Abstract
The cost of bioethanol production from lignocellulosic materials is relatively high because the additional processes of delignification and saccharification are required. Consolidated bioprocessing (CBP) simultaneously uses the multiple processes of delignification, saccharification, and fermentation in a single reactor and has the potential to solve the problem of cost. Some wood-degrading basidiomycetes have lignin- and cellulose-degrading abilities as well as ethanol fermentation ability. The white rot fungus Schizophyllum commune NBRC 4928 was selected as a strong fermenter from a previous study. The lignin-degrading fungus Bjerkandera adusta and polysaccharide-degrading fungus Fomitopsis palustris were respectively added to S. commune ethanol fermentations to help degrade lignocellulosic materials. Bjerkandera adusta produced more ligninase under aerobic conditions, so a switching aeration condition was adopted. The mixed culture of S. commune and B. adusta promoted direct ethanol production from cedar wood. Fomitopsis palustris produced enzymes that released glucose from both carboxymethylcellulose and microcrystalline cellulose. The mixed culture of S. commune and F. palustris did not enhance ethanol production from cedar. The combination of S. commune and cellulase significantly increased the rate of ethanol production. The results suggest that CBP for ethanol production from cellulosic material can be achieved by using multiple fungi in one reactor.
1. Introduction
In bioethanol production using lignocellulosic material derived from non-food biomass, delignification and saccharification are required [1,2]. These pretreatments of biomass are performed by physical methods such as steam explosion [3,4] or by chemical methods such as acid [5,6,7,8] or alkaline hydrolysis [9,10]. However, these methods also produce substances that inhibit ethanol fermentation by yeast [11,12]. Enzymatic processes are considered suitable as a precursor to subsequent fermentation processes even though the overall processing time is extended [13,14]. Wood rot fungi produce enzymes that degrade the main components of plant cells. White rot fungi produce lignin-degrading and cellulose-degrading enzymes, while brown rot fungi produce only cellulase [15,16,17]. These enzymes remove lignin or convert cellulose and hemicellulose to sugars [18]. The increased costs incurred because of the additional processes of ethanol production from lignocellulosic materials is a serious problem. The concept of consolidated bioprocessing (CBP) that simultaneously uses the multiple processes of delignification, saccharification, and fermentation in a single reactor has been proposed [19,20]. Moreover, some wood rot fungi are known to produce ethanol from various sugars including xylose [21,22,23,24]. This suggests that ethanol production by wood rot fungi could be utilized without the need for recombinant gene technology. Therefore, wood rot fungi were thought to have a high potential for use in pretreatment and ethanol fermentation from cellulosic materials. We selected Schizophyllum commune NBRC 4928 as a strong fermenter using glucose and xylose [24] and examined the feasibility of ethanol production from cedar wood using S. commune NBRC 4928. However, the lignin-degrading ability of this fungal strain was weak even though it is a white rot fungus. An exhaustive genomic survey of the wood-degrading enzyme of S. commune NBRC 4928 revealed that this species has few genes related to lignin-degrading enzymes. We tried to improve S. commune ethanol production by using a white rot fungus that is an excellent ligninase producer. Results suggested that ligninase from the support fungus helped to refine the biomass material [25]. Fomitopsis palustris is a species of a brown rot fungus which degrades cellulose and hemicellulose but not lignin. We thought that the brown rot fungus might be able to support saccharification without delignification. In the present study, enhancement by Bjerkandera adusta IWA5b in mixed culture with S. commune NBRC 4928 was examined. In addition, the effect of using Fomitopsis palustris NBRC 30339 as a saccharification agent on ethanol production by S. commune NBRC 4928 was investigated.
2. Materials and Methods
2.1. Fungal Strains and Biomass
Schizophyllum commune NBRC 4928 and Fomitopsis palustris NBRC 30339 used in the study were obtained from the National Biological Research Center of Japan, whose nucleotide sequences of the international transcribed spacer (ITS) and 28s rDNA were analyzed to confirm identification of the strains and have submitted the sequences to the GenBank/DDBJ/EMBL databases (supplemental data). Bjerkandera adusta IWA5b was isolated from the airbone in our laboratory and identified by the ITS and 28S rDNA sequences (supplemental data). The fungal strains were subcultured on potato dextrose agar (Nissui, Tokyo, Japan) plates at 25 °C. Mycelial discs, 12 mm in diameter, were punched out from colonies as inocula. Four mycelial discs were put in a 200-mL flask for the fermentation test.
Japanese cedar (Cryptomeria japonica) wood was used as raw material for ethanol production. Sapwood was cut out and ground into 0.5-mm wood chips using a cutting mill MF10.1 (IKA, Staufen, Germany). The contents of holocellulose, lignin, ethanol-benzene extractives, and ash in the cedar wood were determined according to the Japanese Industrial Standard (P 8012, P 8008, P 8010, and P 8251), where the percentage were 52.8%, 31.4%, 3.2%, and 0.6%, respectively.
2.2. Fermentation Examination
Liquid medium (pH 6.0) containing 20 g/L carbon source, 10 g/L yeast extract, 10 g/L KH2PO4, 2 g/L (NH4)2SO4, and 0.5 g/L MgSO4·7H2O [26] was prepared, and 60 mL of medium was placed in a 200-mL flask containing 5 g of 5.0-mm-diameter glass beads, which were added to each flask to disperse the fungal mat. Glucose and cedar wood were used as a carbon sources. For glucose medium, liquid medium without sugar was first autoclaved and then glucose stock solutions sterilized by filtration using a 0.45-µm membrane filter were added to the medium to give a final concentration of 20 g/L. Semi-anaerobic conditions were generated by an N2 gas purge and a silicon rubber cap with a fermentation airlock. Aerobic conditions were maintained with a sponge cap. The switched aeration condition from aerobic to anaerobic was established by changing the cap of the flask from the sponge to the silicon one. Four discs of mycelia were inoculated in the flask and then incubated on a rotary shaker (90 rpm) at 30 °C. Cellulase Onozuka RS (Yakult, Tokyo, Japan) was used at 1% as an alternative to the fungal cellulase. All experiments were performed in triplicate by using three replicates for each set of conditions.
2.3. Measurement of Enzyme Activity
The liquid medium (pH 6.0) was prepared as above and F. palustris NBRC 30339 was incubated in 60 mL of the medium under anaerobic conditions with glucose or microcrystalline cellulose (MCC) (Sigma-Aldrich, St. Louis, MO, USA) as the carbon source. An aliquot of supernatant was collected periodically to measure cellulase activity. Cellulase activity was assessed by measuring the levels of reducing sugars released from carboxymethylcellulose (CMC) and MCC, respectively, using the 3,5-dinitrosalicylic acid (DNS) colorimetric test [27]. The reaction mixture contained 1% CMC or 1% MCC, 20 mM acetate buffer (pH = 5.0), and 200 µL of the supernatant of culture in a total volume of 1 mL. The mixture was incubated at 40 °C for 180 min and then heated at 95 °C for 10 min to stop the reaction. Reducing sugar in the reacted mixture was determined by the DNS method.
Bjerkandera adusta IWA5b was incubated in a liquid medium with glucose under aerobic and anaerobic conditions to measure the activity of manganese peroxidases (MnPs). An aliquot of supernatant was taken periodically and MnP activity was measured by using guaiacol and hydrogen peroxide [28].
2.4. High-Performance Liquid Chromatography
Samples (1 mL) of supernatant were collected periodically. The supernatant was collected after centrifugation at 11,000 g and filtered with a membrane filter to remove particles. The concentration of ethanol and residual glucose in the sample liquid was determined by HPLC on a Hitachi 7000-D system (Hitachi, Tokyo, Japan) equipped with a differential refractive index detector (L-7490, Hitachi) and a Sugar KS-802 (8.0 × 300 mm) column (Shodex, Tokyo, Japan). The HPLC system was operated at 50 °C using distilled water as the mobile phase at a flow rate of 1.0 mL/min. The limit of detection for the HPLC we used is almost 0.05 g/L.
3. Results
3.1. Ethanol Fermentation Abilities of S. Commune NBRC 4928, B. Adusta IWA5b, and F. Palustris NBRC 30339
The concentrations of ethanol and glucose in the medium using glucose as carbon source are shown in Figure 1. Schizophyllum commune NBRC 4928, which is an excellent ethanol fermenter, converted glucose into ethanol at 80.1% of the theoretical yield on the sixth day. The actual yield (expressed as a percentage of theoretical yield) was computed by dividing the actual yield per 1 g of sugar by the theoretical yield. The theoretical yield of ethanol from glucose was defined as 0.511 g/g-glu. Bjerkandera adusta IWA5b produced ethanol at only 7.1% of the theoretical yield on the 18th day under anaerobic conditions, and 22.0% of the glucose was consumed. However, it produced ethanol at 28.3% of the theoretical yield under aerobic conditions, and glucose consumption was faster. For F. palustris NBRC 30339, the production rate under anaerobic conditions was slower than for S. commune NBRC 4928, but the percentage of theoretical yield was 78.6% on the 28th day.
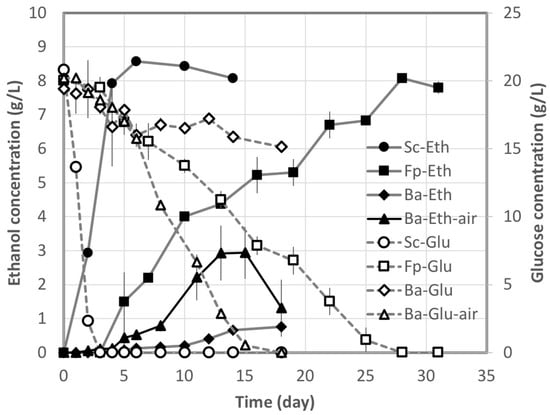
Figure 1.
Ethanol (Eth) production from glucose (Glu) by Schizophyllum commune NBRC 4928, Bjerkandera adusta IWA5b, and Fomitopsis palustris NBRC 30339. Plots and bars show the average and standard deviation obtained from triplicate experiments.
3.2. Production of Lignin- and Cellulose-Degrading Enzymes
Relative activities of MnP produced by B. adusta IWA5b were estimated under three kinds of aeration conditions: aerobic conditions maintained by a sponge cap, anaerobic conditions maintained by N2 gas (>99.99%) and a silicon rubber cap, and switched aeration conditions (Figure 2). More MnP was produced under aerobic conditions, while it was scarcely produced under anaerobic conditions. Bjerkandera adusta IWA5b requires O2 to produce MnP, so a trial with aeration switching was tested. MnP was produced in the aerobic period and its activity was maintained at the same level after switching aeration.
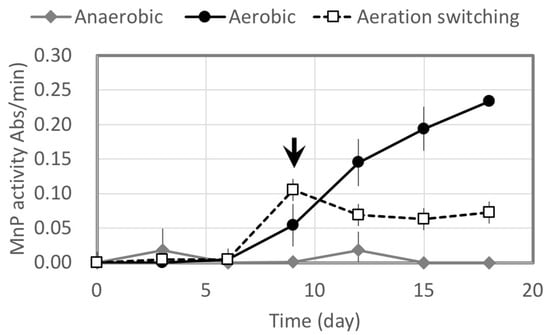
Figure 2.
Relative manganese peroxidase (MnP) activities by B. adusta IWA5b under three kinds of aeration conditions: aerobic condition, anaerobic condition, and switching aeration (at arrow).
Relative cellulase activities were estimated by measuring the reduced sugars released from CMC and MCC (Figure 3). Liquid media were prepared using glucose or CMC as the carbon source. Cellulase was observed to generate glucose from CMC and MCC despite the use of anaerobic conditions, but the level in the MCC medium was very low. The MCC medium was employed to induce cellulase production but no significant effect was observed.
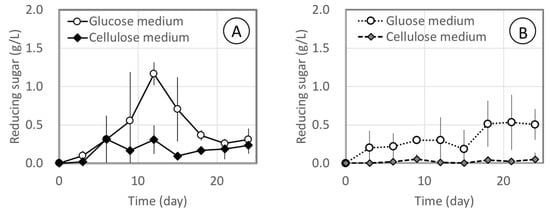
Figure 3.
Relative cellulase activities by F. palustris NBRC 30339 in glucose medium and MCC medium. Cellulase activities were estimated by measuring reducing sugar released from CMC (A) and MCC (B). All experiments were conducted under semi-anaerobic conditions.
3.3. Ethanol Production from Glucose Medium Using Mixed Culture
Ethanol production from glucose was performed using mixed cultures of S. commune NBRC 4928 + B. adusta IWA5b (Sc/Ba) and S. commune NBRC 4928 + F. palustris NBRC 30339 (Sc/Fp) to investigate the effect of the helper fungi on ethanol fermentation by S. commune NBRC 4928 (Figure 4). Two pieces of S. commune and two pieces of B. adusta or F. palustris were inoculated in each flask. Both mixed cultures (Sc/Ba and Sc/Fp) had consumed fully glucose in 6 days and the maximum yields were 67.1% and 80.6%, respectively. The results suggest that the helper fungi did not significantly affect the ethanol yield or the rate of fermentation of S. commune NBRC 4928.
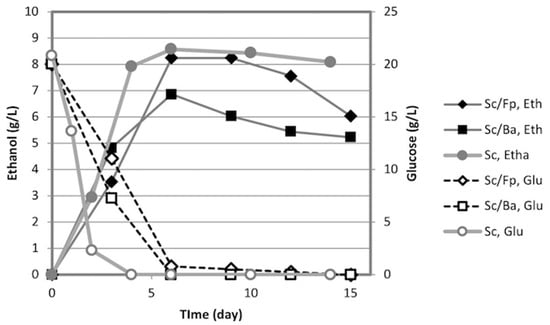
Figure 4.
Ethanol production from glucose by mixed culture of S. commune + B. adusta (Sc/Ba) and that of S. commune + F. palustris (Sc/Fp). All tests were conducted under semi-anaerobic conditions.
3.4. Ethanol Production from Biomass by Mixed Culture
Direct ethanol production from cedar wood was investigated. The experiments were conducted in a single step and the fungi for degrading cedar wood and fermenting ethanol (Sc/Ba or Sc /Fp) were inoculated into the reactor flask at the same time. Little released monosaccharide from cedar wood was detected in the CBP, suggesting that these sugars are consumed quickly for fungal metabolic processes and growth. A combination of S. commune NBRC 4928 and 1% (w/v) cellulase Onozuka was also examined to compare with cedar degradation by fungi (Figure 5). Ethanol from cedar wood was scarcely produced by S. commune alone and the maximum ethanol concentration was 0.01%. However, ethanol concentration increased 15-fold in a mixed culture of Sc/Ba using switching aeration. In this experiment, ethanol concentration increased by the sixth day but then decreased to near 0 g/L after that. The Sc/Fp combination also produced very little ethanol from cedar wood. However, a combination of S. commune and cellulase continued to produce ethanol for 18 days to give a final ethanol concentration of 1.66%. The yield of ethanol converted from cedar wood by the combination of S. commune NBRC 4928 and commercial cellulase was 2.49% on a raw material basis and 4.72% on a holocellulose basis.
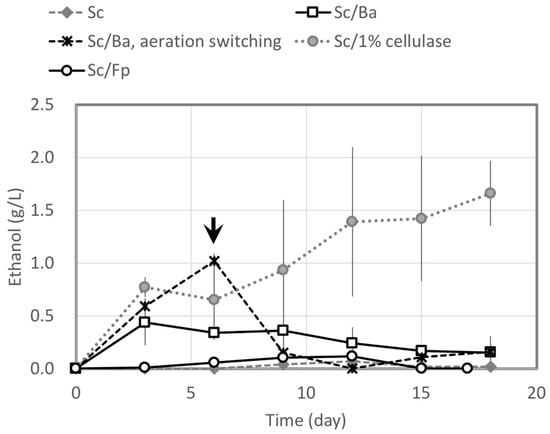
Figure 5.
Ethanol production from cedar wood by S. commune alone, mixed cultures of S. commune + B. adusta (Sc/Ba) and S. commune + F. palustris (Sc/Fp), and S. commune with 1% cellulase. All tests were conducted under semi-anaerobic conditions except for a part of the experiment using Sc/Ba. An arrow shows the switching point of aeration (from aerobic to anaerobic) in the Sc/Ba condition.
4. Discussion
To improve cellulase accessibility to cellulose, B. adusta IWA5b was added to the fermentation flask of S. commune. Bjerkandera adusta is reported to produce MnP and other lignin-degrading enzymes [29,30]. Bjerkandera adusta IWA5b used in the present study was isolated in our laboratory and was selected as the known MnP producer. The fungal strain actively produced MnP under aerobic conditions, but not anaerobically, while S. commune NBRC 4928 produced ethanol more under anaerobic conditions than anaerobically [24]. For ethanol production from cedar wood using the mixed culture of Sc/Ba, the apparent mismatch of aerobic and anaerobic function was addressed by switching the aeration condition from aerobic to anaerobic during fermentation. The switching condition improved ethanol production, but it then decreased with further incubation. Testing for escaped ethanol was conducted using the same flask and silicon cap with an airlock, but no loss of ethanol was detected [24] Therefore, it was considered that one or both fungi consumed the ethanol because of a lack of an available carbon source. Optimization of the O2 supply condition is considered necessary to improve ethanol production using the mixed culture of Sc/Ba.
The cellulase-producing fungus F. palustris NBRC 30339 was added to the fermentation flask of S. commune to enhance ethanol production from cedar wood by degrading cellulose. Fomitopsis palustris secretes enzymes that degrade polysaccharide and causes brown rot decay in the wood [31,32]. However, the results showed almost no difference between the single and mixed cultures, probably because of an insufficient rate of cellulase secretion from F. palustris. This reasoning is supported by the fact that the combination of S. commune and cellulase produced ethanol continuously at a high rate, similar to the Sc/Ba combined fermentation. It is considered that CBP or simultaneous saccharification and fermentation (SSF) would be possible if the concentration of cellulase is sufficiently high.
To produce ethanol from cellulosic biomass by CBP, it is necessary that the processes of lignin decomposition, glycosylation of polysaccharide, and ethanol production from glucose and xylose occur together in the one reaction chamber. The results of this study suggest that CBP for ethanol production from cellulosic material can be achieved by administering multiple microorganisms to a reaction tank without using multifunctional microbes accessed by gene recombination. In the direct ethanol production from cedar wood in the present study, Schizophyllum commune in the CBP added cellulase produced ethanol at 2.49% of the yield on raw material without any processing. That shows a clue for cost reduction of bioethanol production, however, the yield should be improved for the application. Several studies have reported ethanol production from biomass using wood rot fungi. Okamoto et al. reported that the white rot fungus Trametes hirsuta applied to produce ethanol for CBP of crushed rice straw gave the yield of 17% on raw material basis [21]. Maehata et al. reported that Flammulina velutipes in CBP with cellulase performed the ethanol yield of 36% from amorphous cellulose prepared from sugarcane bagasse [33]. Our previous study reported that Schizophyllum commune converted microcrystalline cellulose to ethanol at the yield of 25.9% on the raw material basis. These studies indicate the importance of saccharification of biomass. An effective, low-cost technology of saccharification should be established for cellulosic bioethanol production.
Supplementary Materials
The following are available online at http://www.mdpi.com/2311-5637/5/1/21/s1, supplemental data.
Author Contributions
Conceptualization, S.H.; Data curation, S.H., A.I., and Y.Y.; validation, S.H., A.I., and Y.Y.; formal analysis, S.H. and A.I.; investigation, S.H., I.A., and Y.Y.; resources, S.H., I.A., and Y.Y.; writing—original draft preparation, S.H. and A.I.; writing—review and editing, S.H., A.I., and Y.Y.; visualization, S.H. and A.I.; supervision, S.H.; project administration, S.H.
Funding
This research received no external funding.
Acknowledgments
We express our appreciation to Osamu Ariga of Kochi University of Technology, for his technical support. We thank Austin Schultz from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, G.S.; Pan, X.J.; Zhu, J.Y.; Gleisner, R.; Rockwood, D. Sulfite Pretreatment to Overcome Recalcitrance of Lignocellulose (SPORL) for Robust Enzymatic Saccharification of Hardwoods. Biotechnol. Prog. 2009, 25, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Maurya, D.P.; Singla, A.; Negi, S. An Overview of Key Pretreatment Processes for Biological Conversion of Lignocellulosic Biomass to Bioethanol. 3 Biotech 2015, 5, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.J.; Eijsink, V.G.H. Enzymatic Hydrolysis of Steam-Exploded Hardwood Using Short Processing Times. Biosci. Biotechnol. Biochem. 2010, 74, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.J.; Nguyen, Q.D.; Westereng, B.; Nilsen, P.J.; Eijsink, V.G.H. Screening of Steam Explosion Conditions for Glucose Production from Non-Impregnated Wheat Straw. Biomass Bioenergy 2011, 35, 4879–4886. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Pan, X.J.; Wang, G.S.; Gleisner, R. Sulfite Pretreatment (SPORL) for Robust Enzymatic Saccharification of Spruce and Red Pine. Bioresour. Technol. 2009, 100, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of Promising Technologies for Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, H.; Chen, J.; Lin, Z.; Jin, Q.; Jia, H.; Huang, H. Dilute Sulfuric Acid Cycle Spray Flow-through Pretreatment of Corn Stover for Enhancement of Sugar Recovery. Bioresour. Technol. 2009, 100, 1803–1808. [Google Scholar] [CrossRef]
- Murali, N.; Srinivas, K.; Ahring, B.K.; Murali, N.; Srinivas, K.; Ahring, B.K. Biochemical Production and Separation of Carboxylic Acids for Biorefinery Applications. Fermentation 2017, 3, 22. [Google Scholar] [CrossRef]
- Dixit, G.; Shah, A.R.; Madamwar, D.; Narra, M. High Solid Saccharification Using Mild Alkali-Pretreated Rice Straw by Hyper-Cellulolytic Fungal Strain. Bioresour. Bioprocess. 2015, 2, 46. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, W.; Rosentrater, K.; Yang, M.; Zhang, W.; Rosentrater, K.A. Anhydrous Ammonia Pretreatment of Corn Stover and Enzymatic Hydrolysis of Glucan from Pretreated Corn Stover. Fermentation 2017, 3, 9. [Google Scholar] [CrossRef]
- Parawira, W.; Tekere, M. Biotechnological Strategies to Overcome Inhibitors in Lignocellulose Hydrolysates for Ethanol Production: Review. Crit. Rev. Biotechnol. 2011, 31, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, K.; Bertilsson, M.; Lidén, G. A Short Review on SSF—An Interesting Process Option for Ethanol Production from Lignocellulosic Feedstocks. Biotechnol. Biofuels 2008, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, T. The Degradation of Cellulose and the Production of Cellulase, Xylanase, Mannanase and Amylase by Wood—Attacking Microfungi; Skogshögskolan: Stockholm, Sweden, 1974; Volume 114. [Google Scholar]
- Shinichi, Y.A.N.O. Enzymatic Saccharification and Fermentation Technology for Ethanol Production from Woody Biomass. J. Jpn. Pet. Inst. 2015, 58, 128–134. [Google Scholar]
- Blanchette, R.A. Delignification by Wood-Decay Fungi. Annu. Rev. Phytopathol. 1991, 29, 381–403. [Google Scholar] [CrossRef]
- Blanchette, R.A. Screening Wood Decayed by White Rot Fungi for Preferential Lignin Degradation Screening Wood Decayed by White Rot Fungi for Preferential Lignin Degradationt. Appl. Environ. Microbiol. 1984, 48, 647–665. [Google Scholar] [PubMed]
- Enoki, A.; Takahashi, M.; Tanaka, H.; Fuse, G. Degradation of Lignin-Related Compounds and Wood Components by White-Rot and Brown-Rot Fungi. Mokuzai Gakkaishi 1985, 31, 397–408. [Google Scholar] [CrossRef]
- Ryu, S.-H.; Cho, M.-K.; Kim, M.; Jung, S.-M.; Seo, J.-H. Enhanced Lignin Biodegradation by a Laccase-Overexpressed White-Rot Fungus Polyporus Brumalis in the Pretreatment of Wood Chips. Appl. Biochem. Biotechnol. 2013, 171, 1525–1534. [Google Scholar] [CrossRef]
- Lynd, L.R.; van Zyl, W.H.; McBride, J.E.; Laser, M. Consolidated Bioprocessing of Cellulosic Biomass: An Update. Curr. Opin. Biotechnol. 2005, 16, 577–583. [Google Scholar] [CrossRef]
- Xu, Q.; Singh, A.; Himmel, M.E. Perspectives and New Directions for the Production of Bioethanol Using Consolidated Bioprocessing of Lignocellulose. Curr. Opin. Biotechnol. 2009, 20, 364–371. [Google Scholar] [CrossRef]
- Okamoto, K.; Nitta, Y.; Maekawa, N.; Yanase, H. Direct Ethanol Production from Starch, Wheat Bran and Rice Straw by the White Rot Fungus Trametes Hirsuta. Enzyme Microb. Technol. 2011, 48, 273–277. [Google Scholar] [CrossRef]
- Okamoto, K.; Uchii, A.; Kanawaku, R.; Yanase, H. Bioconversion of Xylose, Hexoses and Biomass to Ethanol by a New Isolate of the White Rot Basidiomycete Trametes Versicolor. Springerplus 2014, 3, 121. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, R.; Ichinose, H.; Honda, M.; Takabatake, K.; Sotome, I.; Takai, T.; Maehara, T.; Okadome, H.; Isobe, S.; Gau, M.; et al. Use of Whole Crop Sorghums as a Raw Material in Consolidated Bioprocessing Bioethanol Production Using Flammulina Velutipes. Biosci. Biotechnol. Biochem. 2009, 73, 1671–1673. [Google Scholar] [CrossRef] [PubMed]
- Horisawa, S.; Nishida, T. Ethanol Production from Lignocellulosic Material by White Rot Fungi. J. Adv. Clean Energy 2014, 1, 71–76. [Google Scholar]
- Horisawa, S.; Ando, H.; Ariga, O.; Sakuma, Y. Direct Ethanol Production from Cellulosic Materials by Consolidated Biological Processing Using the Wood Rot Fungus Schizophyllum commune. Bioresour. Technol. 2015, 197. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Imashiro, K.; Akizawa, Y.; Onimura, A.; Yoneda, M.; Nitta, Y.; Maekawa, N.; Yanase, H. Production of Ethanol by the White-Rot Basidiomycetes Peniophora cinerea and Trametes suaveolens. Biotechnol. Lett. 2010, 32, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Kofujita, H.; Asada, Y.; Kuwahara, M. Alkyl-Aryl Cleavage of Phenolic Beta-O-4 Lignin Substructure Model Compound by Mn(II)-Peroxidase Isolated from Pleurotus ostreatus. Mokuzai gakkaishi 1991, 37, 555–561. [Google Scholar]
- Järvinen, J.; Taskila, S.; Isomäki, R.; Ojamo, H. Screening of White-Rot Fungi Manganese Peroxidases: A Comparison between the Specific Activities of the Enzyme from Different Native Producers. AMB Express 2012, 2, 62. [Google Scholar] [CrossRef]
- Camarero, S.; Martinez, M.J.; Martinez, A.T. A New Versatile Peroxidase. Biochem. Soc. Trans. 2001, 29, 116–122. [Google Scholar]
- Yoon, J.J.; Igarashi, K.; Kajisa, T.; Samejima, M. Purification, Identification and Molecular Cloning of Glycoside Hydrolase Family 15 Glucoamylase from the Brown-Rot Basidiomycete Fomitopsis palustris. FEMS Microbiol. Lett. 2006, 259, 288–294. [Google Scholar] [CrossRef]
- Jeong-Jun, Y.; Cha, C.J.; Kim, Y.S.; Son, D.W.; Kim, Y.K. The Brown-Rot Basidiomycete Fomitopsis palustris Has the Endo-Glucanases Capable of Degrading Microcrystalline Cellulose. J. Microbiol. Biotechnol. 2007, 17, 800–805. [Google Scholar] [CrossRef]
- Maehara, T.; Ichinose, H.; Furukawa, T.; Ogasawara, W.; Takabatake, K.; Kaneko, S. Ethanol Production from High Cellulose Concentration by the Basidiomycete Fungus Flammulina velutipes. Fungal Biol. 2013, 117, 220–226. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).