E.F.; Almazan, O. Biofuels: Environment, technology and food security. Renew. Sustain. Energy Rev. 2009, 13, 1275–1287. [Google Scholar] [CrossRef]
- Balat, M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review. Energy Convers. Manag. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Pereira, S.R.; Portugal-Nunes, D.J.; Evtuguin, D.V.; Serafim, L.S.; Xavier, A.M.R.B. Advances in ethanol production from hardwood spent sulphite liquors. Process Biochem. 2013, 48, 272–282. [Google Scholar] [CrossRef]
- Lawford, H.G.; Rousseau, J.D. Production of ethanol from pulp mill hardwood and softwood spent sulfite liquors by genetically engineered E. coli. Appl. Biochem. Biotechnol. 1993, 39–40, 667–685. [Google Scholar] [CrossRef]
- Marques, A.P.; Evtuguin, D.V.; Magina, S.; Amado, F.M.L.; Prates, A. Chemical composition of spent liquors from acidic magnesium-based sulphite pulping of Eucalyptus globulus. J. Wood Chem. Technol. 2009, 29, 322–336. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Suzuki, M. Phylogenetic analysis of ascomycete yeasts that form coenzyme q-9 and the proposal of the new genera Babjeviella, Meyerozyma, Millerozyma, Priceomyces, and Scheffersomyces. Mycoscience 2010, 51, 2–14. [Google Scholar] [CrossRef]
- Delgenes, J.P.; Moletta, R.; Navarro, J.M. The effect of aeration on d-xylose fermentation by Pachysolen tannophilus, Pichia stipitis, Kluyveromyces marxianus and Candida shehatae. Biotechnol. Lett. 1986, 8, 897–900. [Google Scholar] [CrossRef]
- Limtong, S.; Sumpradit, T.; Kitpreechavanich, V.; Tuntirungkij, M.; Seki, T.; Yoshida, T. Effect of acetic acid on growth and ethanol fermentation of xylose fermenting yeast and Saccharomyces cerevisiae. Kasetsart J. (Nat. Sci.) 2000, 34, 64–73. [Google Scholar]
- Pereira, S.R.; Ivanuša, T.; Evtuguin, D.V.; Serafim, L.S.; Xavier, A.M.R.B. Biological treatment of eucalypt spent sulphite liquors: A way to boost the production of second generation bioethanol. Bioresour. Technol. 2012, 103, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.R.; Sànchez i Nogué, V.; Frazão, C.J.R.; Serafim, L.S.; Gorwa-Grauslund, M.F.; Xavier, A.M.R.B. Adaptation of scheffersomyces stipitis to hardwood spent sulfite liquor by evolutionary engineering. Biotechnol. Biofuels 2015, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nigam, J.N. Ethanol production from hardwood spent sulfite liquor using an adapted strain of pichia stipitis. J. Industrial Microbiol. Biotechnol. 2001, 26, 145–150. [Google Scholar] [CrossRef]
- Delgenes, J.P.; Moletta, R.; Navarro, J.M. Fermentation of d-xylose, d-glucose, l-arabinose mixture by pichia stipitis: Effect of the oxygen transfer rate on fermentation performance. Biotechnol. Bioeng. 1989, 34, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.A.; Mussatto, S.I.; Roberto, I.C.; Teixeira, J.A. Fermentation medium and oxygen transfer conditions that maximize the xylose conversion to ethanol by Pichia stipitis. Renew. Energy 2012, 37, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Skoog, K.; Hahn-Hagerdal, B. Effect of oxygenation on xylose fermentation by Pichia stipitis. Appl. Environ. Microbiol. 1990, 56, 3389–3394. [Google Scholar] [PubMed]
- Skoog, K.; Hahn-Hagerdal, B.; Degn, H.; Jacobsen, J.P.; Jacobsen, H.S. Ethanol reassimilation and ethanol tolerance in Pichia stipitis cbs 6054 as studied by 13c nuclear magnetic resonance spectroscopy. Appl. Environ. Microbiol. 1992, 58, 2552–2558. [Google Scholar] [PubMed]
- Furlan, S.A.; Bouilloud, P.; De Castro, H.F. Influence of oxygen on ethanol and xylitol production by xylose fermenting yeasts. Process Biochem. 1994, 29, 657–662. [Google Scholar] [CrossRef]
- Silva, J.P.A.; Mussatto, S.I.; Roberto, I.C. The influence of initial xylose concentration, agitation, and aeration on ethanol production by Pichia stipitis from rice straw hemicellulosic hydrolysate. Appl. Biochem. Biotechnol. 2010, 162, 1306–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, M.; Tohma, T.; Itaya, T.; Fujii, M. Ethanol production from a mixture of glucose and xylose by co-culture of pichia stipitis and a respiratory-deficient mutant of Saccharormyces cerevisiae. J. Ferment. Bioeng. 1997, 83, 364–370. [Google Scholar] [CrossRef]
- Bellido, C.; González-Benito, G.; Coca, M.; Lucas, S.; García-Cubero, M.T. Influence of aeration on bioethanol production from ozonized wheat straw hydrolysates using Pichia stipitis. Bioresour. Technol. 2013, 133, 51–58. [Google Scholar] [CrossRef] [PubMed]
- du Preez, J.C.; van Driessel, B.; Prior, B.A. D-xylose fermentation by Candida shehatae and Pichia stipitis at low dissolved oxygen levels in fed-batch cultures. Biotechnol. Lett. 1989, 11, 131–136. [Google Scholar] [CrossRef]
- Wise, W.S. The measurement of the aeration of culture media. J. Gen. Microbiol. 1951, 5, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Schaepe, S.; Kuprijanov, A.; Sieblist, C.; Jenzsch, M.; Simutis, R.; Lübbert, A. kLa of stirred tank bioreactors revisited. J. Biotechnol. 2013, 168, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Laplace, J.M.; Delgenes, J.P.; Moletta, R.; Navarro, J.M. Alcoholic fermentation of glucose and xylose by Pichia stipitis, Candida shehatae, Saccharomyces cerevisiae and Zymomonas mobilis: Oxygen requirement as a key factor. Appl. Microbiol. Biotechnol. 1991, 36, 158–162. [Google Scholar] [CrossRef]
- Silva, J.P.A.; Mussatto, S.I.; Roberto, I.C.; Teixeira, J.A. Ethanol production from xylose by pichia stipitis nrrl y-7124 in a stirred tank bioreactor. Braz. J. Chem. Eng. 2011, 28, 151–156. [Google Scholar] [CrossRef]
- Su, Y.K.; Willis, L.B.; Jeffries, T.W. Effects of aeration on growth, ethanol and polyol accumulation by Spathaspora passalidarum nrrl y-27907 and Scheffersomyces stipitis nrrl y-7124. Biotechnol.Bioeng. 2015, 112, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Xavier, A.M.R.B.; Correia, M.F.; Pereira, S.R.; Evtuguin, D.V. Second-generation bioethanol from eucalypt sulphite spent liquor. Bioresour. Technol. 2010, 101, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.L.A.; Silva, C.M.; Xavier, A.M.R.B.; Evtuguin, D.V. Fractionation of sulphite spent liquor for biochemical processing using ion exchange resins. J. Biotechnol. 2012, 162, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. Ii: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Ejim, L.; Mirza, I.A.; Capone, C.; Nazi, I.; Jenkins, S.; Chee, G.L.; Berghuis, A.M.; Wright, G.D. New phenolic inhibitors of yeast homoserine dehydrogenase. Bioorg. Med. Chem. 2004, 12, 3825–3830. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.M.; Modig, T.; Petersson, A.; Hähn-Hägerdal, B.; Lidén, G.; Gorwa-Grauslund, M.F. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J. Chem. Technol. Biotechnol. 2007, 82, 340–349. [Google Scholar] [CrossRef]
- Díaz, M.J.; Ruiz, E.; Romero, I.; Cara, C.; Moya, M.; Castro, E. Inhibition of pichia stipitis fermentation of hydrolysates from olive tree cuttings. World J. Microbiol. Biotechnol. 2009, 25, 891–899. [Google Scholar] [CrossRef]
- Nigam, J.N. Ethanol production from wheat straw hemicellulose hydrolysate by Pichia stipitis. J. Biotechnol. 2001, 87, 17–27. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, J.; Zhu, Y.; Zhang, L.; Yong, Q.; Xu, Y.; Li, X.; Yu, S. Cause analysis of the effects of acid-catalyzed steam-exploded corn stover prehydrolyzate on ethanol fermentation by Pichia stipitis cbs 5776. Bioprocess Biosyst. Eng. 2014, 37, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Mahler, G.; Nudel, C. Effect of magnesium ions on fermentative and respirative functions in pichia stipitis under oxygen-restricted growth. Microbiol. Res. 2000, 155, 31–35. [Google Scholar] [CrossRef]
- Mahler, G.F.; Guebel, D.V. Influence of magnesium concentration on growth, ethanol and xylitol production by Pichia stipitis nrrl y-7124. Biotechnol. Lett. 1994, 16, 407–412. [Google Scholar] [CrossRef]


| Fermentation Medium | Single-Stage Aeration Experiments | Two-Stage Aeration Experiments | |
|---|---|---|---|
| 1st Stage | 2nd Stage | ||
| Synthetic medium | DOT 1% | DOT 50% | 0 mLAir min−1 and 250 rpm |
| DOT 2.5% | DOT 50% | 50 mLAir min−1 and 150 rpm | |
| DOT 10% | DOT 50% | DOT 1% | |
| DOT 25% | |||
| DOT 50% | |||
| 60% HSSL/40% synthetic medium (v/v) | - | DOT 50% | 0 mLAir min−1 and 250 rpm |
| Medium | Synthetic Medium | 60% HSSL | ||||||
|---|---|---|---|---|---|---|---|---|
| Experiment | DOT 50%—0 mLAir min−1 and 250 rpm | DOT 50%—50 mLAir min−1 and 150 rpm | DOT 50%—DOT 1% | DOT 50%—0 mLAir min−1 and 250 rpm | ||||
| Stage | 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd |
| µmax (h−1) | 0.43 | - | 0.44 | - | 0.43 | - | 0.07 | - |
| [Biomass]max (g L−1) | 6.38 | 6.58 | 5.36 | 6.06 | 5.57 | 10.2 | 6.47 | - |
| [Ethanol]max (g L−1) | - | 15.8 | - | 11.4 | - | 1.67 | - | 12.2 |
| [Xylitol]max (g L−1) | - | 5.10 | - | 3.72 | - | 0.698 | - | 2.66 |
| rglucose (g L−1 h−1) | 0.58 | - | 0.51 | - | 0.53 | - | 0.03 | - |
| rxylose (g L−1 h−1) | 0.31 | 0.52 | - | 0.72 | - | 0.42 | - | 0.11 |
| racetic acid (g L−1 h−1) | - | - | - | - | - | - | 0.08 | - |
| Consumed sugars (%) | 16.9 | 82.2 | 10.4 | 84.2 | 12.6 | 86.4 | 5.7 | 66.8 |
| rethanol (g L−1 h−1) | - | 0.21 | - | 0.21 | - | 0.09 | - | 0.04 |
| rxylitol (g L−1 h−1) | - | 0.068 | - | 0.073 | - | 0.038 | - | 0.004 |
| Ybiomass/substrate (g g−1) | 0.78 | - | 1.13 | 0.02 | 0.93 | - | 0.65 | - |
| Yethanol/substrate (g g−1) | - | 0.40 | - | 0.29 | - | 0.06 | - | 0.39 |
| Yxylitol/xylose (g g−1) | - | 0.13 | - | 0.10 | - | 0.03 | - | 0.03 |
| Experiment | DOT 50%—0 mLAir min−1 and 250 rpm | DOT 50%—50 mLAir min−1 and 150 rpm | DOT 50%—DOT 1% |
|---|---|---|---|
| [Ethanol]max (g L−1) | 15.8 | 11.4 | 1.67 |
| rethanol (g L−1 h−1) | 0.18 | 0.18 | 0.04 |
| Yethanol/substrate (g g−1) | 0.33 | 0.25 | 0.04 |
| Conversion Efficiency (%) | 64.1 | 49.4 | 7.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henriques, T.M.; Pereira, S.R.; Serafim, L.S.; Xavier, A.M.R.B. Two-Stage Aeration Fermentation Strategy to Improve Bioethanol Production by Scheffersomyces stipitis. Fermentation 2018, 4, 97. https://doi.org/10.3390/fermentation4040097
Henriques TM, Pereira SR, Serafim LS, Xavier AMRB. Two-Stage Aeration Fermentation Strategy to Improve Bioethanol Production by Scheffersomyces stipitis. Fermentation. 2018; 4(4):97. https://doi.org/10.3390/fermentation4040097
Chicago/Turabian StyleHenriques, Tiago M., Susana R. Pereira, Luísa S. Serafim, and Ana M. R. B. Xavier. 2018. "Two-Stage Aeration Fermentation Strategy to Improve Bioethanol Production by Scheffersomyces stipitis" Fermentation 4, no. 4: 97. https://doi.org/10.3390/fermentation4040097