Touch-Induced Transcriptional Changes in Flower Buds of a Non-Model Horticultural Plant Dianthus hybrida
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and RNA Extraction
2.2. RNA-seq Analysis
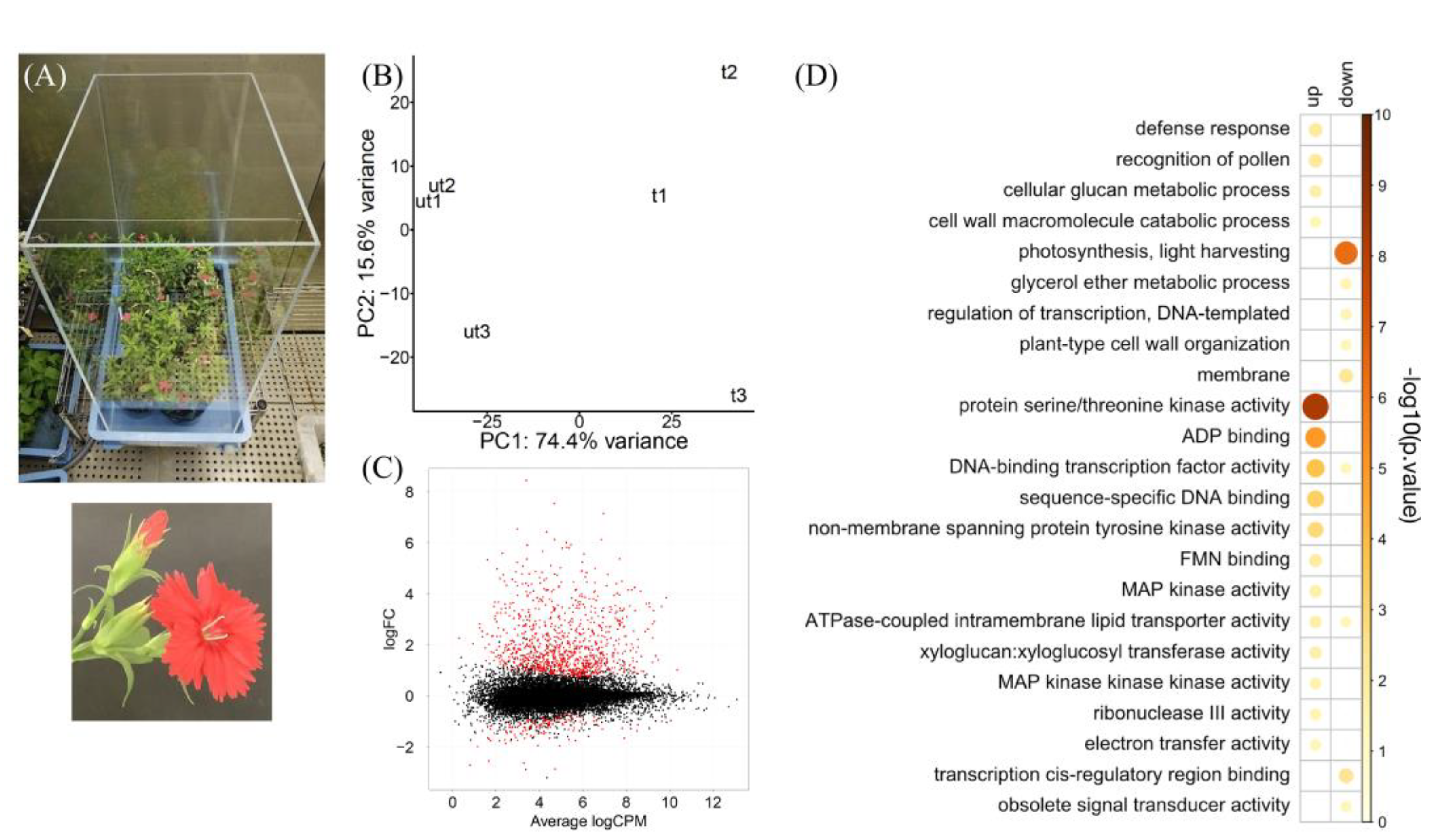
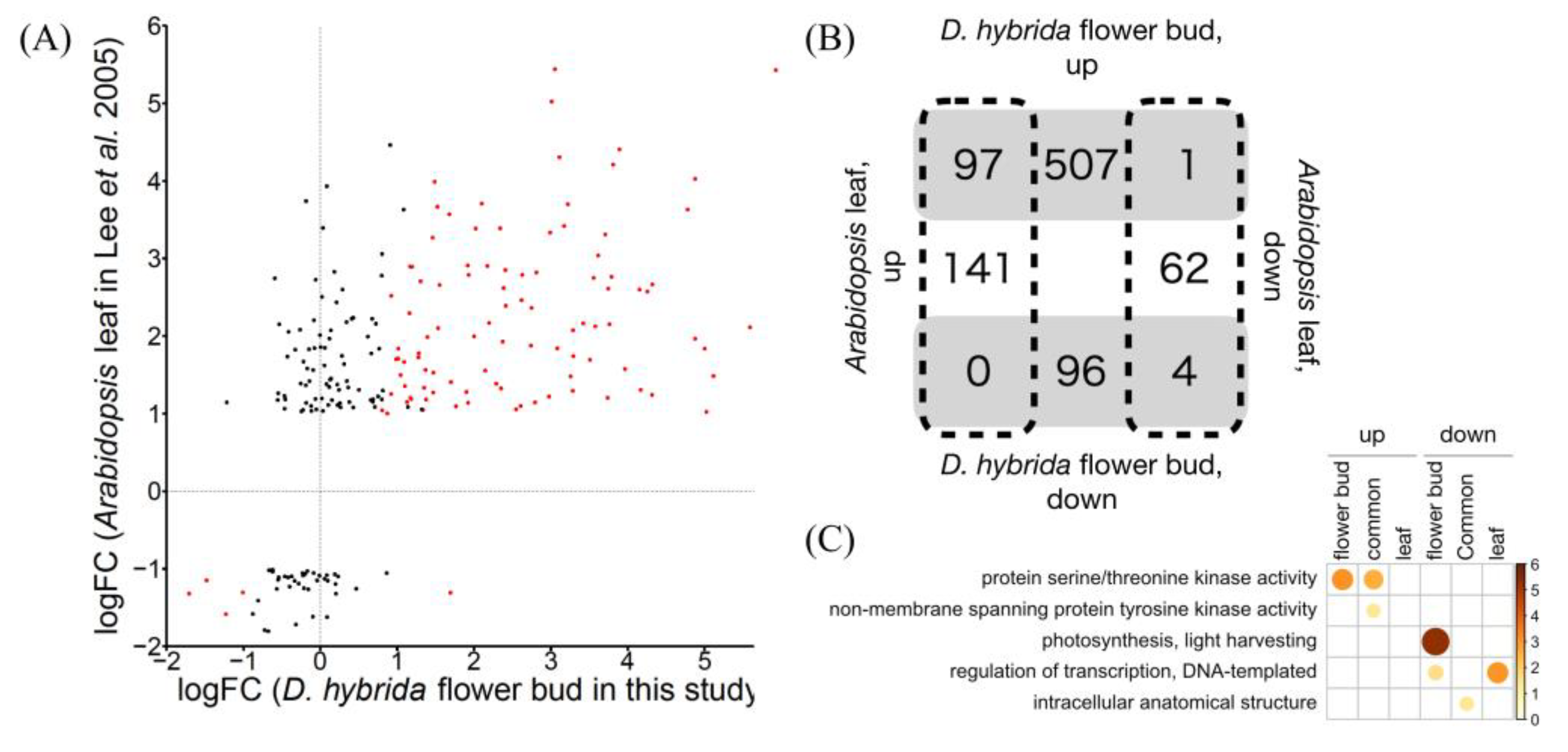
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Braam, J.; Davis, R.W. Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell 1990, 60, 357–364. [Google Scholar] [CrossRef]
- Braam, J. In touch: Plant responses to mechanical stimuli. New Phytol. 2005, 165, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Willmer, P.; Stanley, D.A.; Steijven, K.; Matthews, I.M.; Nuttman, C.V. Bidirectional flower color and shape changes allow a second opportunity for pollination. Curr. Biol. 2009, 19, 919–923. [Google Scholar] [CrossRef] [Green Version]
- Veits, M.; Khait, I.; Obolski, U.; Zinger, E.; Boonman, A.; Goldshtein, A.; Saban, K.; Seltzer, R.; Ben-Dor, U.; Estlein, P.; et al. Flowers respond to pollinator sound within minutes by increasing nectar sugar concentration. Ecol. Lett. 2019, 22, 1483–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholson, C.C.; Bales, J.W.; Palmer-Fortune, J.E.; Nicholson, R.G. Darwin’s bee-trap: The kinetics of Catasetum, a new world orchid. Plant Signal. Behav. 2008, 3, 19–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, M.R.; Campbell, A.K.; Smith, S.M.; Trewavas, A.J. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 1991, 352, 524–526. [Google Scholar] [CrossRef]
- Zhu, X.; Feng, Y.; Liang, G.; Liu, N.; Zhu, J.K. Aequorin-based luminescence imaging reveals stimulus- and tissue-specific Ca2+ dynamics in Arabidopsis Plants. Mol. Plant 2013, 6, 444–455. [Google Scholar] [CrossRef] [Green Version]
- Harper, J.F.; Breton, G.; Harmon, A. Decoding Ca2+ Signals through plant protein kinases. Annu. Rev. Plant Biol. 2004, 55, 263–288. [Google Scholar] [CrossRef]
- Kim, M.C.; Chung, W.S.; Yun, D.J.; Cho, M.J. Calcium and calmodulin-mediated regulation of gene expression in plants. Mol. Plant 2009, 2, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Luan, S. The CBL-CIPK network in plant calcium signaling. Trends Plant Sci. 2009, 14, 37–42. [Google Scholar] [CrossRef]
- DeFalco, T.A.; Bender, K.W.; Snedden, W.A. Breaking the code: Ca2+ sensors in plant signalling. Biochem. J. 2009, 425, 27–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudla, J.; Batistič, O.; Hashimoto, K. Calcium signals: The lead currency of plant information processing. Plant Cell 2010, 22, 541–563. [Google Scholar] [CrossRef] [PubMed]
- Hunter, T. Protein kinases and phosphatases: The yin and yang of protein phosphorylation and signaling. Cell 1995, 80, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Soderling, T.R. The Ca2+–calmodulin-dependent protein kinase cascade. Trends Biochem. Sci. 1999, 24, 232–236. [Google Scholar] [CrossRef]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Yang, Z.; Qing, D.; Ren, F.; Liu, S.; Zheng, Q.; Liu, J.; Zhang, W.; Dai, C.; Wu, M.; et al. Quantitative and functional posttranslational modification proteomics reveals that TREPH1 plays a role in plant touch-delayed bolting. Proc. Natl. Acad. Sci. USA 2018, 115, E10265–E10274. [Google Scholar] [CrossRef] [Green Version]
- Sistrunk, M.L.; Antosiewicz, D.M.; Purugganan, M.M.; Braam, J. Arabidopsis TCH3 encodes a novel Ca2+ binding protein and shows environmentally induced and tissue-specific regulation. Plant Cell 1994, 6, 1553–1565. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.R.; Johnson, K.A.; Braam, J.; James, M.N.G. Comparative modeling of the three-dimensional structure of the calmodulin-related TCH2 protein from Arabidopsis. Proteins 1997, 27, 144–153. [Google Scholar] [CrossRef]
- McCormack, E.; Braam, J. Calmodulins and related potential calcium sensors of Arabidopsis. New Phytol. 2003, 159, 585–598. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Polisensky, D.H.; Braam, J. Genome-wide identification of touch- and darkness-regulated Arabidopsis Genes: A focus on calmodulin-like and XTH genes. New Phytol. 2005, 165, 429–444. [Google Scholar] [CrossRef]
- Rose, J.K.C.; Braam, J.; Fry, S.C.; Nishitani, K. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: Current perspectives and a new unifying nomenclature. Plant Cell Physiol. 2002, 43, 1421–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chehab, E.W.; Yao, C.; Henderson, Z.; Kim, S.; Braam, J. Arabidopsis touch-induced morphogenesis is jasmonate mediated and protects against pests. Curr. Biol. 2012, 22, 701–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harbaugh, D.T.; Nepokroeff, M.; Rabeler, R.K.; McNeill, J.; Zimmer, E.A.; Wagner, W.L. A new lineage-based tribal classification of the family Caryophyllaceae. Int. J. Plant Sci. 2010, 171, 185–198. [Google Scholar] [CrossRef] [Green Version]
- Onozaki, T.; Mato, M.; Shibata, M.; Ikeda, H. Differences in flower color and pigment composition among white carnation (Dianthus caryophyllus L.) Cultivars. Sci. Hortic. 1999, 82, 103–111. [Google Scholar] [CrossRef]
- Okamura, M.; Yasuno, N.; Ohtsuka, M.; Tanaka, A.; Shikazono, N.; Hase, Y. Wide variety of flower-color and -shape mutants regenerated from leaf cultures irradiated with ion beams. Nucl. Instrum. Methods Phys. Res. B 2003, 206, 574–578. [Google Scholar] [CrossRef]
- Okamura, M.; Umemoto, N.; Onishi, N. Breeding glittering carnations by an efficient mutagenesis system. Plant Biotechnol. 2012, 29, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Okamura, M.; Nakayama, M.; Umemoto, N.; Cano, E.A.; Hase, Y.; Nishizaki, Y.; Sasaki, N.; Ozeki, Y. Crossbreeding of a metallic color carnation and diversification of the peculiar coloration by ion-beam irradiation. Euphytica 2013, 191, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Yagi, M.; Kosugi, S.; Hirakawa, H.; Ohmiya, A.; Tanase, K.; Harada, T.; Kishimoto, K.; Nakayama, M.; Ichimura, K.; Onozaki, T.; et al. Sequence analysis of the genome of carnation (Dianthus caryophyllus L.). DNA Res. 2014, 21, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Simko, V.R. Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.92). Available online: https://github.com/taiyun/corrplot (accessed on 1 September 2022).
- Zhang, D.; Liu, D.; Lv, X.; Wang, Y.; Xun, Z.; Liu, Z.; Li, F.; Lu, H. The cysteine protease CEP1, a key executor involved in tapetal programmed cell death, regulates pollen development in Arabidopsis. Plant Cell 2014, 26, 2939–2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwish, E.; Ghosh, R.; Ontiveros-Cisneros, A.; Tran, H.C.; Petersson, M.; de Milde, L.; Broda, M.; Goossens, A.; van Moerkercke, A.; Khan, K.; et al. Touch signaling and thigmomorphogenesis are regulated by complementary CAMTA3- and JA-dependent pathways. Sci. Adv. 2022, 8, eabm2091. [Google Scholar] [CrossRef]
- Bilgin, D.D.; Zavala, J.A.; Zhu, J.; Clough, S.J.; Ort, D.R.; Delucia, E.H. Biotic stress globally downregulates photosynthesis genes. Plant Cell Environ. 2010, 33, 1597–1613. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishijima, R.; Sanjaya, A.; Shinoyama, H.; Kazama, Y. Touch-Induced Transcriptional Changes in Flower Buds of a Non-Model Horticultural Plant Dianthus hybrida. Horticulturae 2022, 8, 918. https://doi.org/10.3390/horticulturae8100918
Nishijima R, Sanjaya A, Shinoyama H, Kazama Y. Touch-Induced Transcriptional Changes in Flower Buds of a Non-Model Horticultural Plant Dianthus hybrida. Horticulturae. 2022; 8(10):918. https://doi.org/10.3390/horticulturae8100918
Chicago/Turabian StyleNishijima, Ryo, Alvin Sanjaya, Harue Shinoyama, and Yusuke Kazama. 2022. "Touch-Induced Transcriptional Changes in Flower Buds of a Non-Model Horticultural Plant Dianthus hybrida" Horticulturae 8, no. 10: 918. https://doi.org/10.3390/horticulturae8100918
APA StyleNishijima, R., Sanjaya, A., Shinoyama, H., & Kazama, Y. (2022). Touch-Induced Transcriptional Changes in Flower Buds of a Non-Model Horticultural Plant Dianthus hybrida. Horticulturae, 8(10), 918. https://doi.org/10.3390/horticulturae8100918








