Physico-Chemical Characteristics and Amino Acid Content Evaluation of Citric Acid by-Product Produced by Microbial Fermentation as a Potential Use in Animal Feed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Physical Characteristic Measurement
2.3. Microscopy Compound
2.4. Chemical Composition
2.5. Citric Acid Measurement
2.6. Amino Acid Determination
2.7. Aflatoxins and Fumonisin Measurement
2.8. Statistical Analysis
3. Results
3.1. Physical Characteristics
3.2. Microscopy Compound
3.3. Chemical Composition
3.4. Amino Acid Composition
3.5. Mycotoxins Contamination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Show, P.L.; Oladele, K.O.; Siew, Q.Y.; Aziz Zakry, F.A.; Lan, J.C.W.; Ling, T.C. Overview of citric acid production from Aspergillus niger. Front. Life Sci. 2015, 8, 271–283. [Google Scholar] [CrossRef] [Green Version]
- Dhillon, G.; Brar, S.; Dhillon, S.; Verma, M. Bioproduction and extraction optimization of citric acid from Aspergillus niger by rotating drum type solid-state bioreactor. Ind. Crops Prod. 2013, 41, 78–84. [Google Scholar] [CrossRef]
- Alsudani, A.A.; Al-Shibli, M.K. Citric acid production from some local isolates of the fungus Aspergillus niger by rice husks filtrate medium. Int. J. Recent Sci. Res. 2015, 6, 5625–5633. [Google Scholar]
- Khalil, M.; Berawi, M.A.; Heryanto, R.; Rizalie, A. Waste to energy technology: The potential of sustainable biogas production from animal waste in Indonesia. Renew. Sustain. Energy Rev. 2019, 105, 323–331. [Google Scholar] [CrossRef]
- Tanpong, S.; Cherdthong, A.; Tengjaroenkul, B.; Tengjaroenkul, U.; Wongtangtintharn, S. Evaluation of physical and chemical properties of citric acid industrial waste. Trop. Anim. Health Prod. 2019, 105, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H. Animal nutrition in a 360-degree view and a framework for future R&D work: Towards sustainable livestock production. Anim. Prod. Sci. 2016, 56, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- Syamsu, J.A.; Yusuf, M.; Abdullah, A. Evaluation of physical properties of feedstuffs in supporting the development of feed mill at farmers group scale. J. Adv. Agric. Technol. 2015, 2, 147–150. [Google Scholar] [CrossRef] [Green Version]
- AAFCO. Association of American Feed Control Officials; Association of American Feed Control Officials Inspection and Sampling Committee: Champaign, IL, USA, 2014. [Google Scholar]
- Vasconcelos, L.M.; Brito, A.C.; Carmo, C.D.; Oliveira, P.H.G.A.; Oliveira, E.J. Phenotypic diversity of starch granules in cassava germplasm. Genet. Mol. Res. 2017, 16, 1–15. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists; Official Methods of Analysis, 15th ed.; Association ofAnalytical Chemists: Arlington, TX, USA, 1990. [Google Scholar]
- Ezea, I.; Chiejina, N.V.; Ogbonna, J.C. Biological production of citric acid in solid state cultures of Aspergillus niger. ChemXpress 2016, 8, 201–207. [Google Scholar]
- Nimbalkar, M.S.; Pai, S.R.; Pawar, N.V.; Oulkar, D.; Dixit, G.B. Free amino acid profiling in grain Amaranth using LC–MS/MS. Food Chem. 2012, 134, 2565–2569. [Google Scholar] [CrossRef]
- Thiele, B.; Hupert, M.; Santiago-Schübel, B.; Oldiges, M.; Hofmann, D. Direct analysis of underivatized amino acids in plant extracts by LC-MS/MS (Improved Method). Methods Mol. Biol. 2019, 2030, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Chumroenphat, T.; Somboonwatthanakul, I.; Saensouk, S. Changes in curcuminoids and chemical components of turmeric (Curcuma longa L) under freeze-drying and low-temperature drying methods. Food Chem. 2021, 339, 128121. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.; Trucksess, M. Determination of Aflatoxins in Botanical Roots by a Modification of AOAC Official MethodSM 991.31: Single-Laboratory Validation. J. AOAC Int. 2010, 93, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Sirhan, A.Y.; Tan, G.H.; Al-Shunnaq, A.; Abdulra’uf, L.; Wong, R.C.S. QuEChERS-HPLC method for aflatoxin detection of domestic and imported food in Jordan. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 321–342. [Google Scholar] [CrossRef]
- Nwabanne, J.T. Drying characteristics and engineering properties of fermented ground cassava. African J. Biotechnol. 2009, 8, 873–876. [Google Scholar] [CrossRef]
- Ndou, S.P.; Gous, R.M.; Chimonyo, M. Prediction of scaled feed intake in weaner pigs using physico-chemical properties of fibrous feeds. Br. J. Nutr. 2013, 110, 774–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, S.; Herrman, T. MF2051 Evaluating Particle Size; Kansas State University: Manhattan, Kansas, 1976; pp. 1–6. [Google Scholar]
- Fitzpatrick, J.; Iqbal, T.; Delaney, C.; Twomey, T.; Keogh, M.K. Effect of powder properties on the flowability of milk powders with different fat contents. J. Food Eng. 2004, 64, 435–444. [Google Scholar] [CrossRef]
- Amidon, G.; Meyer, P.J.; Mudie, D. Particle, Powder, and Compact Characterization. In Developing Solid Oral Dosage Forms: Pharmaceutical Theory and Practice, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 271–293. ISBN 9780128024478. [Google Scholar]
- Papagianni, M.; Boonpooh, Y.; Mattey, M.; Kristiansen, B. Substrate inhibition kinetics of Saccharomyces cerevisiae in fed-batch cultures operated at constant glucose and maltose concentration levels. J. Ind. Microbiol. Biotechnol. 2007, 34, 301–309. [Google Scholar] [CrossRef]
- Max, B.; Salgado, J.; Rodríguez, N.; Cortes, S.; Converti, A.; Domínguez, J. Biotechnological production of citric acid. Braz. J. Microbiol. 2010, 41, 862–875. [Google Scholar] [CrossRef] [Green Version]
- Angumeenal, A.R.; Venkappayya, D. An overview of citric acid production. LWT-Food Sci. Technol. 2013, 50, 367–370. [Google Scholar] [CrossRef]
- Vu, T.V.; Delgado-Saborit, J.M.; Harrison, R.M. Review: Particle number size distributions from seven major sources and implications for source apportionment studies. Atmos. Environ. 2015, 122, 114–132. [Google Scholar] [CrossRef]
- Nutrition, C.A. Impacts of feed texture and particle size on broiler and layer feeding patterns. In Proceedings of the XXIV World’s Poultry Congress, Bahia, Brazil, 9 August 2012; pp. 1–8. [Google Scholar]
- Kiarie, E.G.; Mills, A. Role of Feed Processing on Gut Health and Function in Pigs and Poultry: Conundrum of Optimal Particle Size and Hydrothermal Regimens. Front. Vet. Sci. 2019, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammed, H. 2 Effect of utilization organic acid supplement on broiler (ROS-308). Int. J. Adv. Res. Biol. Sci. 2016, 3, 76–81. [Google Scholar]
- Tanpong, S. Utilization of Citric Acid by Product from Industrial Waste Used as Poultry Feed. Ph.D. Thesis, Khon Kaen University, Khon Kaen, Thailand, 2019. [Google Scholar]
- Juliano, B. Rice in human nutrition; International Rice Research Institute, Food and Agriculture Organization of the United Nations: Rome, Italy, 1993; pp. 31–34. [Google Scholar]
- Wu, G.; Bazer, F.; Dai, Z.; Li, D.; Wang, J.; Wu, Z. Amino acid nutrition in animals: Protein synthesis and beyond. Annu. Rev. Anim. Biosci. 2014, 2, 387–417. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, D.; Panneerselvam, A.; Thajuddin, N. Evaluation of aflatoxicosis in hens fed with commercial poultry feed. Turkish J. Vet. Anim. Sci. 2009, 33, 385–391. [Google Scholar] [CrossRef]

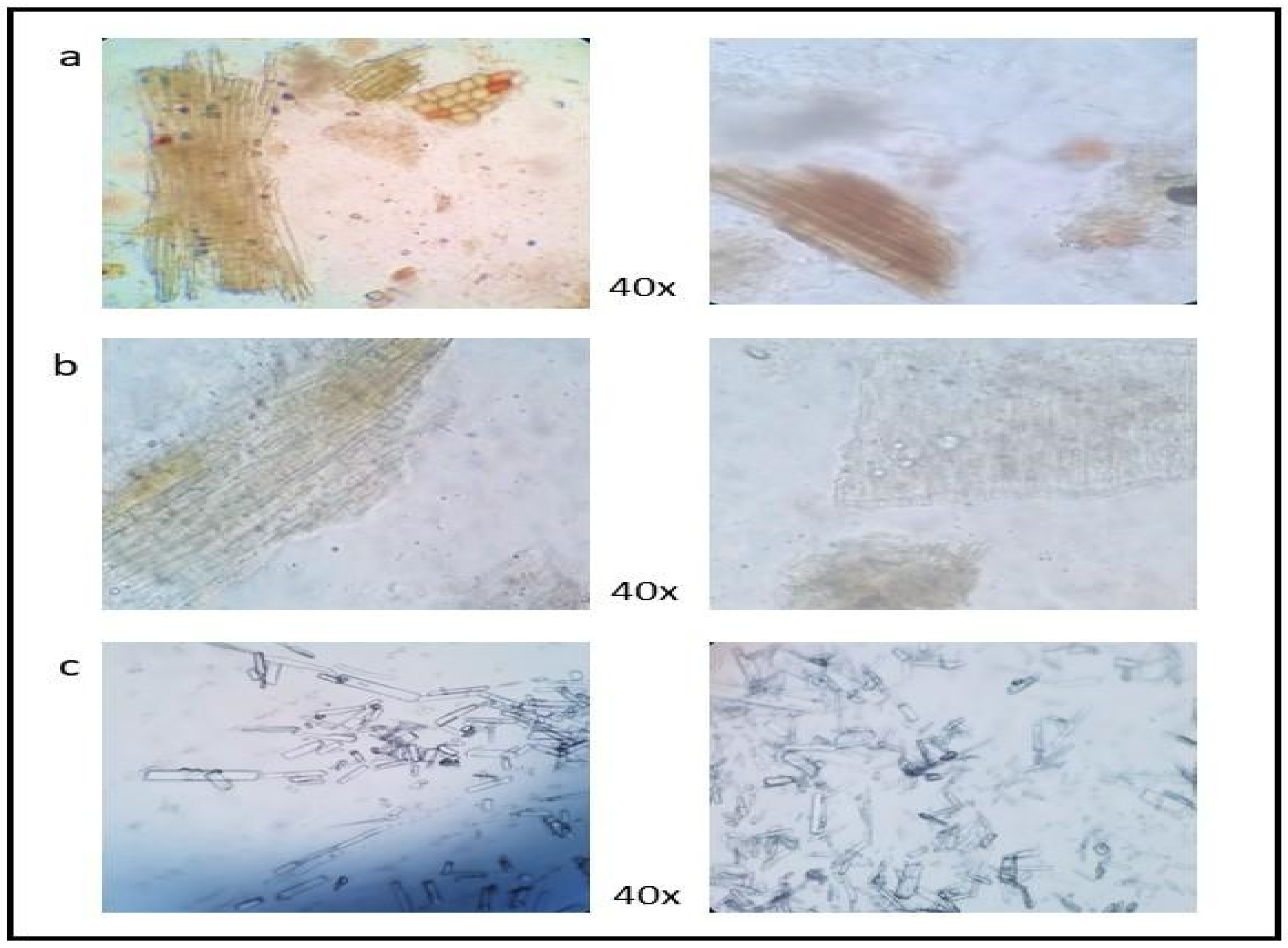
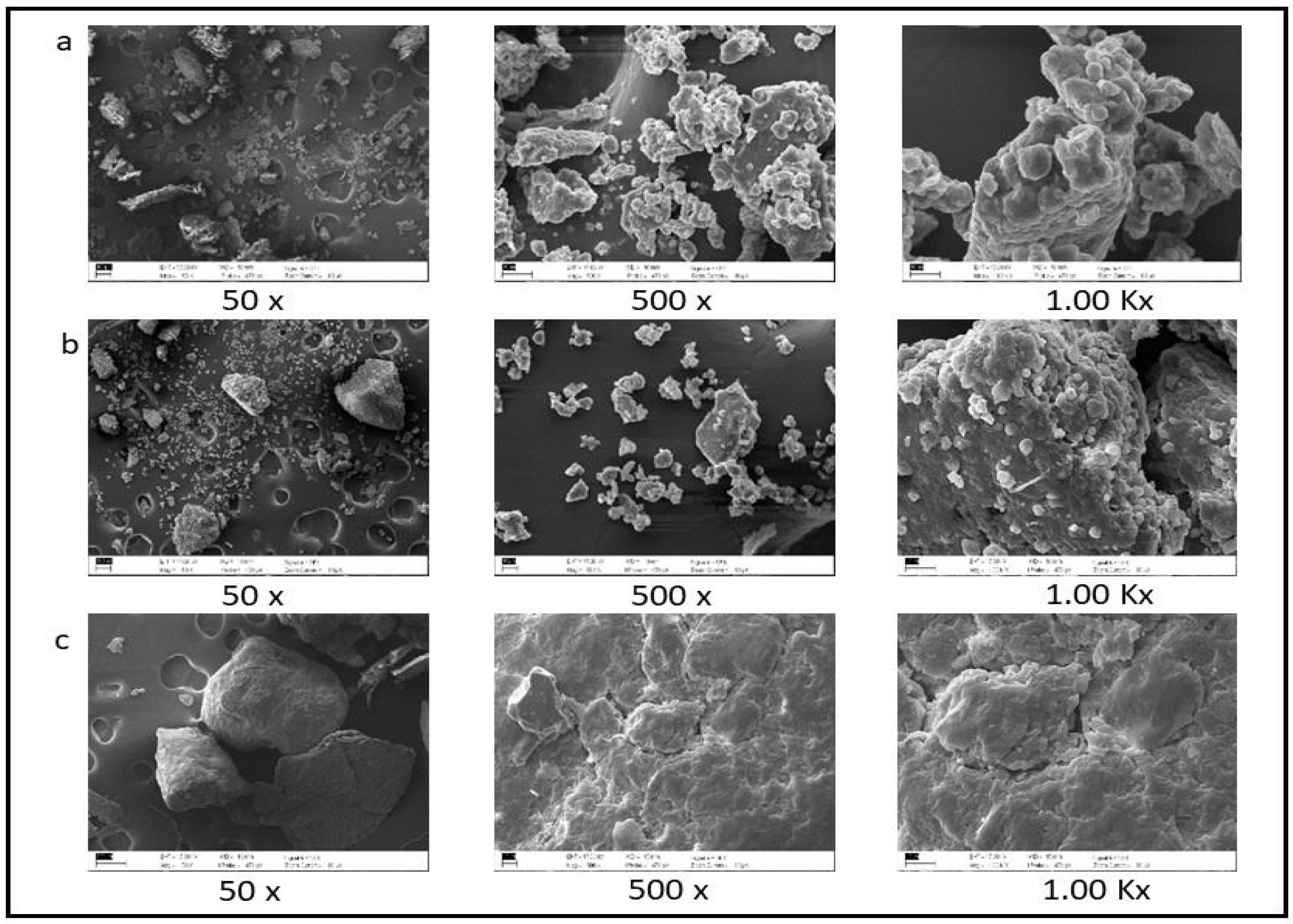
| Parameter | CABR | BR | RB | SEM | p-Value |
|---|---|---|---|---|---|
| Bulk density (g/L) | 549.65 b | 868.12 a | 453.78 c | 5.867 | 0.0001 |
| Angle of repose (o) | 40.6 b | 39.45 a | 50.6 c | 0.380 | 0.0001 |
| Color | |||||
| L* | 45.02 b | 78.37 a | 76.90 c | 0.397 | 0.0001 |
| A* | 5.64 a | 1.66 b | 1.20 c | 0.080 | 0.0001 |
| B* | 13.88 c | 18.89 a | 17.92 b | 0.147 | 0.0001 |
| Sieve Mesh no. | Sieve Size (μm) | Sample (g) | Retain (%) | SEM | Cumulative (%) | Passing (%) | Dgw 1 (μm) | SEM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CABR | BR | RB | CABR | BR | RB | CABR | BR | RB | CABR | BR | RB | CABR | BR | RB | ||||
| 20 | 850 | 19.92 | 99.92 | 31.21 | 20.09 c | 99.78 a | 31.09 b | 1.56 | 20.09 | 99.78 | 31.09 | 79.91 | 0.22 | 68.91 | 232 c | 600 a | 338 b | 5.915 |
| 40 | 425 | 22.82 | 0.19 | 40.3 | 23.01 b | 0.20 c | 40.15 a | 0.87 | 43.01 | 99.98 | 71.25 | 56.9 | 0.02 | 28.75 | ||||
| 60 | 250 | 19.35 | 0.00 | 19.37 | 19.51 b | 0.01 b | 19.3 | 0.82 | 62.61 | 99.98 | 90.54 | 37.39 | 0.02 | 9.46 | ||||
| 80 | 180 | 14.26 | 0.00 | 7.58 | 14.38 a | 0.00 c | 7.55 b | 0.92 | 76.98 | 99.99 | 98.1 | 23.01 | 0.01 | 1.9 | ||||
| 100 | 150 | 8.91 | 0.00 | 01.20 | 7.77 a | 0.00 c | 1.20 b | 0.09 | 84.75 | 99.99 | 99.3 | 15.25 | 0.01 | 0.7 | ||||
| Pan | 15.12 | 0.01 | 0.70 | 15.25 a | 0.01 c | 0.70 b | 0.20 | 100.00 | 100.00 | 100.00 | 0 | 0 | 0 | |||||
| Total | 100.42 | 100.14 | 100.37 | 100 | 100 | 100 | ||||||||||||
| Parameter | CABR | BR | RB | SEM | p-Value |
|---|---|---|---|---|---|
| Moisture (%) | 8.26 b | 9.35 a | 8.43 b | 0.114 | 0.0012 |
| Ash (%) | 9.35 a | 0.49 c | 0.49 b | 0.154 | 0.0001 |
| Soluble ash (%) | 5.20 b | 0.49 c | 6.88 a | 0.148 | 0.0002 |
| Insoluble ash (%) | 4.15 a | 0.00 c | 0.39 b | 0.013 | 0.0001 |
| Ether extract (%) | 3.98 b | 1.41 c | 14.28 a | 0.161 | 0.0001 |
| Crude fiber (%) | 11.97 a | 0.10 b | 0.76 b | 0.297 | 0.0002 |
| Nitrogen-free extract (%) | 46.64 c | 82.20 a | 55.89 b | 0.428 | 0.0001 |
| Ca (%) | 0.43 b | 0.76 a | 0.76 a | 0.050 | 0.0001 |
| Phosphorus (%) | 0.07 a | 0.04 b | 0.01 c | 0.003 | 0.0001 |
| Crude protein (%) | 19.80 a | 6.47 c | 13.37 b | 0.159 | 0.0001 |
| Gross energy (kcal/kg) | 4,005.72 b | 3,780.52 c | 4,287.11 a | 7.430 | 0.0001 |
| pH | 4.77 b | 6.46 a | 6.51 a | 0.053 | 0.0001 |
| Citric acid content (%) | 3.3 | - | - |
| Amino Acid (g/kg) | CABR | BR | RB | SEM | p-Value |
|---|---|---|---|---|---|
| Essential amino acid | |||||
| Leucine | 3.72 b | 2.72 c | 4.42 a | 17.23 | 0.0051 |
| Valine | 2.70 a | 1.80 b | 1.53 c | 7.59 | 0.0012 |
| Isoleucine | 2.49 b | 1.71 c | 3.48 a | 11.34 | 0.0013 |
| Phenylamine | 1.53 b | 1.20 b | 3.63 a | 33.20 | 0.0098 |
| Threonine | 1.44 a | 0.87 b | 0.82 b | 4.24 | 0.0012 |
| Histidine | 0.92 b | 4.03 a | 0.93 b | 36.47 | 0.0052 |
| Tryptophan | 0.39 c | 1.13 a | 0.61 b | 18.13 | 0.0565 |
| Lysine | 0.31 c | 0.87 b | 1.88 a | 7.24 | 0.0005 |
| Methionine | 0.05 c | 0.96 a | 0.37 b | 50.41 | 0.3216 |
| Non-essential amino acid | |||||
| Tyrosine | 5.50 c | 6.81 b | 12.99 a | 30.37 | 0.0003 |
| Glycine | 0.77 a | 0.77 | 0.28 b | 4.83 | 0.0003 |
| Proline | 2.96 b | 1.49 c | 7.58 a | 24.04 | 0.0003 |
| Alanine | 9.07 a | 4.26 b | 2.47 c | 12.72 | 0.0001 |
| Cysteine | 0.05 b | 0.06 a | 0.07 a | 0.51 | 0.1828 |
| Arginine | 3.77 b | 3.05 b | 5.82 a | 53.64 | 0.0293 |
| Aspartic acid | 0.81 a | 0.38 b | 0.13 c | 4.28 | 0.0013 |
| Glutamic acid | 1.99 a | 0.67 c | 0.99 b | 7.37 | 0.0008 |
| Serine | 0.07 c | 0.09 b | 0.11 a | 1.08 | 0.0738 |
| Asparagine | 0.32 b | 5.07 a | 0.66 b | 33.23 | 0.0013 |
| Glutamine | 0.31 c | 0.90 b | 1.97 a | 6.90 | 0.0004 |
| Parameter | Observation | LOD |
|---|---|---|
| Aflatoxin B1 | ND | 0.8 μg/kg |
| Aflatoxin B2 | ND | 0.8 μg/kg |
| Aflatoxin G1 | ND | 0.8 μg/kg |
| Aflatoxin G2 | ND | 0.8 μg/kg |
| Fumonisin B1 | ND | 100.00 μg/kg |
| Fumonisin B2 | ND | 100.00 μg/kg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oryza.S, M.; Wongtangtintharn, S.; Tengjaroenkul, B.; Cherdthong, A.; Tanpong, S.; Bunchalee, P.; Pootthachaya, P.; Reungsang, A.; Polyorach, S. Physico-Chemical Characteristics and Amino Acid Content Evaluation of Citric Acid by-Product Produced by Microbial Fermentation as a Potential Use in Animal Feed. Fermentation 2021, 7, 149. https://doi.org/10.3390/fermentation7030149
Oryza.S M, Wongtangtintharn S, Tengjaroenkul B, Cherdthong A, Tanpong S, Bunchalee P, Pootthachaya P, Reungsang A, Polyorach S. Physico-Chemical Characteristics and Amino Acid Content Evaluation of Citric Acid by-Product Produced by Microbial Fermentation as a Potential Use in Animal Feed. Fermentation. 2021; 7(3):149. https://doi.org/10.3390/fermentation7030149
Chicago/Turabian StyleOryza.S, Mutyarsih, Sawitree Wongtangtintharn, Bundit Tengjaroenkul, Anusorn Cherdthong, Sirisak Tanpong, Pasakorn Bunchalee, Padsakorn Pootthachaya, Alissara Reungsang, and Sineenart Polyorach. 2021. "Physico-Chemical Characteristics and Amino Acid Content Evaluation of Citric Acid by-Product Produced by Microbial Fermentation as a Potential Use in Animal Feed" Fermentation 7, no. 3: 149. https://doi.org/10.3390/fermentation7030149










