Microalgal Biodiesel: A Challenging Route toward a Sustainable Aviation Fuel
Abstract
:1. Introduction
2. Current Aviation Fuel Production
2.1. Aviation Fuel Specification
2.2. Kerosene
2.3. Routes to SAF Production
3. Microalgae as a Potential Fuel Feedstock
4. Potential Areas for Growing Microalgae
5. Current Challenges in Cultivating High-Oil Microalgae
5.1. Strain Selection
5.2. Nutrient Cost
5.3. Growing Platforms and Harvesting
5.4. Optimising Oil Production
- (i)
- Diacylglycerol and phosphosphatidylcholine (PC) interconversion catalysed by cholinephosphotransferase.
- (ii)
- Acyl exchange between the acyl-CoA pool and position sn-2 of PC catalysed by lyso phosphtidylcholine:acyl-CoA acyltransferase.
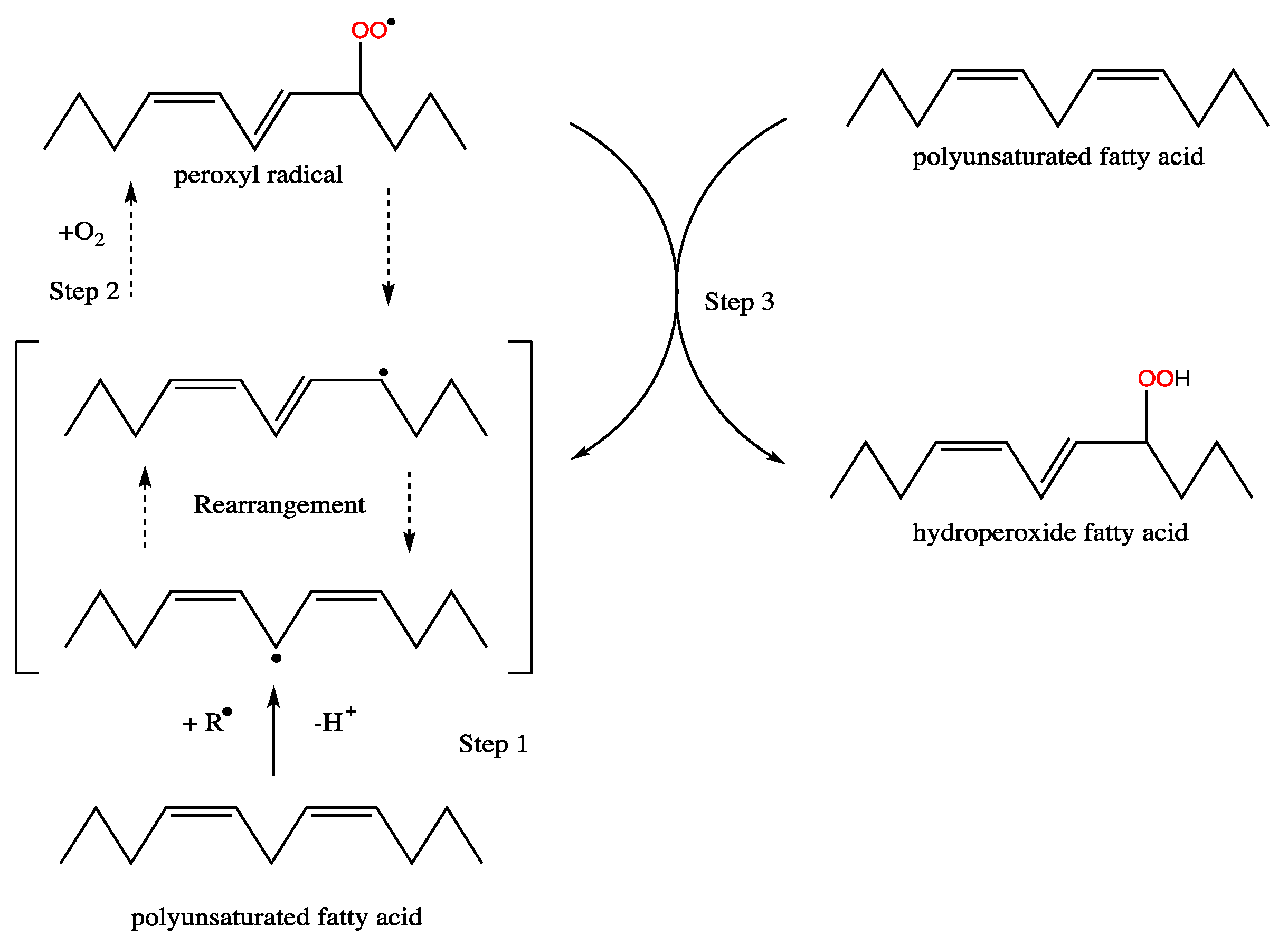
5.5. Fatty Acid Composition and Oxidative Stability of Derived Biodiesels
5.6. Non-Triacylglycerol Lipid Sources from Microalgae
6. Energy and Economics of Algae-Based Aviation Biofuels
7. Aviation Flights Undertaken Using Microalgal Biofuel
8. Future Work
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| ATAG | Air Transport Action Group |
| ASTM | American Society for Testing and Materials |
| ATJ | Alcohol-to-jet |
| GHG | Greenhouse gas |
| AVGAS | Aviation gasoline |
| CO2 | Carbon dioxide |
| CO2 eq | Carbon dioxide equivalent |
| CORSIA | Carbon Offsetting and Reduction Scheme for International Aviation |
| CH | Catalytic hydrotreating |
| CCS-APR | Catalytic conversion of sugars by aqueous phase reforming |
| DAG | Diacylglycerol |
| DAGAT | Diacylglycerol acyltransferase |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| EU ETS | EU Emissions Trading Scheme |
| EFR | Effective radiative forcing |
| FAME | Fatty acid methyl esters |
| FT-SPK | Fischer-Tropsch synthetic paraffinic kerosene |
| GCAM | Global change assessment model |
| GLA | Gamma linolenic acid |
| HFS-SIP | Hydro-processing of fermented sugars–synthetic isoparaffinic kerosene |
| HEFA | Hydro-processed esters and fatty acids |
| ICAO | International Civil Aviation Organization |
| IEA | International Energy Agency |
| ILUC | Indirect land use change |
| LA | Linoleic acid |
| LNA | Linolenic acid |
| LPA | Lyso-phosphatidic acid |
| LPC | Lyso-phophatidylcholine |
| MAG | Monoacylglycerol |
| Mt | Million tonnes |
| MJSP | Minimum jet fuel selling price |
| PA | Phosphatidic acid |
| PC | Phosphatidylcholine |
| PDAT | Phosphatidylcholine: diacylglycerol acyltransferase |
| PUFAs | Polyunsaturated fatty acids |
| RF | Radiative forcing |
| SPK | Synthetic paraffinic kerosene |
| SAF | Sustainable aviation fuel |
| TAGs | Triacylglycerols |
| UCO | Used cooking oil |
References
- Lokke, S.; Aramendia, E.; Malskaer, J. A review of public opinion on liquid biofuels in the EU: Current knowledge and future challenges. Biomass Bioenergy 2021, 150, 106094. [Google Scholar]
- Varotsos, C.; Krapivin, V.; Mkrtchyan, F.; Zhou, X.R. On the effects of aviation on carbon-methane cycles and climate change during the period 2015–2100. Atmos. Pollut. Res. 2021, 12, 184–194. [Google Scholar]
- Chiaramonti, D. Sustainable Aviation Fuels: The challenge of decarbonization. Innov. Solut. Energy Transit. 2019, 158, 1202–1207. [Google Scholar]
- Chiaramonti, D.; Talluri, G.; Scarlat, N.; Prussi, M. The challenge of forecasting the role of biofuel in EU transport decarbonisation at 2050: A meta-analysis review of published scenarios. Renew. Sustain. Energy Rev. 2021, 139, 110715. [Google Scholar]
- Carlsson, F.; Hammar, H. Incentive-based regulation of CO2 emissions from international aviation. J. Air Transp. Manag. 2002, 8, 365–372. [Google Scholar]
- Hassan, M.; Pfaender, H.; Mavris, D. Probabilistic assessment of aviation CO2 emission targets. Transp. Res. Part D Transp. Environ. 2018, 63, 362–376. [Google Scholar]
- Bailis, R.E.; Bake, J.E. Greenhouse Gas Emissions and Land Use Change from Jatropha Curcas-Based Jet Fuel in Brazil. Environ. Sci. Technol. 2010, 44, 8684–8691. [Google Scholar]
- Lehr, U.; Nitsch, J.; Kratzat, M.; Lutz, C.; Edler, D. Renewable energy and employment in Germany. Energ. Policy 2008, 36, 108–117. [Google Scholar] [CrossRef]
- Agency, I.E. Market Report Series Renewables 2018, Analysis and Forecast to 2023. 2022. Available online: https://www.iea.org/reports/renewables-2018 (accessed on 25 May 2023).
- IATA Forecast Predicts 8.2 Billion Air Travelers in 2037. 2018. Available online: https://www.iata.org/en/pressroom/pr/2018-10-24-02/ (accessed on 20 March 2023).
- Abrantes, I.; Ferreira, A.F.; Silva, A.; Costa, M. Sustainable aviation fuels and imminent technologies—CO2 emissions evolution towards 2050. J. Clean. Prod. 2021, 313, 127937. [Google Scholar]
- Sustainable Aviation Fuel Report. Sustainable Aviation Fuel Road-Map 2020. 2020. Available online: https://www.sustainableaviation.co.uk/wp-content/uploads/2020/02/SustainableAviation_FuelReport_20200231.pdf (accessed on 1 April 2023).
- Biofuelwatch. ICAO Aviation Biofuels Report. 2017. Available online: https://www.biofuelwatch.org.uk/wp-content/uploads/Aviation-biofuels-report.pdf (accessed on 20 April 2023).
- CMR GWONGa. Jet Biofuels from Algae, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Mitici, M.; Pereira, M.; Oliviero, F. Electric flight scheduling with battery-charging and battery-swapping opportunities. EURO J. Transp. Logist. 2022, 11, 100074. [Google Scholar]
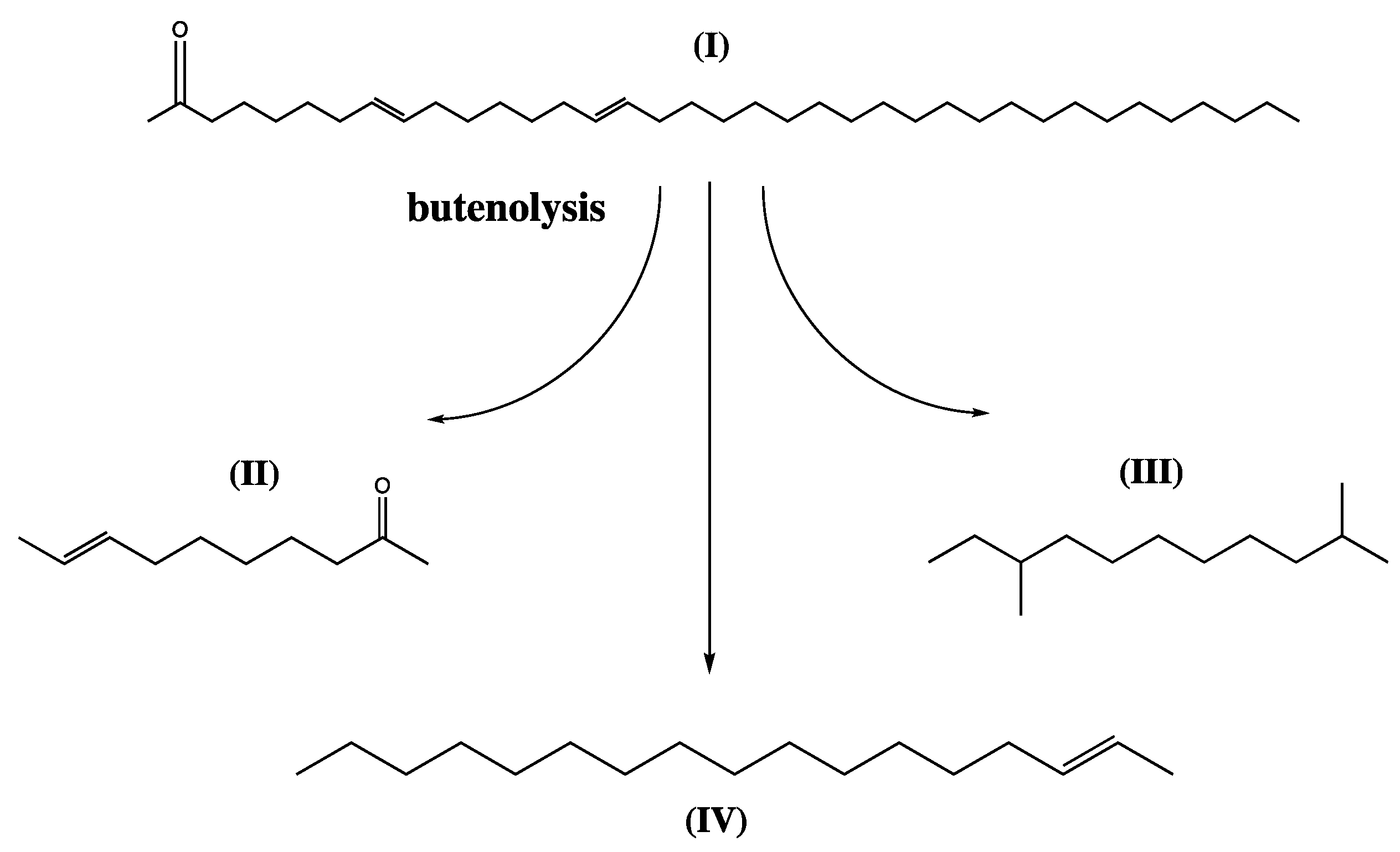
- O’Neil, G.W.; Culler, A.R.; Williams, J.R.; Burlow, N.P.; Gilbert, G.J.; Carmichael, C.A.; Nelson, R.K.; Swarthout, R.F.; Reddy, C.M. Production of Jet Fuel Range Hydrocarbons as a Coproduct of Algal Biodiesel by Butenolysis of Long-Chain Alkenones. Energy Fuels 2015, 29, 922–930. [Google Scholar]
- Paek, S.W.; Kim, S.; Raj, R.V. Optimal Endurance and Range of Electric Aircraft with Battery Degradation. Trans. Jpn. Soc. Aeronaut. Space Sci. 2020, 63, 62–65. [Google Scholar] [CrossRef]
- Bauen, A.; Bitossi, N.; German, L.; Harris, A.; Leow, K. Sustainable Aviation Fuels Status, challenges and prospects of drop-in liquid fuels, hydrogen and electrification in aviation. Johns. Matthey Technol. Rev. 2020, 64, 263–278. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, J. Estimation of Liquid Hydrogen Fuels in Aviation. Aerospace 2022, 9, 564. [Google Scholar]
- Ajjawi, I.; Verruto, J.; Aqui, M.; Soriaga, L.B.; Coppersmith, J.; Kwok, K.; Peach, L.; Orchard, E.; Kalb, R.; Xu, W.; et al. Lipid production in Nannochloropsis gaditana is doubled by decreasing expression of a single transcriptional regulator. Nat. Biotechnol. 2017, 35, 647–652. [Google Scholar]
- Aliyu, A.; Lee, J.G.M.; Harvey, A.P. Microalgae for biofuels via thermochemical conversion processes: A review of cultivation, harvesting and drying processes, and the associated opportunities for integrated production. Bioresour. Technol. Rep. 2021, 14, 100676. [Google Scholar]
- Deane, P.G.B.; Shea, R.O. Biofuels for Aviation: Technology Brief; International Renewable Energy Agency (IRENA): Masdar City, United Arab Emirates, 2017. [Google Scholar]
- Jia, T.H.; Zhang, X.W.; Liu, Y.; Gong, S.; Deng, C.; Pan, L.; Zou, J.-J. A comprehensive review of the thermal oxidation stability of jet fuels. Chem. Eng. Sci. 2021, 229, 116157. [Google Scholar]
- Qavi, I.; Jiang, L.L.; Akinyemi, O.S. Near-field spray characterization of a high-viscosity alternative jet fuel blend C-3 using a flow blurring injector. Fuel 2021, 293, 120350. [Google Scholar]
- Beal, C.M.; Cuellar, A.D.; Wagner, T.J. Sustainability assessment of alternative jet fuel for the U.S. Department of Defense. Biomass Bioenergy 2021, 144, 105881. [Google Scholar] [CrossRef]
- Dutta, S.; Madav, V.; Joshi, G.; Naik, N.; Kumar, S. Directional synthesis of aviation-, diesel-, and gasoline range hydrocarbon fuels by catalytic transformations of biomass components: An overview. Fuel 2023, 347, 128437. [Google Scholar]
- Wang, W.C.; Tao, L. Bio-jet fuel conversion technologies. Renew. Sustain. Energy Rev. 2016, 53, 801–822. [Google Scholar]
- US Department of Energy; Sustainable Aviation Fuel; Review of Technical Pathways. 2020. Available online: https://www.energy.gov/sites/prod/files/2020/09/f78/beto-sust-aviation-fuel-sep-2020.pdf (accessed on 4 March 2023).
- Vozka, P.; Kilaz, G. A review of aviation turbine fuel chemical composition-property relations. Fuel 2020, 268, 117391. [Google Scholar]
- Wei, H.J.; Liu, W.Z.; Chen, X.Y.; Yang, Q.; Li, J.S.; Chen, H.P. Renewable bio-jet fuel production for aviation: A review. Fuel 2019, 254, 115599. [Google Scholar]
- Kang, D.; Kim, D.; Kalaskar, V.; Violi, A.; Boehman, A.L. Experimental characterization of jet fuels under engine relevant conditions-Part 1: Effect of chemical composition on autoignition of conventional and alternative jet fuels. Fuel 2019, 239, 1388–1404. [Google Scholar]
- Ardo, F.M.; Lim, J.W.; Ramli, A.; Lam, M.K.; Kiatkittipong, W.; Abdelfattah, E.A.; Shahid, M.K.; Usman, A.; Wongsakulphasatch, S.; Sahrin, N.T. A review in redressing challenges to produce sustainable hydrogen from microalgae for aviation industry. Fuel 2022, 330, 125646. [Google Scholar]
- Prussi, M.; O’Connell, A.; Lonza, L. Analysis of current aviation biofuel technical production potential in EU28. Biomass Bioenergy 2019, 130, 105371. [Google Scholar] [CrossRef]
- Mathimani, T.; Mallick, N. A comprehensive review on harvesting of microalgae for biodiesel—Key challenges and future directions. Renew. Sustain. Energy Rev. 2018, 91, 1103–1120. [Google Scholar]
- Rajvanshi, S.; Sharma, M.P. Micro Algae: A Potential Source of Biodiesel. J. Sustain. Bioenergy Syst. 2012, 2, 49–59. [Google Scholar]
- Shokravi, Z.; Shokravi, H.; Atabani, A.E.; Lau, W.J.; Chyuan, O.H.; Ismail, A.F. Impacts of the harvesting process on microalgae fatty acid profiles and lipid yields: Implications for biodiesel production. Renew. Sustain. Energy Rev. 2022, 161, 112410. [Google Scholar]
- Fasaei, F.; Bitter, J.H.; Slegers, P.M.; van Boxtel, A.J.B. Techno-economic evaluation of microalgae harvesting and dewatering systems. Algal Res. 2018, 31, 347–362. [Google Scholar]
- Lin, C.H.; Chen, Y.K.; Wang, W.C. The production of bio-jet fuel from palm oil derived alkanes. Fuel 2020, 260, 116345. [Google Scholar]
- Doliente, S.S.; Narayan, A.; Tapia, J.F.D.; Samsatli, N.J.; Zhao, Y.R.; Samsatli, S. Bio-aviation Fuel: A Comprehensive Review and Analysis of the Supply Chain Components. Front. Energy Res. 2020, 8, 110. [Google Scholar]
- Yu, W.B.; Zhao, F.Y. Formulating of model-based surrogates of jet fuel and diesel fuel by an intelligent methodology with uncertainties analysis. Fuel 2020, 268, 117393. [Google Scholar]
- Bosnjakovic, M.; Sinaga, N. The Perspective of Large-Scale Production of Algae Biodiesel. Appl. Sci. 2020, 10, 8181. [Google Scholar]
- Zheng, X.S.R.D. Fuel Burn of New Commercial Jet Aircraft: 1960 to 2019; Int Counc Clean Transp: Washington, DC, USA, 2020. [Google Scholar]
- Bwapwa, J.K.; Anandraj, A.; Trois, C. Possibilities for conversion of microalgae oil into aviation fuel: A review. Renew. Sustain. Energy Rev. 2017, 80, 1345–1354. [Google Scholar]
- Griffiths, G.; Hossain, A.K.; Sharma, V.; Duraisamy, G. Key Targets for Improving Algal Biofuel Production. Clean. Technol. 2021, 3, 711–742. [Google Scholar]
- Li, P.Y.; Wang, X.; Luo, Y.Q.; Yuan, X.G. Sustainability evaluation of microalgae biodiesel production process integrated with nutrient close-loop pathway based on emergy analysis method. Bioresour. Technol. 2022, 346, 126611. [Google Scholar]
- Patnaik, R.; Mallick, N. Microalgal Biodiesel Production: Realizing the Sustainability Index. Front. Bioeng. Biotech. 2021, 9, 620777. [Google Scholar]
- Craggs, R.J.; Heubeck, S.; Lundquist, T.J.; Benemann, J.R. Algal biofuels from wastewater treatment high rate algal ponds. Water Sci. Technol. 2011, 63, 660–665. [Google Scholar]
- Zhang, L.; Wang, S.; Han, J.C.; Yang, G.P.; Pan, K.H.; Xu, J.L. Manipulation of triacylglycerol biosynthesis in Nannochloropsis oceanica by overexpressing an Arabidopsis thaliana diacylglycerol acyltransferase gene. Algal Res. 2022, 61, 102590. [Google Scholar]
- Paul, T.; Sinharoy, A.; Baskaran, D.; Pakshirajan, K.; Pugazhenthi, G.; Lens, P.N.L. Bio-oil production from oleaginous microorganisms using hydrothermal liquefaction: A biorefinery approach. Crit. Rev. Environ. Sci. Technol. 2022, 52, 356–394. [Google Scholar] [CrossRef]
- Prasad, R.; Gupta, S.K.; Shabnam, N.; Oliveira, C.Y.B.; Nema, A.K.; Ansari, F.A.; Bux, F. Role of Microalgae in Global CO2 Sequestration: Physiological Mechanism, Recent Development, Challenges, and Future Prospective. Sustainability 2021, 13, 13601. [Google Scholar]
- Chu, R.Y.; Hu, D.; Zhu, L.D.; Li, S.X.; Yin, Z.H.; Yu, Y.J. Recycling spent water from microalgae harvesting by fungal pellets to re-cultivate Chlorella vulgaris under different nutrient loads for biodiesel production. Bioresour. Technol. 2022, 344, 126227. [Google Scholar] [CrossRef] [PubMed]
- Vasistha, S.; Khanra, A.; Clifford, M.; Rai, M.P. Current advances in microalgae harvesting and lipid extraction processes for improved biodiesel production: A review. Renew. Sustain. Energy Rev. 2021, 137, 110498. [Google Scholar]
- Song, X.T.; Kong, F.Y.; Liu, B.F.; Song, Q.Q.; Ren, N.Q.; Ren, H.Y. Thallium-mediated NO signaling induced lipid accumulation in microalgae and its role in heavy metal bioremediation. Water Res. 2023, 239, 120027. [Google Scholar]
- Mofijur, M.; Ashrafur Rahman, S.M.; Nguyen, L.N.; Mahlia, T.M.I.; Nghiem, L.D. Selection of microalgae strains for sustainable production of aviation biofuel. Bioresour. Technol. 2022, 345, 126408. [Google Scholar] [CrossRef]
- Ahn, Y.; Park, S.; Ji, M.K.; Ha, G.S.; Jeon, B.H.; Choi, J. Biodiesel production potential of microalgae, cultivated in acid mine drainage and livestock wastewater. J. Environ. Manage. 2022, 314, 115031. [Google Scholar]
- Patlakas, P.; Stathopoulos, C.; Flocas, H.; Kalogeri, C.; Kallos, G. Regional Climatic Features of the Arabian Peninsula. Atmosphere 2019, 10, 220. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.-H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M.; et al. Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances, and future prospects. Environ. Sci. Ecotechnol. 2023, 13, 100205. [Google Scholar]
- Awad, N.; Vega-Estévez, S.; Griffiths, G. Salicylic acid and aspirin stimulate growth of Chlamydomonas and inhibit lipoxygenase and chloroplast desaturase pathways. Plant Physiol. Biochem. 2020, 149, 256–265. [Google Scholar] [CrossRef]
- Arora, K.; Kaur, P.; Kumar, P.; Singh, A.; Patel, S.K.S.; Li, X.; Yang, Y.-H.; Bhatia, S.K.; Kulshrestha, S. Valorization of Wastewater Resources Into Biofuel and Value-Added Products Using Microalgal System. Front. Energy Res. 2021, 9, 646571. [Google Scholar]
- Muhammad, G.; Alam, M.A.; Mofijur, M.; Jahirul, M.I.; Lv, Y.; Xiong, W.; Ong, H.C.; Xu, J. Modern developmental aspects in the field of economical harvesting and biodiesel production from microalgae biomass. Renew. Sustain. Energy Rev. 2021, 135, 110209. [Google Scholar]
- Kim, B.-H.; Choi, J.-E.; Cho, K.; Kang, Z.; Ramanan, R.; Moon, D.-G.; Kim, H.-S. Influence of Water Depth on Microalgal Production, Biomass Harvest, and Energy Consumption in High Rate Algal Pond Using Municipal Wastewater. J. Microbiol. Biotechnol. 2018, 28, 630–637. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar]
- Bharathiraja, B.; Iyyappan, J.; Gopinath, M.; Jayamuthunagai, J.; PraveenKumar, R. Transgenicism in algae: Challenges in compatibility, global scenario and future prospects for next generation biofuel production. Renew. Sustain. Energy Rev. 2022, 154, 111829. [Google Scholar] [CrossRef]
- Bellou, S.; Baeshen, M.N.; Elazzazy, A.M.; Aggeli, D.; Sayegh, F.; Aggelis, G. Microalgal lipids biochemistry and biotechnological perspectives. Biotechnol. Adv. 2014, 32, 1476–1493. [Google Scholar] [CrossRef]
- Sun, X.M.; Geng, L.J.; Ren, L.J.; Ji, X.J.; Hao, N.; Chen, K.Q.; Huang, H. Influence of oxygen on the biosynthesis of polyunsaturated fatty acids in microalgae. Bioresour. Technol. 2018, 250, 868–876. [Google Scholar] [CrossRef]
- Carlsson, A.S.; Yilmaz, J.L.; Green, A.G.; Stymne, S.; Hofvander, P. Replacing fossil oil with fresh oil—With what and for what? Eur. J. Lipid Sci. Technol. 2011, 113, 812–831. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.; Li, C.-L.; Zhu, S.-N.; Wang, Z.-M.; Zeng, E.Y. Lipid accumulation and eicosapentaenoic acid distribution in response to nitrogen limitation in microalga Eustigmatos vischeri JHsu-01 (Eustigmatophyceae). Algal Res. 2020, 48, 101910. [Google Scholar] [CrossRef]
- Song, X.T.; Liu, B.F.; Kong, F.Y.; Song, Q.Q.; Ren, N.Q.; Ren, H.Y. Lipid accumulation by a novel microalga Parachlorella kessleri R-3 with wide pH tolerance for promising biodiesel production. Algal Res. 2023, 69, 102925. [Google Scholar] [CrossRef]
- Osorio, J.H.M.; Pollio, A.; Frunzo, L.; Lens, P.N.L.; Esposito, G. A Review of Microalgal Biofilm Technologies: Definition, Applications, Settings and Analysis. Front. Chem. Eng. 2021, 3, 737710. [Google Scholar] [CrossRef]
- Fica, Z.T.; Sims, R.C. Algae-based biofilm productivity utilizing dairy wastewater: Effects of temperature and organic carbon concentration. J. Biol. Eng. 2016, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Zhang, L.J.; Xu, G.; Li, F.; Li, X.K. A review on biodiesel production from microalgae: Influencing parameters and recent advanced technologies. Front. Microbiol. 2022, 13, 737710. [Google Scholar] [CrossRef]
- Mediboyina, M.K.; Banuvalli, B.K.; Chauhan, V.S.; Mudliar, S.N. Comparative life cycle assessment of autotrophic cultivation of Scenedesmus dimorphus in raceway pond coupled to biodiesel and biogas production. Bioprocess. Biosyst. Eng. 2020, 43, 233–247. [Google Scholar] [CrossRef]
- Arguelles, E.D.L.R. NITROGEN STARVATION INDUCED LIPID ACCUMULATION BY Chlorococcum infusionum (EAU-10) AS POTENTIAL RENEWABLE SOURCE OF LIPID FOR BIODIESEL PRODUCTION. J. Microbiol. Biotechnol. Food Sci. 2022, 11, e1931. [Google Scholar] [CrossRef]
- Chu, F.; Cheng, J.; Zhang, X.; Ye, Q.; Chen, S.; Zhou, J.; Cen, K. Transcriptome and key gene expression related to carbon metabolism and fatty acid synthesis of Chlorella vulgaris under a nitrogen starvation and phosphorus repletion regime. J. Appl. Phycol. 2019, 31, 2881–2893. [Google Scholar] [CrossRef]
- Mou, Y.; Liu, N.; Su, K.; Li, X.; Lu, T.; Yu, Z.; Song, M. The growth and lipid accumulation of Scenedesmus quadricauda under nitrogen starvation stress during xylose mixotrophic/heterotrophic cultivation. Environ. Sci. Pollut. Res. 2022, 30, 98934–98946. [Google Scholar] [CrossRef]
- Valledor, L.; Furuhashi, T.; Recuenco-Muñoz, L.; Wienkoop, S.; Weckwerth, W. System-level network analysis of nitrogen starvation and recovery in Chlamydomonas reinhardtii reveals potential new targets for increased lipid accumulation. Biotechnol. Biofuels 2014, 7, 171. [Google Scholar] [CrossRef]
- Chungjatupornchai, W.; Fa-aroonsawat, S. Enhanced triacylglycerol production in oleaginous microalga Neochloris oleoabundans by co-overexpression of lipogenic genes: Plastidial LPAAT1 and ER-located DGAT2. J. Biosci. Bioeng. 2021, 131, 124–130. [Google Scholar] [CrossRef]
- Azizi, S.; Bayat, B.; Tayebati, H.; Hashemi, A.; Pajoum Shariati, F. Nitrate and phosphate removal from treated wastewater by Chlorella vulgaris under various light regimes within membrane flat plate photobioreactor. Env. Prog. Sustain. 2021, 40, e13519. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Thelen, J.J.; Fedosejevs, E.; Harwood, J.L. The lipid biochemistry of eukaryotic algae. Prog. Lipid Res. 2019, 74, 31–68. [Google Scholar] [PubMed]
- Barros, A.; Pereira, H.; Campos, J.; Marques, A.; Varela, J.; Silva, J. Heterotrophy as a tool to overcome the long and costly autotrophic scale-up process for large scale production of microalgae. Sci. Rep. 2019, 9, 13935. [Google Scholar] [CrossRef] [PubMed]
- Jareonsin, S.; Pumas, C. Advantages of Heterotrophic Microalgae as a Host for Phytochemicals Production. Front. Bioeng. Biotech. 2021, 9, 628597. [Google Scholar]
- Shi, M.C.; Yu, L.H.; Shi, J.A.; Liu, J. A conserved MYB transcription factor is involved in regulating lipid metabolic pathways for oil biosynthesis in green algae. New Phytol. 2022, 235, 576–594. [Google Scholar] [CrossRef]
- Sagun, J.V.; Yadav, U.P.; Alonso, A.P. Progress in understanding and improving oil content and quality in seeds. Front. Plant Sci. 2023, 14, 1116894. [Google Scholar]
- Zafar, S.; Li, Y.-L.; Li, N.-N.; Zhu, K.-M.; Tan, X.-L. Recent advances in enhancement of oil content in oilseed crops. J. Biotechnol. 2019, 301, 35–44. [Google Scholar]
- Sun, X.-M.; Ren, L.-J.; Zhao, Q.-Y.; Ji, X.-J.; Huang, H. Enhancement of lipid accumulation in microalgae by metabolic engineering. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2019, 1864, 552–566. [Google Scholar]
- Brar, A.; Kumar, M.; Soni, T.; Vivekanand, V.; Pareek, N. Insights into the genetic and metabolic engineering approaches to enhance the competence of microalgae as biofuel resource: A review. Bioresour. Technol. 2021, 339, 125597. [Google Scholar]
- Riaz, I.; Shafiq, I.; Jamil, F.; Al-Muhtaseb, A.H.; Akhter, P.; Shafique, S.; Park, Y.-K.; Hussain, M. A review on catalysts of biodiesel (methyl esters) production. Catal. Rev. 2022, 1–53. [Google Scholar] [CrossRef]
- Acien, F.G.; Fernandez, J.M.; Magan, J.J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, C.F.; Südfeld, C.; Naduthodi, M.I.; Weusthuis, R.A.; Barbosa, M.J.; Wijffels, R.H.; D’adamo, S. Genetic engineering of microalgae for enhanced lipid production. Biotechnol. Adv. 2021, 52, 107836. [Google Scholar]
- Fivga, A.; Speranza, L.G.; Branco, C.M.; Ouadi, M.; Hornung, A. A review on the current state of the art for the production of advanced liquid biofuels. Aims Energy 2019, 7, 46–76. [Google Scholar]
- Avdeev, Y.G.; Kuznetsov, Y.I. Inhibitor protection of steel corrosion in acid solutions at high temperatures. A review. Part 2. Int. J. Corros. Scale Inhib. 2020, 9, 867–902. [Google Scholar]
- Frankel, E.N. Lipid Oxidation; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Shahidi, F.; Hossain, A. Role of Lipids in Food Flavor Generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- Cheng, P.; Okada, S.; Zhou, C.; Chen, P.; Huo, S.; Li, K.; Addy, M.; Yan, X.; Ruan, R.R. High-value chemicals from Botryococcus braunii and their current applications—A review. Bioresour. Technol. 2019, 291, 121911. [Google Scholar]
- Chacko, A.R.; Amster, D.E.; Johnson, T.E.; Newman, S.R.; Gladchuk, A.V.; Sohn, C.J.; Prunkard, D.E.; Yakelis, N.A.; Freeman, J.O. High-throughput screen for sorting cells capable of producing the biofuel feedstock botryococcene. Org. Biomol. Chem. 2019, 17, 3195–3201. [Google Scholar] [CrossRef]
- Estevam, B.R.; Pinto, L.F.R.; Maciel, R.; Fregolente, L.V. Potential applications of Botryococcus terribilis: A review. Biomass Bioenergy 2022, 165, 106582. [Google Scholar]
- Liao, S.A.; Wang, K.J.; Huang, Y.S. Extended chain length alkenoates differentiate three Isochrysidales groups. Org. Geochem. 2021, 161, 104303. [Google Scholar] [CrossRef]
- Yin, S.; Jin, W.; Zhou, X.; Han, W.; Gao, S.; Chen, C.; Ding, W.; He, Z.; Chen, Y.; Jiang, G. Enhancing harvest of biodiesel-promising microalgae using Daphnia domesticated by amino acids. Environ. Res. 2022, 212, 113465. [Google Scholar]
- Lin, W.Z.; Chen, L.N.; Tan, Z.X.; Deng, Z.Q.; Liu, H. Application of filamentous fungi in microalgae-based wastewater remediation for biomass harvesting and utilization: From mechanisms to practical application. Algal Res. 2022, 62, 102614. [Google Scholar] [CrossRef]
- Said, Z.; Nguyen, T.H.; Sharma, P.; Li, C.; Ali, H.M.; Nguyen, V.N.; Pham, V.V.; Ahmed, S.F.; Van, D.N.; Truong, T.H. Multi-attribute optimization of sustainable aviation fuel production-process from microalgae source. Fuel 2022, 324, 124759. [Google Scholar] [CrossRef]
- Haghpanah, T.; Sobati, M.A.; Pishvaee, M.S. Multi-objective superstructure optimization of a microalgae biorefinery considering economic and environmental aspects. Comput. Chem. Eng. 2022, 164, 107894. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-Derived Pigments for the Food Industry. Mar. Drugs 2023, 21, 82. [Google Scholar] [CrossRef]
- Vazquez-Romero, B.; Perales, J.A.; Pereira, H.; Barbosa, M.; Ruiz, J. Techno-economic assessment of microalgae production, harvesting and drying for food, feed, cosmetics, and agriculture. Sci. Total Environ. 2022, 837, 155742. [Google Scholar] [CrossRef]
- Morales, M.; Aflalo, C.; Bernard, O. Microalgal lipids: A review of lipids potential and quantification for 95 phytoplankton species. Biomass Bioenergy 2021, 150, 106108. [Google Scholar]
- Shetty, P.; Gitau, M.M.; Maróti, G. Salinity Stress Responses and Adaptation Mechanisms in Eukaryotic Green Microalgae. Cells 2019, 8, 1657. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S. Bioremediation of heavy metals using microalgae: Recent advances and mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.-P.; Han, B.; Yu, X. Coupling of abiotic stresses and phytohormones for the production of lipids and high-value by-products by microalgae: A review. Bioresour. Technol. 2019, 274, 549–556. [Google Scholar] [CrossRef]
- LaPanse, A.J.; Krishnan, A.; Posewitz, M.C. Adaptive Laboratory Evolution for algal strain improvement: Methodologies and applications. Algal Res. 2021, 53, 102122. [Google Scholar] [CrossRef]
- Fajardo, C.; De Donato, M.; Carrasco, R.; Martínez-Rodríguez, G.; Mancera, J.M.; Fernández-Acero, F.J. Advances and challenges in genetic engineering of microalgae. Rev. Aquac. 2020, 12, 365–381. [Google Scholar] [CrossRef]




| Regulating Agency | Country | Standards/Resolution | Commercial Name |
|---|---|---|---|
| Agência Nacional do Petróleo, Gás Natural Biocombustíveis | Brazil | Resolution No. 37 | Jet A-1 |
| Federal Aviation Administration | USA | ASTM D1655/ASTM 6615 | Jet A, Jet A1/Jet B |
| Transport Canada Civil Aviation | Canada | CAN/CGSB-3.23 CAN/CGSB-3.22 | Jet A/A1/Jet B |
| Civil Aviation Authority | UK | DefStan 91–91 | Jet A1 |
| European Aviation Safety Agency | EU | AFQRJOS | Jet A1 |
| Federal Air Transport Agency | Russia | GOST 10,227/GOST R 52,050 | TS-1/Jet A1 |
| Civil Aviation Administration of China | China | GB 6537 | No. 3 |
| Properties | Unit | ASTM 1655-4a | Def Stan 91-91 | ANPn°37 |
|---|---|---|---|---|
| Density | g/mL | 0.775–0.840 | 0.775–0.840 | 0.771–0.836 (20 °C) |
| Viscosity at 20 °C | mm2/s | 8.0 max | 8.0 max | 8.0 max |
| Acid value | mgKOH/g | 0.100 | 0.0012 | 0.015 |
| Flash point | °C | 38 min | 38 min | 38–40 min |
| Heat of combustion | MJ/kg | 42.8 min | 42.8 min | 42.8 min |
| Freezing point | °C | −47 | −47 | −47 |
| Sulphur | % | 0.3 | 0.3 | 0.3 |
| Aromatics | % | 25 | 25 | 25 |
| Smoke point | Mm | 25 min | 25 min | 25 min |
| JFTOT Delta P (260 °C) | mmHg | 25 | 25 | 25 |
| Conductivity | pS/m | 50–450 | 50–600 | 50–600 |
| Maximum boiling point | °C | 300 max | 300 max | 300 max |
| Compound | Formula | Type | Chemical Structure |
|---|---|---|---|
| n-octane | C8H18 | n-paraffin | 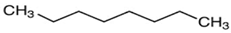 |
| 2-Methylheptane | C8H18 | Isoparaffin | 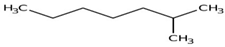 |
| 1-Methyl-1-ethylcyclopentane | C8H14 | Cycloparaffin |  |
| Ethyl-cyclohexane | C8H16 | Cycloparaffin | 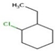 |
| o-Xylene | C8H10 | Aromatic | 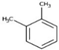 |
| p-Xylene | C8H10 | Aromatic | 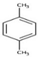 |
| Cis-Decalin | C10H18 | Cycloparaffin | 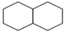 |
| Tetralin | C10H12 | Aromatic |  |
| Naphthalene | C10H8 | Aromatic | 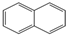 |
| n-Dodecane | C12H26 | n-paraffin | 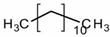 |
| 2-Methylundecane | C12H26 | Isoparaffin | 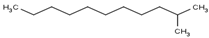 |
| 1-Ethylnaftalene | C12H12 | Aromatic | 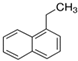 |
| n-Hexylbenzene | C12H18 | Aromatic | 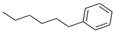 |
| n-Hexadecane | C16H34 | n-paraffin | 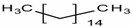 |
| 2-Methylpentadecane | C16H34 | Isoparaffin | 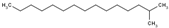 |
| n-Decylbenzene | C16H26 | Aromatic |  |
| Methods | Description |
|---|---|
| FT-SPK | Fischer-Tropsch synthetic paraffinic kerosene (FT-SPK). Biomass is converted into syngas and then biofuels via the FT process. ASTM approved the approach in 2009, and the UK MOD Def-Stan (91-91) approved it in 2010. FT-SPK aviation biofuel can be blended up to 50% with fossil jet fuel. |
| HEFA | Hydro-processed fatty acid esters and free fatty acid (HEFA). Hydrogen is used to transform liquid feedstock, including vegetable oils, cooking oil, and tallow, into green diesel, which can then be isomerised and separated to produce a jet fraction. In 2011, the route was certified for a 50% blend with fossil jet fuel. |
| HFS-SIP | Hydro-processing of fermented sugars–synthetic isoparaffinic kerosene (HFS-SIP). Sugars can be transformed to hydrocarbons using modified yeasts. The current permitted technique creates a C15 hydrocarbon terpenoid, farnesene. ASTM authorised this technology in 2014, and it can be combined with fossil jet fuel up to 10%. |
| FT-SPK/A | This is a modified FT-SPK process. Light aromatics are alkylated to yield a hydrocarbon mix with an aromatic component. This method was authorised in 2015 and can blend up to 50%. |
| ATJ-SPK | Alcohol-to-jet-synthetic paraffinic kerosene (ATJ-SPK). Hydro-processing, dehydration, and oligomerisation are used to convert alcohols (iso-butanol) into hydrocarbon. A certified process allows a maximum 50% blending. |
| Co-processing | Biological liquid feedstock, such as fats, oil, and other residues, can be blended with fossil crude oil by 5% (v/v) to carry out the refining process. This process was approved in April 2018 by ASTM and certified with ASTM D1655. |
| CCS-APR | Catalytic conversion of sugars by aqueous phase reforming. |
| CH | Catalytic hydrotreating of liquid to jet fuels. |
| CATJ-SKA | Catalytic upgrading of alcohol intermediate-catalytic ATJ-synthetic kerosene with aromatics. |
| ATJ-SPK expansion | Catalytic upgrading of ethanol. |
| HEFA expansion | Direct use of a wider cut of HEFA with renewable diesel. |
| HDCJ UOP-Eco-refining™ | Pyrolysis-hydrotreated de-polymerised cellulose. Blending vegetable biodiesels with petroleum-based fuels. |
| Microalgal Species | Oil Content (wt. % Dry Weight) |
|---|---|
| Schizochytrium sp. | 50–77 |
| Botryococcus braunii | 25–75 |
| Nannochloropsis sp. | 31–68 |
| Neochloris oleoabundans | 35–54 |
| Nitzschia sp. | 45–47 |
| Cylindrotheca sp. | 16–37 |
| Nannochloris sp. | 20–35 |
| Isochrysis sp. | 25–33 |
| Phaeodactylum tricornutum | 20–30 |
| Crop | Oil (L/Hectare) |
|---|---|
| Microalgae | 95,200 |
| Palm | 5950 |
| Jatropha | 4348 |
| Coconut | 2689 |
| Castor | 1413 |
| Sunflower | 952 |
| Tung | 640 |
| Soy | 446 |
| Oil Content of Algal Cells (% Dry Weight) | Oil Yield L/ha/Year | Biodiesel L/ha/Year | Land Area (km2) Required for 30% Replacement of Fossil Fuel |
|---|---|---|---|
| 30 | 58,700 | 51,927 | 11,345 |
| 50 | 97,833 | 86,582 | 6805 |
| 70 | 136,967 | 121,104 | 4863 |
| Name | Type | Area | Temp. Range (°C) | Rainfall (mm/Year) | Topography |
|---|---|---|---|---|---|
| Antarctic | Polar | 14,000,000 | +10 to −89 | 50–165 | Almost entirely covered in ice sheets. |
| Sahara | Subtropical | 9,200,000 | +47 to +5 | 25–120 | Extensive gravel-covered plains, abrupt mountains, sand dunes, large oasis depressions, and shallow seasonally inundated basins. |
| Great Australian | Subtropical | 2,700,000 | +40 to 16 | 40–300 | Salt lakes, sandy areas, grassy plains, and rocky areas. |
| Arabian | Subtropical | 2,330,000 | +55 to +8 | 20–500 | Vast light-coloured sand terrain, mountain ranges, black lava flows, and desert dunes. |
| Gobi | Interior | 1,295,000 | +45 to −40 | 152–194 | Cold desert with frost on plateau, 900–1500 m in height. |
| Kalahari | Subtropical | 900,000 | +45 to −12 | 100–500 | Featureless. Gently undulating sand covered 900 m above sea level. |
| Patagonia | Rain shadow | 673,000 | +13 to −33 | 127–203 | Alternating tablelands and massifs dissected by river valleys and canyons. |
| Sonoran | Subtropical | 310,000 | +48 to +4 | 30–265 | Mountain ranges and valleys. Trees common on rocky slopes and native cacti. Saguaros abundant. |
| Thar | Subtropical | 200,000 | +50 to 0 | 100–500 | Undulating sandy dunes separated by sandy plains and low barren hills, which rise abruptly from surrounding plains. |
| Namib | Coastal | 160,000 | +35 to +1 | 5–85 | Broad expanse of hyper-arid gravel and dunes that stretch along the entire coastline. Fog is frequent. |
| Atacama | Coastal | 140,000 | +32 to −2 | 0–1 | Stony terrain, salt lakes, sand, and felsic lava. |
| Danakil | Subtropical | 137,000 | +50 to +25 | 25–200 | Volcanic lakes formed by lava blockages of valleys, and salt lakes flanked by mountains to the east. |
| Mojave | Subtropical | 124,000 | +36 to +2 | 89–250 | Mountains and basins with spare vegetation. Dry riverbeds, towering sand dunes, and lava flows. |
| Ferlo | Subtropical | 70,000 | +43 to +32 | 300–400 | Plains and sand dunes, scattered rocks, and small valleys with clay soil. Traversed by tributaries of Senegal River. |
| Autotrophic Microalgae | Heterotrophic Microalgae | |
|---|---|---|
| Advantages | Low initial costs, as CO2 and H2O are relatively inexpensive, natural resources. Can reduce the amount of atmospheric CO2. Easier to genetically modify than other microalgae. | Higher yield of lipids; therefore, the cost of extracting and harvesting oils is lower. Growth is not limited by sunlight. Yield is 5.5 times larger compared to autotrophic microalgae. Shorter scale-up time than autotrophic microalgae. Requires 12 times less land for cultivation. |
| Disadvantages | High sunlight for photosynthesis is needed for growth. Areas where microalgae are grown are restricted to latitudes 30° above and below the equator and coastal regions with a flat topography. Mutual shading of cells. Lower yields of lipids extracted. | High initial cost to provide organic carbon sources, such as glucose and acetate. |
| Methods of Cultivating Microalgae | ||
|---|---|---|
| Open Raceway Pond | Photobioreactors | |
| Design characteristics | Closed-loop oval-shaped channels. Channels filled with water to a depth of 0.25–0.4 m, constructed using concrete. Circulation of nutrients using stirrer paddles. | Array of transparent tubes. Tubes have a diameter of 0.1 m, made from plastic or glass. Circulation of nutrients using a pump or airlift technology. Use an external light source. |
| Advantages | Easy to construct. Low construction, operating, and maintenance costs. Lower energy demands. Can use non-arable land. | Increased exposure to light. Larger biomass and lipid productivity. Low water consumption. Low risk of contamination. |
| Disadvantages | Susceptible to contamination, predators, and harsh weather conditions. Requires a large amount of land. Water loss is an issue. Hard to control the temperature. | High construction, operating, and maintenance costs. |
| Feedstock | MJSP (US$/Gallon) |
|---|---|
| Camelina oil | 1.63–4.62 |
| Soybean oil | 3.82–4.39 |
| Jatropha oil | 5.42–5.74 |
| Waste oil | 2.36–4.73 |
| Microalgae | 31.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, V.; Hossain, A.K.; Duraisamy, G.; Griffiths, G. Microalgal Biodiesel: A Challenging Route toward a Sustainable Aviation Fuel. Fermentation 2023, 9, 907. https://doi.org/10.3390/fermentation9100907
Sharma V, Hossain AK, Duraisamy G, Griffiths G. Microalgal Biodiesel: A Challenging Route toward a Sustainable Aviation Fuel. Fermentation. 2023; 9(10):907. https://doi.org/10.3390/fermentation9100907
Chicago/Turabian StyleSharma, Vikas, Abul Kalam Hossain, Ganesh Duraisamy, and Gareth Griffiths. 2023. "Microalgal Biodiesel: A Challenging Route toward a Sustainable Aviation Fuel" Fermentation 9, no. 10: 907. https://doi.org/10.3390/fermentation9100907






