Biochar-Derived Anode Materials for Lithium-Ion Batteries: A Review
Abstract
:1. Introduction
2. Synthesis Methods
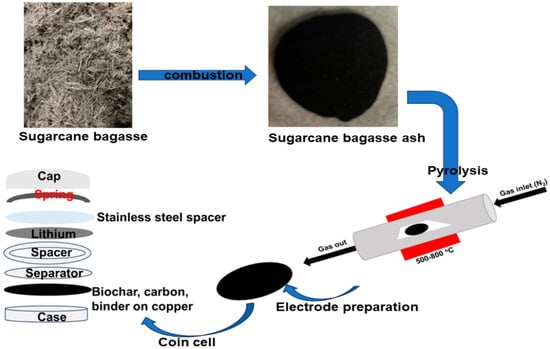
2.1. Pyrolysis
2.2. Activation
3. Biochar Characteristics
3.1. Elemental Composition of Biochar
3.2. pH and Surface States
3.3. Surface Area
4. Energy Storage Systems
4.1. Operational Principles of LIBs
4.2. Biochar in LIBs
| Biomass. | Rate Capability (mA g−1) | Cycles | Capacity (mAh g−1) | Capacity Retention | Ref. |
|---|---|---|---|---|---|
| Spruce tree | 100, 500, 1000 | 100, 1000, 5000 | 370, 332.4, 319 | 98.50 | [42] |
| Sisal fiber | - | - | 492/646 (T) | - | [63] |
| Sugarcane bagasse | 100 | 600 | 1662 | 80.2 | [89] |
| MacroAlgae | - | 500 | 740 | - | [76] |
| Bamboo chopsticks | - | 300 | 710 | - | [87] |
| Soybean Milk | 50 | 100 | 1084 | - | [83] |
| Gelatin | - | 200 | 600 | - | [90] |
| Human hair | 50, 100 | 50 | 700, 610 | - | [80] |
4.3. Prospects
- I.
- The chemistry of various biomass sources should be further and extensively studied to better understand the low cost and quality of biomass to yield significant and promising battery performance.
- II.
- Surface science study is also required to better take impurities or residuals to a negligible level in biomass-derived carbon. Sophisticated techniques to explain the chemistry of these carbonaceous nanomaterials from atomic and molecular structure will aid in theoretical and experimental studies.
- III.
- A window of opportunity in this area is to opt for a tailored atomic structure of carbon derived from biomass. In the future, upcoming authors may look at other forms of biomass that could potentially offer improved performance.
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From lithium-ion to sodium-ion batteries: Advantages, challenges, and surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Kim, Y. Technologies of lithium recycling from waste lithium-ion batteries: A review. Mater. Adv. 2021, 2, 3234–3250. [Google Scholar] [CrossRef]
- Xu, D.; Liang, M.; Qi, S.; Sun, W.; Lv, L.P.; Du, F.H.; Wang, B.; Chen, S.; Wang, Y.; Yu, Y. Progress and prospects of tunable organic molecules for organic lithium-ion batteries. ACS Nano 2020, 15, 47–80. [Google Scholar] [CrossRef] [PubMed]
- Kane, S.; Storer, A.; Xu, W.; Ryan, C.; Stadie, N.P. Biochar as a renewable substitute for carbon black in lithium ion battery electrodes. ACS Sustain. Chem. Eng. 2022, 10, 12226–12233. [Google Scholar] [CrossRef]
- Molaiyan, P.; Dos Reis, G.S.; Karuppiah, D.; Subramaniyam, C.M.; Garcia-Alvarado, F.; Lassi, U. Recent progress in biomass-derived carbon materials for lithium ion and Na ion batteries: A review. Batteries 2023, 9, 116. [Google Scholar] [CrossRef]
- Muddasar, M.; Mushtaq, M.; Beaucamp, A.; Kennedy, T.; Culebras, M.; Collins, M.N. Synthesis of sustainable lignin precursors for hierarchical porous carbons and their efficient performance in energy storage applications. ACS Sustain. Chem. Eng. 2024, 12, 2352–2363. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Murai, T.; Zaghib, K. Vapor-grown carbon fiber anode for cylindrical lithium ion rechargeable batteries. J. Power Sources 1999, 77, 110–115. [Google Scholar] [CrossRef]
- Li, W.; Huang, J.; Feng, L.; Cao, L.; Ren, Y.; Li, R.; Xu, Z.; Li, J.; Yao, C. Controlled synthesis of macroscopic three-dimensional hollow reticulate hard carbon as long-life anode materials for Na-ion batteries. J. Alloys Compd. 2017, 716, 210–219. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, J.; Liu, X.; Wang, F.; Wang, L.; Shi, C.; Huang, L.; Feng, X.; Chen, X.; Xu, L.; et al. Self-adaptive strain-relaxation optimization for high-energy lithium storage material through crumpling of graphene. Nat. Commun. 2014, 5, 4565. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, Y.; Lei, H.; Wang, C.; Zhao, Y.; Huo, E.; Lin, X.; Zhang, Q.; Qian, M.; Mateo, W.; et al. Synthesis of graphene-like carbon from biomass pyrolysis and its applications. Chem. Eng. J. 2020, 399, 125808. [Google Scholar] [CrossRef]
- Jin, C.; Nai, J.; Sheng, O.; Yuan, H.; Zhang, W.; Tao, X.; Lou, X.W.D. Biomass-based materials for green lithium secondary batteries. Energy Environ. Sci. 2021, 14, 1326–1379. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, K.; Hu, Z.; Tao, Z.; Mai, L.; Kang, Y.M.; Chou, S.L.; Chen, J. Recent developments on and prospects for electrode materials with hierarchical structures for lithium-ion batteries. Adv. Energy Mater. 2018, 8, 1701415. [Google Scholar] [CrossRef]
- Mahmood, N.; Tang, T.; Hou, Y. Nanostructured anode materials for lithium ion batteries: Progress, challenge and perspective. Adv. Energy Mater. 2016, 6, 1600374. [Google Scholar] [CrossRef]
- Das, R.; Panda, S.N. Preparation and Applications of Biochar-Based Nanocomposite: A review. J. Anal. Appl. Pyrolysis 2022, 167, 105691. [Google Scholar] [CrossRef]
- You, S.; Ok, Y.S.; Chen, S.S.; Tsang, D.C.; Kwon, E.E.; Lee, J.; Wang, H.C. A Critical Review on Sustainable Biochar System through Gasification: Energy and environmental applications. Bioresour. Technol. 2017, 246, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, F.; Zhang, Q.; Liu, G.; Xue, C. Controllable preparation of green biochar-based high-performance supercapacitors. Ionics 2022, 28, 2525–2561. [Google Scholar] [CrossRef]
- Salimi, P.; Tieuli, S.; Taghavi, S.; Venezia, E.; Fugattini, S.; Lauciello, S.; Prato, M.; Marras, S.; Li, T.; Signoretto, M.; et al. Sustainable lithium-ion batteries based on metal-free tannery waste biochar. Green Chem. 2022, 24, 4119–4129. [Google Scholar] [CrossRef]
- Bartoli, M.; Arrigo, R.; Malucelli, G.; Tagliaferro, A.; Duraccio, D. Recent advances in biochar polymer composites. Polymers 2022, 14, 2506. [Google Scholar] [CrossRef]
- Malyan, S.K.; Kumar, S.S.; Fagodiya, R.K.; Ghosh, P.; Kumar, A.; Singh, R.; Singh, L. Biochar for environmental sustainability in the energy-water-agroecosystem nexus. Renew. Sustain. Energy Rev. 2021, 149, 111379. [Google Scholar] [CrossRef]
- Sun, C.; Du, A.; Deng, G.; Zhao, X.; Pan, J.; Fu, X.; Liu, J.; Cui, L.; Wang, Q. Naturally nitrogen-doped self-encapsulated biochar materials based on mouldy wheat flour were used for silicon anode in lithium-ion batteries. Electrochim. Acta 2023, 450, 142269. [Google Scholar] [CrossRef]
- Anand, A.; Kumar, V.; Kaushal, P. Biochar and its twin benefits: Management of crop residues and mitigation of climate change in India. Renew. Sustain. Energy Rev. 2022, 156, 111959. [Google Scholar] [CrossRef]
- Kang, Z.; Jia, X.; Zhang, Y.; Kang, X.; Ge, M.; Liu, D.; Wang, C.; He, Z. A review on application of biochar in the removal of pharmaceutical pollutants through adsorption and persulfate-based AOPs. Sustainability 2022, 14, 10128. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, M.; Pang, Y.; Wu, H.; Ding, S. Layered NiPS3 nanoparticles anchored on two-dimensional nitrogen-doped biochar nanosheets for ultra-high-rate sodium-ion storage. Compos. Commun. 2022, 29, 100988. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Abdulkareem, S.A.; Ighalo, J.O.; Onifade, D.V.; Sanusi, S.K. Thermochemical co-conversion of sugarcane bagasse-LDPE hybrid waste into biochar. Arab. J. Sci. Eng. 2021, 46, 6391–6397. [Google Scholar] [CrossRef]
- Ma, C.; Wang, H.; Zhao, X.; Wang, X.; Miao, Y.; Cheng, L.; Wang, C.; Wang, L.; Yue, H.; Zhang, D. Porous Bamboo-Derived Carbon as Selenium Host for Advanced Lithium/Sodium–Selenium Batteries. Energy Technol. 2020, 8, 1901445. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, F.; Bai, T.; Long, B.; Liao, Q.; Ren, Y.; Yang, J. Interconnected highly graphitic carbon nanosheets derived from wheat stalk as high-performance anode materials for lithium ion batteries. Green Chem. 2016, 18, 2078–2088. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Ighalo, J.O.; Onifade, D.V. Biochar production from elephant grass (Pernisetum purpureum) using an updraft biomass gasifier with retort heating. Biofuels 2019, 12, 1283–1290. [Google Scholar] [CrossRef]
- Zheng, S.; Luo, Y.; Zhang, K.; Liu, H.; Hu, G.; Qin, A. Nitrogen and phosphorus co-doped mesoporous carbon nanosheets derived from bagasse for lithium-ion batteries. Mater. Lett. 2021, 290, 129459. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Ighalo, J.O.; Onifade, D.V. Production of biochar from plantain fibres (Musa paradisiaca) fibers using an updraft biomass gasifier with retort heating. Combust. Sci. Technol. 2021, 193, 60–74. [Google Scholar] [CrossRef]
- Adelodun, A.A.; Adeniyi, A.G.; Ighalo, J.O.; Onifade, D.V.; Arowoyele, L.T. Thermochemical conversion of oil palm fiberLDPE hybrid waste to biochar. Biofuels Bioprod. Biorefining 2020, 14, 1313–1323. [Google Scholar] [CrossRef]
- Sun, J.; Shu, X.; Guan, J.; Tong, G.; Ding, H.; Chen, L.; Zhou, N.; Shuai, Y. N, P, and O-coated biochar from phytoremediation residues: A promising cathode material for Li–S batteries. Nanotechnology 2022, 33, 215403. [Google Scholar]
- Li, M.; Zhang, H.; Xiao, T.; Wang, S.; Zhang, B.; Chen, D.; Su, M.; Tang, J. Low-cost biochar derived from corncob as an oxygen reduction catalyst in air cathode microbial fuel cells. Electrochim. Acta 2018, 283, 780–788. [Google Scholar] [CrossRef]
- Ren, B.; Zhang, X.; Wang, B.; Li, Y.; Zeng, X.; Zhang, X.; Fan, M.; Yang, X. Designed the formation of hierarchical core-shell NiCo2S4@ NiMoO4 arrays on cornstalk biochar as battery-type electrodes for hybrid supercapacitors. J. Alloys Compd. 2023, 937, 168403. [Google Scholar] [CrossRef]
- Gu, X.X.; Kuang, L.Y.; Lin, J.; Qiao, S.; Ma, S.; Li, Y.; Wang, Q.; Dai, J.H.; Zhou, X.; Zhou, H.Y.; et al. Highly porous nitrogen-doped biochar nanosheets for high-performance Li–Se batteries. Rare Met. 2023, 42, 822–829. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Chang, Q.; Wu, Y.; Lei, W.; Zou, Y.; Ma, Z.; Pan, Y. Porous biochar nanosheets loaded with Fe3C particles accelerate electrochemical reactions and their applications in Li–S batteries. Sustain. Energy Fuels 2021, 5, 4346–4354. [Google Scholar] [CrossRef]
- Magnacca, G.; Guerretta, F.; Vizintin, A.; Benzi, P.; Valsania, M.C.; Nistic, R. Preparation, characterisation, and environmental/electrochemical energy storage testing of low-cost biochar from natural chitin obtained by pyrolysis under mild conditions. Appl. Surf. Sci. 2018, 427, 883–893. [Google Scholar] [CrossRef]
- Senthil, C.; Lee, C.W. Biomass-derived biochar materials as sustainable energy sources for electrochemical energy storage devices. Renew. Sustain. Energy Rev. 2021, 137, 110464. [Google Scholar] [CrossRef]
- Reis, G.S.D.; Oliveira, H.P.D.; Larsson, S.H.; Thyrel, M.; Claudio Lima, E. A short review on the electrochemical performance of hierarchical and nitrogen-doped activated biocarbon-based electrodes for supercapacitors. Nanomaterials 2021, 11, 424. [Google Scholar] [CrossRef]
- Moralı, U.; Demiral, H.; Şensöz, S. Optimization of activated carbon production from sunflower seed extracted meal: Taguchi design of experiment approach and analysis of variance. J. Clean. Prod. 2018, 189, 602–611. [Google Scholar] [CrossRef]
- Thue, P.S.; Umpierres, C.S.; Lima, E.C.; Lima, D.R.; Machado, F.M.; Dos Reis, G.S.; da Silva, R.S.; Pavan, F.A.; Tran, H.N. Single-step pyrolysis for producing magnetic activated carbon from tucumã (Astrocaryum aculeatum) seed and nickel (II) chloride and zinc (II) chloride. Application for removal of nicotinamide and propanolol. J. Hazard. Mater. 2020, 398, 122903. [Google Scholar] [CrossRef]
- Simoes dos Reis, G.; Mayandi Subramaniyam, C.; Cárdenas, A.D.; Larsson, S.H.; Thyrel, M.; Lassi, U.; Garcia-Alvarado, F. Facile synthesis of sustainable activated biochars with different pore structures as efficient additive-carbon-free anodes for lithium-and sodium-ion batteries. ACS Omega 2022, 7, 42570–42581. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, M.; Zhu, K.; Ni, J.; Wang, S.; Cao, B.; Zhong, S.; Zhou, J.; Wang, S. Facile synthesis of nitrogen-doped interconnected porous carbons derived from reed and chlorella for high-performance supercapacitors. Fuel Process. Technol. 2022, 238, 107466. [Google Scholar] [CrossRef]
- Hou, S.; Cai, X.; Wu, H.; Yu, X.; Peng, M.; Yan, K.; Zou, D. Nitrogen-doped graphene for dye-sensitized solar cells and the role of nitrogen states in triiodide reduction. Energy Environ. Sci. 2013, 6, 3356–3362. [Google Scholar] [CrossRef]
- Wei, M.; Marrakchi, F.; Yuan, C.; Cheng, X.; Jiang, D.; Zafar, F.F.; Fu, Y.; Wang, S. Adsorption modeling, thermodynamics, and DFT simulation of tetracycline onto mesoporous and high-surface-area NaOH-activated macroalgae carbon. J. Hazard. Mater. 2022, 425, 127887. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, F. Naturally derived nanostructured materials from biomass for rechargeable lithium/sodium batteries. Nano Energy 2015, 17, 91–103. [Google Scholar] [CrossRef]
- Vivekanandhan, S. Biochar supercapacitors: Recent developments in materials and methods. Green Sustain. Adv. Mater. Appl. 2018, 2, 223–249. [Google Scholar]
- Azam, M.A.; Safie, N.E.; Ahmad, A.S.; Yuza, N.A.; Zulkifli, N.S.A. Recent advances of silicon, carbon composites, and tin oxide as new anode materials for lithium-ion battery: A comprehensive review. J. Energy Storage 2021, 33, 102096. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Liu, B.; Su, Z. A critical review on the application and recent developments of post-modified biochar in supercapacitors. J. Clean. Prod. 2021, 310, 127428. [Google Scholar] [CrossRef]
- Wang, T.; Kretschmer, K.; Choi, S.; Pang, H.; Xue, H.; Wang, G. Methods for the fabrication of porous carbon materials and separator membranes for lithium–sulphur batteries: Development and future perspectives. Small Methods 2017, 1, 1700089. [Google Scholar] [CrossRef]
- Leng, L.; Liu, R.; Xu, S.; Mohamed, B.A.; Yang, Z.; Hu, Y.; Chen, J.; Zhao, S.; Wu, Z.; Peng, H.; et al. An overview of sulphur-functional groups in biochar from biomass pyrolysis. J. Environ. Chem. Eng. 2022, 10, 107185. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.; Ok, Y.S.; Gao, B. Biochar Technology in Wehrmacht Treatment: Critical review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef] [PubMed]
- Goud, M.; Raval, F. A sustainable biochar-based shape stable composite phase change material for thermal management of a lithium ion battery system and hybrid neural network modelling for heat flow prediction. J. Energy Storage 2022, 56, 106163. [Google Scholar]
- Venkatachalam, C.D.C.; Sekar, S.; Sengottian, M.; Ravichandran, S.R.; Bhuvaneshwaran, P. Critical review of the production, activation, and morphological characteristic study of functionalised biochar. J. Energy Storage 2023, 67, 107525. [Google Scholar] [CrossRef]
- Abed Hussein, B.; Mahdi, A.B.; Emad Izzat, S.; Acwin Dwijendra, N.K.; Romero Parra, R.M.; Barboza Arenas, L.A.; Mustafa, Y.F.; Yasin, G.; Thaeer Hammid, A. Production, structural properties nano biochar and effects nano biochar in soil: A review. Egypt. J. Chem. 2022, 65, 607–618. [Google Scholar]
- Adeniyi, A.G.; Ighalo, J.O.; Onifade, D.V. Biochar from the thermochemical conversion of orange peel (Citrus sinensis) peel and Albedo: Product quality and potential applications. Chem. Afr. 2020, 3, 439–448. [Google Scholar] [CrossRef]
- Kalinke, C.; de Oliveira, P.R.; Bonacin, J.A.; Janegitz, B.C.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. State-of-the-art technologies and perspectives in the use of biochar for electrochemical and electroanalytical applications. Green Chem. 2021, 23, 5272–5301. [Google Scholar] [CrossRef]
- Pramanik, A.; Chattopadhyay, S.; De, G.; Mahanty, S. Efficient energy storage in mustard husk-derived porous spherical carbon nanostructures. Mater. Adv. 2021, 2, 7463–7472. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, K.X.; Li, G.D.; Chen, J.S. Hierarchical porous carbon derived from rice straw for lithium-ion batteries with high-rate performance. Electrochem. Commun. 2009, 11, 130–133. [Google Scholar] [CrossRef]
- Lotfabad, E.M.; Ding, J.; Cui, K.; Kohandehghan, A.; Kalisvaart, W.P.; Hazelton, M.; Mitlin, D. High-density sodium and lithium-ion battery anodes from banana peels. ACS Nano 2014, 8, 7115–7129. [Google Scholar] [CrossRef]
- Drews, M.; Büttner, J.; Bauer, M.; Ahmed, J.; Sahu, R.; Scheu, C.; Vierrath, S.; Fischer, A.; Biro, D. Hard carbon anodes for lithium ion batteries. ChemElectroChem 2021, 8, 4750–4761. [Google Scholar] [CrossRef]
- Kietisirirojana, N.; Tunkasiri, T.; Pengpat, K.; Khamman, O.; Intatha, U.; Eitssayeam, S. Synthesis of mesoporous carbon powder from gold beard grass pollen for use as an anode for lithium ion batteries. Microporous Mesoporous Mater. 2022, 331, 111565. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, K.; Tian, N.; Qin, A.; Liao, L.; Du, R.; Wei, C. Biomass carbon derived from sisal fibre as anode material for lithium-ion batteries. Mater. Lett. 2015, 142, 193–196. [Google Scholar] [CrossRef]
- Xiao, Y.; Raheem, A.; Ding, L.; Chen, W.H.; Chen, X.; Wang, F.; Lin, S.L. Pretreatment, modification and applications of sewage sludge-derived biochar for resource recovery-A review. Chemosphere 2022, 287, 131969. [Google Scholar] [CrossRef]
- Iwuozor, K.A.; Emenike, E.C.; Ighalo, J.O.; Omoarukhe, F.I.; Omuku, E.E.; Adeniyi, A.G. Review of the thermochemical conversion of sugarcane bagasse into biochar. Clean. Mater. 2022, 6, 100162. [Google Scholar] [CrossRef]
- Yuan, S.; Lai, Q.; Duan, X.; Wang, Q. Carbon-based materials as anode materials for lithium-ion batteries and lithium-ion capacitors: A review. J. Energy Storage 2023, 61, 106716. [Google Scholar] [CrossRef]
- Du, A.; Li, H.; Chen, X.; Han, Y.; Zhu, Z.; Chu, C. Recent research progress of silicon-based anode materials for lithium-ion batteries. ChemistrySelect 2022, 7, e202201269. [Google Scholar] [CrossRef]
- Haghighi Mood, S.; Pelaez-Samaniego, M.R.; Garcia-Perez, M. Perspectives of engineered biochar for environmental applications: A review. Energy Fuels 2022, 36, 7940–7986. [Google Scholar] [CrossRef]
- Mian, M.M.; Alam, N.; Ahommed, M.S.; He, Z.; Ni, Y. Emerging applications of sludge biochar-based catalysts for environmental remediation and energy storage: A review. J. Clean. Prod. 2022, 360, 132131. [Google Scholar] [CrossRef]
- Uday, V.; Harikrishnan, P.S.; Deoli, K.; Zitouni, F.; Mahlknecht, J.; Kumar, M. Current trends in the production, morphology, and real-world environmental applications of biochar for the promotion of sustainability. Bioresour. Technol. 2022, 359, 127467. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterisation, stability, and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar]
- Ling, H.Y.; Chen, H.; Wu, Z.; Hencz, L.; Qian, S.; Liu, X.; Liu, T.; Zhang, S. Sustainable bioderived materials for addressing critical problems of next-generation high-capacity lithium ion batteries. Mater. Chem. Front. 2021, 5, 5932–5953. [Google Scholar] [CrossRef]
- Amalina, F.; Abd Razak, A.S.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Biochar production techniques utilising biomass waste-derived materials and environmental applications—A review. J. Hazard. Mater. Adv. 2022, 7, 100134. [Google Scholar] [CrossRef]
- Ryu, D.J.; Oh, R.G.; Seo, Y.D.; Oh, S.Y.; Ryu, K.S. Recovery and electrochemical performance in lithium secondary batteries of biochar derived from rice straw. Environ. Sci. Pollut. Res. 2015, 22, 10405–10412. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Wang, H.; Liu, S.; Mao, N.; Liu, X.; Shi, J.; Liu, W.; Chen, S.; Wang, X. N, O-codoped hierarchical porous carbons derived from algae for high-capacity supercapacitors and battery anodes. J. Mater. Chem. A 2016, 4, 5973–5983. [Google Scholar] [CrossRef]
- Salimi, P.; Norouzi, O.; Pourhoseini, S.E.M.; Bartocci, P.; Tavasoli, A.; Di Maria, F.; Pirbazari, S.M.; Bidini, G.; Fantozzi, F. Magnetic biochar obtained through catalytic pyrolysis of macroalgae: A promising anode material for Li-ion batteries. Renew. Energy 2019, 140, 704–714. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L., III. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef]
- Liu, T.; Luo, R.; Qiao, W.; Yoon, S.H.; Mochida, I. Microstructure of carbon derived from mangrove charcoal and its application in Li-ion batteries. Electrochim. Acta 2010, 55, 1696–1700. [Google Scholar] [CrossRef]
- Isaev, I.; Salitra, G.; Soffer, A.; Cohen, Y.S.; Aurbach, D.; Fischer, J. A new approach for the preparation of anodes for Li-ion batteries based on activated hard carbon cloth with pore design. J. Power Sources 2003, 119, 28–33. [Google Scholar] [CrossRef]
- Saravanan, K.R.; Kalaiselvi, N. Nitrogen containing bio-carbon as a potential anode for lithium batteries. Carbon 2015, 81, 43–53. [Google Scholar] [CrossRef]
- Kaskhedikar, N.A.; Maier, J. Lithium storage in carbon nanostructures. Adv. Mater. 2009, 21, 2664–2680. [Google Scholar] [CrossRef]
- Shi, L.; Chen, Y.; Song, H.; Li, A.; Chen, X.; Zhou, J.; Ma, Z. Preparation and lithium-storage performance of a novel hierarchical porous carbon from sucrose using Mg-Al layered double hydroxides as template. Electrochim. Acta 2017, 231, 153–161. [Google Scholar] [CrossRef]
- Guo, S.; Chen, Y.; Shi, L.; Dong, Y.; Ma, J.; Chen, X.; Song, H. Nitrogen-doped biomass-based ultra-thin carbon nanosheets with interconnected framework for High-Performance Lithium-Ion Batteries. Appl. Surf. Sci. 2018, 437, 136–143. [Google Scholar] [CrossRef]
- Wei, Y.; Tao, Y.; Kong, Z.; Liu, L.; Wang, J.; Qiao, W.; Ling, L.; Long, D. Unique electrochemical behavior of heterocyclic selenium–sulfur cathode materials in ether-based electrolytes for rechargeable lithium batteries. Energy Storage Mater. 2016, 5, 171–179. [Google Scholar] [CrossRef]
- Norouzi, O.; Di Maria, F. Catalytic effect of functional and Fe composite biochars on biofuel and biochemical derived from the pyrolysis of green marine biomass. Fermentation 2018, 4, 96. [Google Scholar] [CrossRef]
- Javanbakht, M.; Omidvar, H.; Hosen, M.S.; Hubin, A.; Van Mierlo, J.; Berecibar, M. Development, retainment, and assessment of the graphite-electrolyte interphase in Li-ion batteries regarding the functionality of SEI-forming additives. Iscience 2022, 25, 103862. [Google Scholar]
- Pol, V.G.; Thackeray, M.M. Spherical carbon particles and carbon nanotubes prepared by autogenic reactions: Evaluation as anodes in lithium electrochemical cells. Energy Environ. Sci. 2011, 4, 1904–1912. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, J.; Ai, W.; Fan, Z.; Shen, X.; Zou, C.; Liu, J.; Zhang, H.; Yu, T. Evolution of disposable bamboo chopsticks into uniform carbon fibers: A smart strategy to fabricate sustainable anodes for Li-ion batteries. Energy Environ. Sci. 2014, 7, 2670–2679. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Peng, Y.; Wang, X.; Wang, N.; Wang, J.; Zhao, J. Nitrogen-doped biomass-based hierarchical porous carbon with large mesoporous volume for application in energy storage. Chem. Eng. J. 2018, 348, 850–859. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, Z.; Zhang, X.; Ren, G.; Lai, Y.; Liu, Y.; Li, J. Highly ordered nitrogen-rich mesoporous carbon derived from biomass waste for high-performance lithium–sulfur batteries. Carbon 2015, 84, 399–408. [Google Scholar] [CrossRef]
- Roy, J.J.; Rarotra, S.; Krikstolaityte, V.; Zhuoran, K.W.; Cindy, Y.D.I.; Tan, X.Y.; Carboni, M.; Meyer, D.; Yan, Q.; Srinivasan, M. Green recycling methods to treat lithium-ion batteries E-waste: A circular approach to sustainability. Adv. Mater. 2022, 34, 2103346. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Liu, X.; Wang, X.; Yu, K.; Liang, C. Jute fiber based micro-mesoporous carbon: A biomass derived anode material with high-performance for lithium-ion batteries. Mater. Sci. Eng. B 2021, 265, 115015. [Google Scholar] [CrossRef]
- Panda, M.R.; Kathribail, A.R.; Modak, B.; Sau, S.; Dutta, D.P.; Mitra, S. Electrochemical properties of biomass-derived carbon and its composite along with Na2Ti3O7 as potential high-performance anodes for Na-ion and Li-ion batteries. Electrochim. Acta 2021, 392, 139026. [Google Scholar] [CrossRef]
- Hernández-Rentero, C.; Marangon, V.; Olivares-Marín, M.; Gómez-Serrano, V.; Caballero, Á.; Morales, J.; Hassoun, J. Alternative lithium-ion battery using biomass-derived carbons as environmentally sustainable anode. J. Colloid Interface Sci. 2020, 573, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yin, J.; Lin, Z.; Lin, H.; Lu, H.; Wang, Y.; Huang, W. Facile preparation of 3D hierarchical porous carbon from lignin for the anode material in lithium ion battery with high rate performance. Electrochim. Acta 2015, 176, 1136–1142. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, Z.; Liang, J.; Liang, C. Natural biomass-derived porous carbons from buckwheat hulls used as anode for lithium-ion batteries. Diam. Relat. Mater. 2021, 119, 108553. [Google Scholar] [CrossRef]
- Feng, D.; Li, Y.; Qin, X.; Zheng, L.; Guo, B.; Dai, W.; Song, N.; Liu, L.; Xu, Y.; Tang, Z.; et al. Biomass derived porous carbon anode materials for lithium-ion batteries with high electrochemical performance. Int. J. Electrochem. Sci. 2024, 19, 100488. [Google Scholar] [CrossRef]
- Deng, W.N.; Li, Y.H.; Xu, D.F.; Zhou, W.; Xiang, K.X.; Chen, H. Three-dimensional hierarchically porous nitrogen-doped carbon from water hyacinth as selenium host for high-performance lithium–selenium batteries. Rare Met. 2022, 41, 3432–3445. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Edvinsson, T.; Kwong, P. Biochar for electrochemical applications. Curr. Opin. Green Sustain. Chem. 2020, 23, 25–30. [Google Scholar] [CrossRef]
- Zeng, G.; Wang, Y.; Lou, X.; Chen, H.; Jiang, S.; Zhou, W. Vanadium oxide/carbonized chestnut needle composites as cathode materials for advanced aqueous zinc-ion batteries. J. Energy Storage 2024, 77, 109859. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Amirkhiz, B.S.; Tan, X.; Xu, Z.; Wang, H.; Olsen, B.C.; Holt, C.M.; Mitlin, D. Carbonized chicken eggshell membranes with 3D architectures as high-performance electrode materials for supercapacitors. Adv. Energy Mater. 2012, 2, 431–437. [Google Scholar] [CrossRef]











| Biomass Waste | Optimal Temperature (°C) | Duration (min) | Product Yield (wt%) | References |
|---|---|---|---|---|
| Sugarcane bagasse ash | 349 | 70 | 16.67 | [30] |
| Orange Peel | 300 | 120 | - | [31] |
| Orange albedo | 300 | 120 | - | [31] |
| Elephant grass | 300 | 120 | 14.29 | [32] |
| Palm oil fibres | 387 | 80 | 15.9 | [33] |
| Plantain fibres | 220 | 150 | 6.98 | [34] |
| Roles of Biomass-Derived Carbon in LIBs | Biochar Types | Properties of Biomass | Ref. |
|---|---|---|---|
| Anode material | Hard/soft carbon Activated carbon | SP3 hybridised carbon sites, porosity, conductivity. | [38] |
| Dopant | Chitosan | Foreign atoms N and O | [38] |
| Binder | Chitin, Chitosan | Polymeric composition, chemically stable | [38] |
| Separator | Cellulose | Nonconductive, mechanically flexible, chemically stable | [38] |
| Current collector | Carbon cloth | Conductive thin film, mechanically flexible, and chemically organised | [38] |
| Feedstock | Synthetic Route | Initial Capacity (mA h g−1) | Ref. |
|---|---|---|---|
| Mustard seed | Hydrothermal | ~822 | [5] |
| Rice straws | High temperature | 2041 | [59] |
| Bagasse | Hydrothermal | 2347.56 | [29] |
| Banana peel | High temperature | ~2150 | [60] |
| Wood | Ball milling and pyrolysis | ~315 | [61] |
| Grass pollen | Pyrolysis | 788.99 | [62] |
| Coffee waste | Dry mechanochemical grinding | 764 | [63] |
| Sisal fibre | Hydrothermal | 646 | [64] |
| Source of Biomass | Morphology | Specific Surface Area (m2 g−1) | Initial Discharge–Charge Capacity Yield (mAh g−1) | Cycles/Rate Capability (mAh g−1) | Ref. |
|---|---|---|---|---|---|
| Wood | Hard | 61 | 400/250 | 100/4 C | [92] |
| Sisal fiber | Honeycomb | 103.5 | 1037/414 | - | [93] |
| Jute fiber | Micro–meso | 1028.6 | 1173/534.1 | 310.4/1.86 | [94] |
| Plant tree leaves | Porous | 518.6 | 906.2/460.4 | 242.7/2 A | [63] |
| Tamarind plant seeds | porous | 103.5 | 1037/414 | - | [95] |
| Cherry pit | Dejected | 1662 | 1300/300 | 70/1.86 A | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seroka, N.S.; Luo, H.; Khotseng, L. Biochar-Derived Anode Materials for Lithium-Ion Batteries: A Review. Batteries 2024, 10, 144. https://doi.org/10.3390/batteries10050144
Seroka NS, Luo H, Khotseng L. Biochar-Derived Anode Materials for Lithium-Ion Batteries: A Review. Batteries. 2024; 10(5):144. https://doi.org/10.3390/batteries10050144
Chicago/Turabian StyleSeroka, Ntalane Sello, Hongze Luo, and Lindiwe Khotseng. 2024. "Biochar-Derived Anode Materials for Lithium-Ion Batteries: A Review" Batteries 10, no. 5: 144. https://doi.org/10.3390/batteries10050144