Leaching of Metals from Spent Lithium-Ion Batteries †
Abstract
:1. Introduction
2. Experimental
2.1. Material
2.2. Methods
3. Results and Discussion
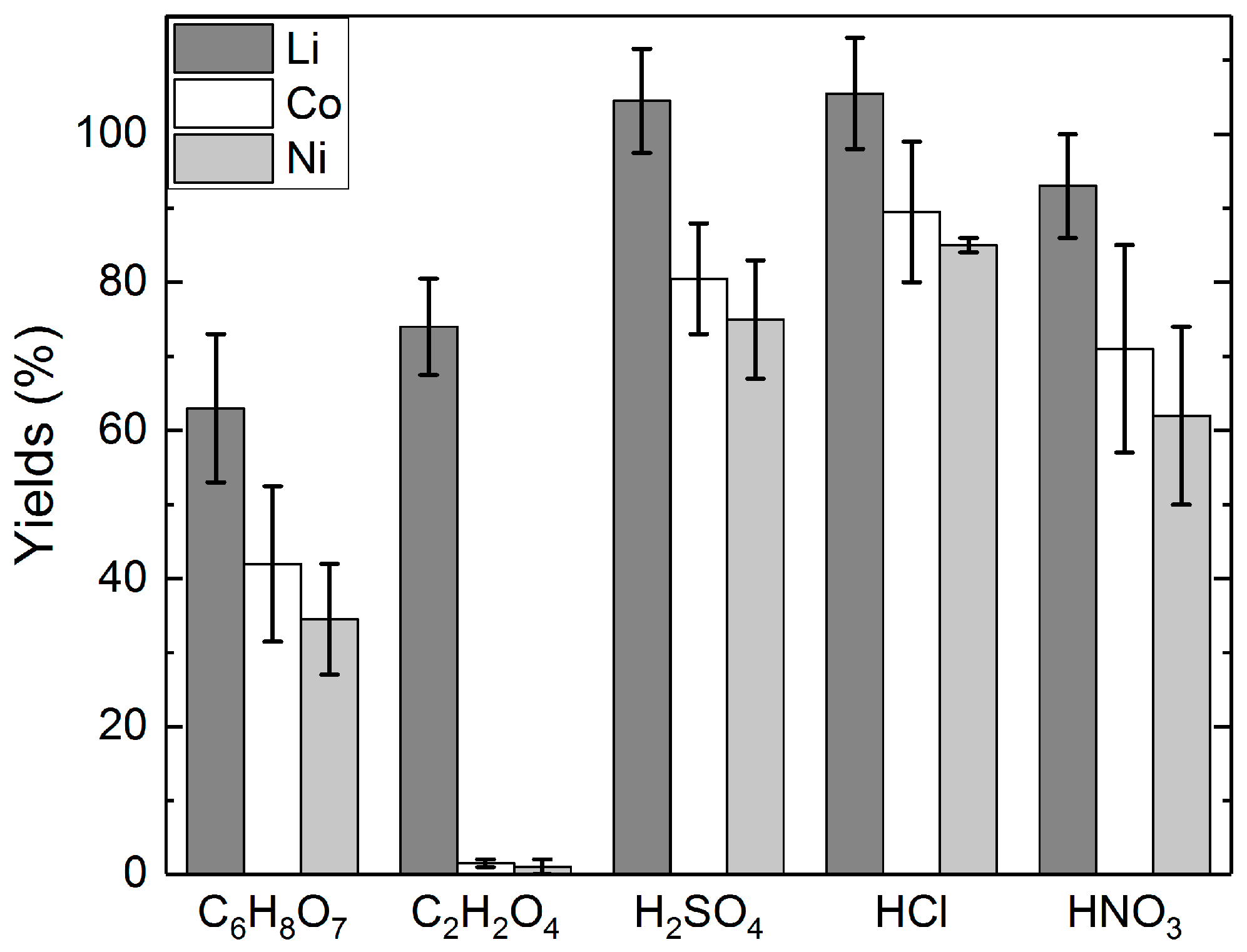
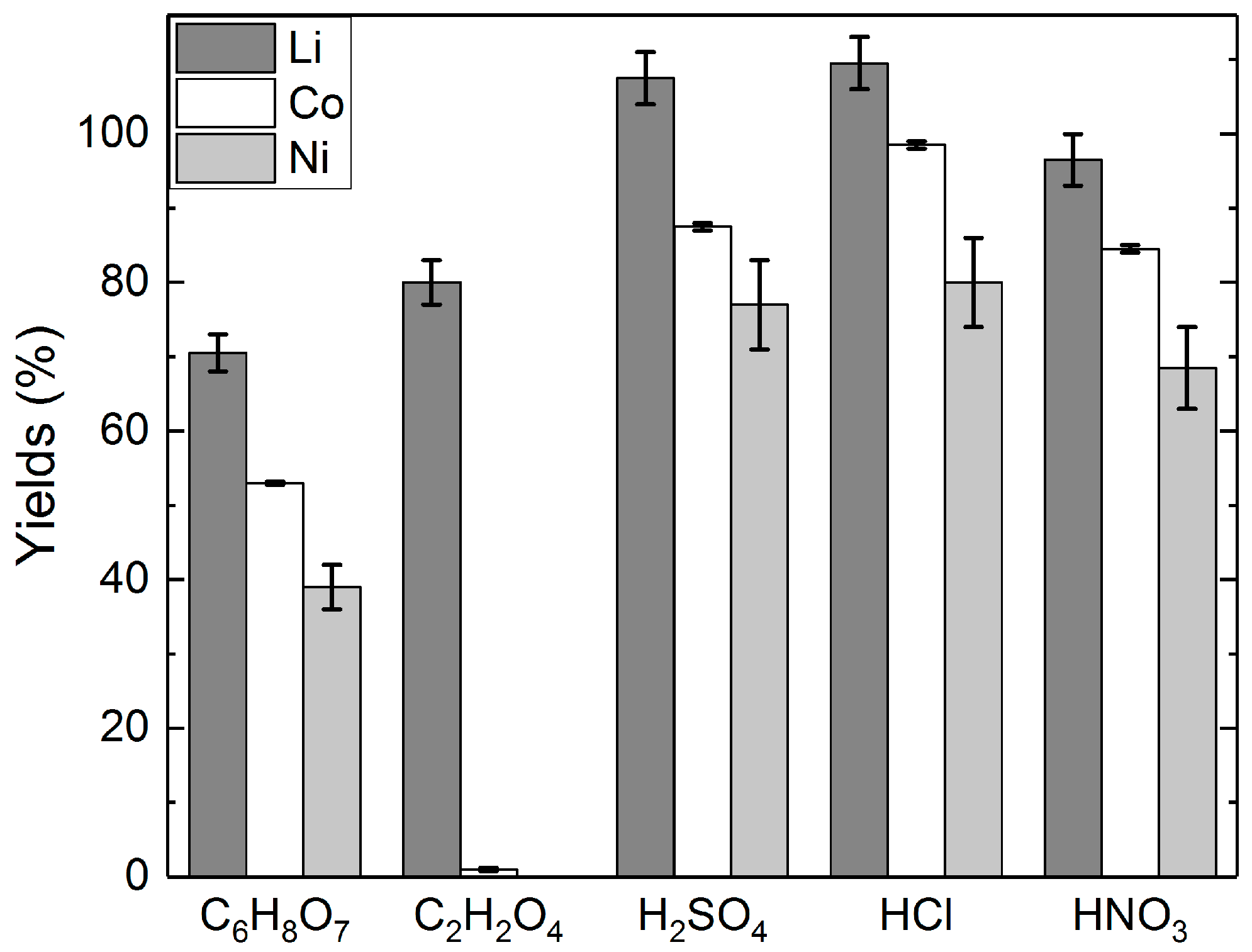
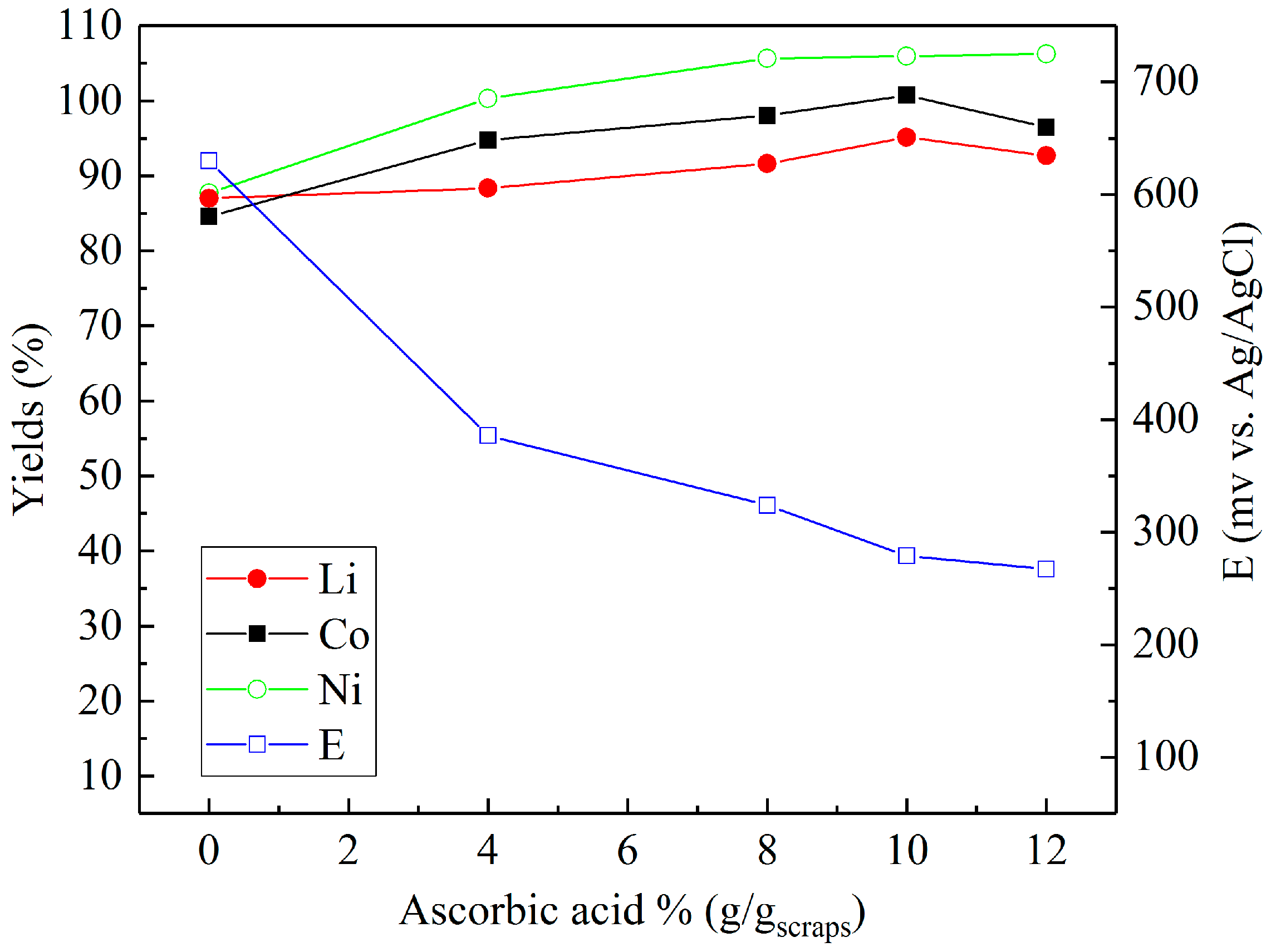
3.1. Long-Term Leaching Tests with Different Acids
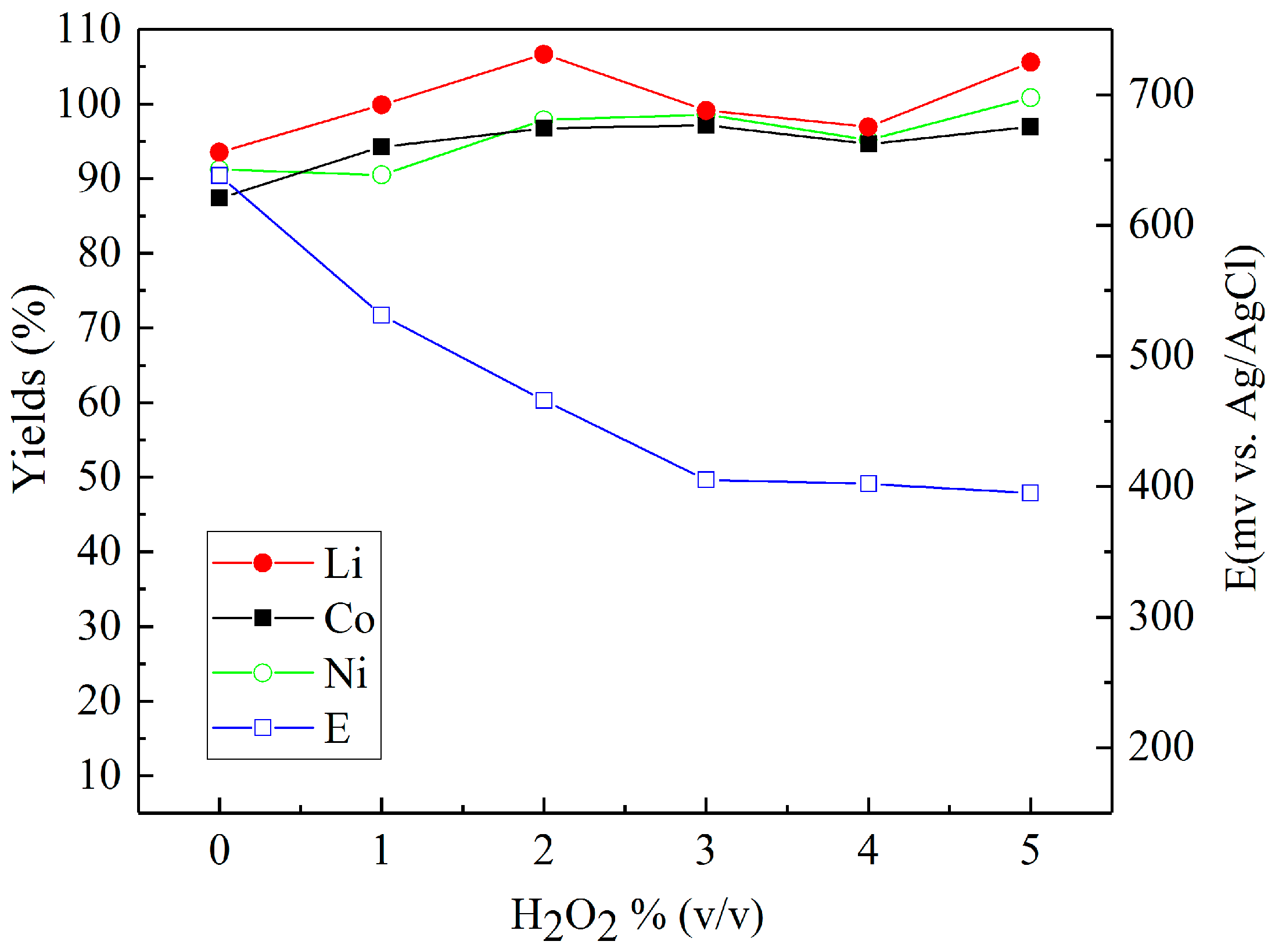
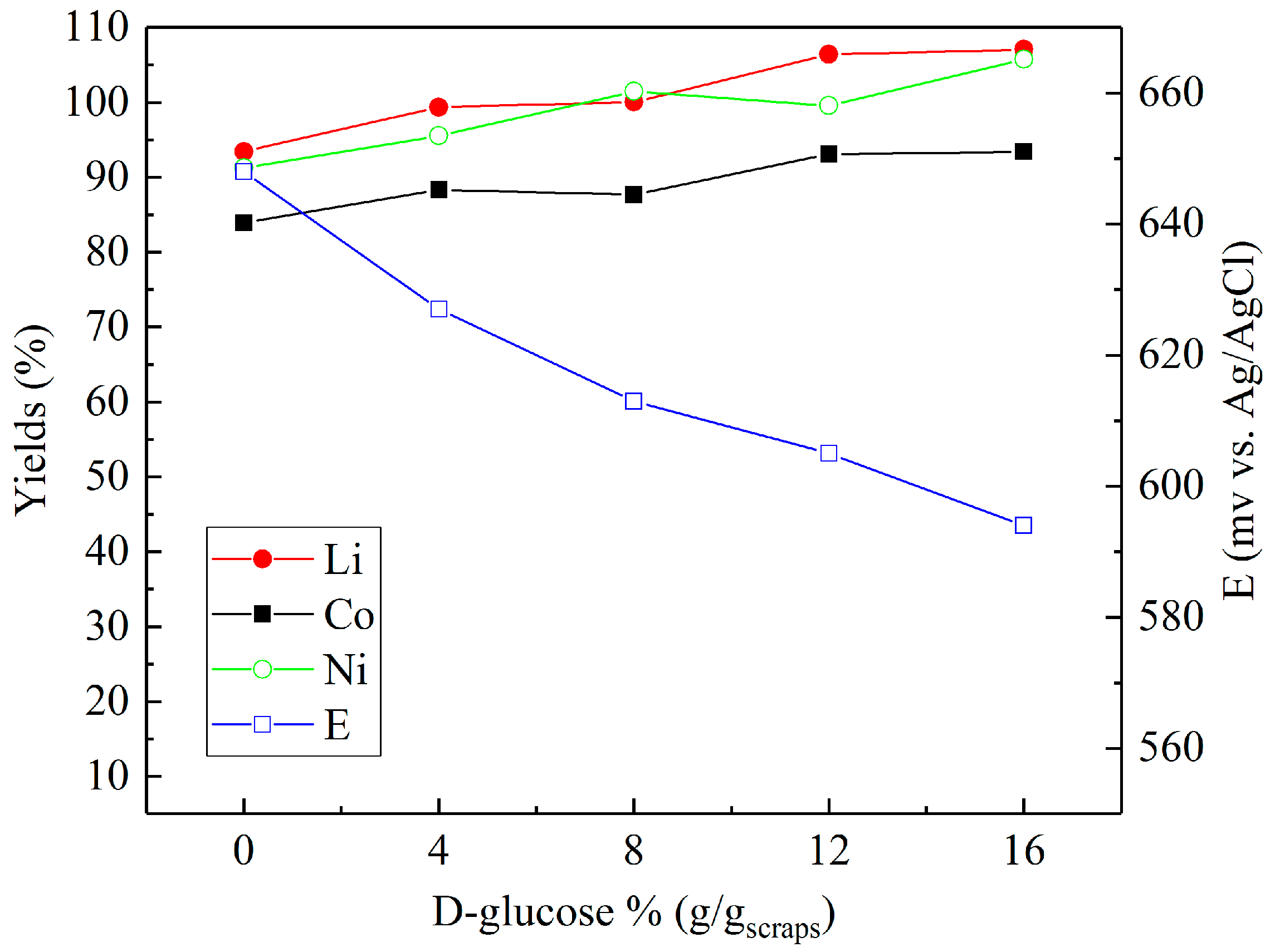
3.2. Leaching in Sulfuric Acid with Different Reducing Agents
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Christmann, P.; Gloaguen, E.; Labbé, J.F.; Melleton, J.; Piantone, P. Global Lithium Resources and Sustainability Issues. In Lithium Process Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–40. [Google Scholar]
- Graedel, T.E.; Allwood, J.; Birat, J.P.; Buchert, M.; Hagelüken, C.; Reck, B.K.; Sibley, S.F.; Sonnemann, G. UNEP 2011 Recycling Rates of Metals–A Status Report, A Report of the Working Group on the Global Metal Flows to the International Resource Panel; United Nations Environment Programme: Nairobi, Kenya, 2011; p. 44. ISBN 978-92-807-3161-3. [Google Scholar]
- Swiatowska, J.; Barboux, P. Lithium Battery Technologies: From the Electrodes to the Batteries. In Lithium Process Chemistry, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 125–166. ISBN 9780128016862. [Google Scholar]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Hannis, S.; Minks, A. Cobalt; British Geological Survey: Nottingham, UK, 2009; pp. 1–19. [Google Scholar]
- Brown, T.; Walters, A.; Idoine, N.; Gunn, G.; Shaw, R.A.; Rayner, D. Lithium; British Geological Survey: Nottingham, UK, 2016; pp. 1–39. [Google Scholar]
- Zhang, P.; Yokoyama, T.; Itabashi, O.; Suzuki, T.M.; Inoue, K. Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries. Hydrometallurgy 1998, 47, 259–271. [Google Scholar] [CrossRef]
- Joulié, M.; Lacournet, R.; Billy, E. Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries. J. Power Sources 2014, 247, 551–555. [Google Scholar] [CrossRef]
- Kang, J.; Senanayake, G.; Sohn, J.; Shin, S.M. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy 2010, 100, 168–171. [Google Scholar] [CrossRef]
- Lee, C.K.; Rhee, K.I. Preparation of LiCoO2 from spent lithium-ion batteries. J. Power Sources 2002, 109, 17–21. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J. Hazard Mater. 2010, 176, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, T. Hydrometallurgical process for the recovery of metal values from spent lithium-ion batteries in citric acid media. Waste Manag. Res. 2014, 32, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Qiu, K. Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag. 2012, 32, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, J.; Shen, B. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid. J. Hazard Mater. 2015, 295, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sun, R.; Xu, J.; Chen, Z.; Kang, M. Recovery of cobalt from spent lithium ion batteries using sulphuric acid leaching followed by solid–liquid separation and solvent extraction. RSC Adv. 2016, 6, 85303–85311. [Google Scholar] [CrossRef]
- Wang, J.; Chen, M.; Chen, H.; Luo, T.; Xu, Z. Leaching study of spent Li-ion batteries. Procedia Environ. Sci. 2012, 16, 443–450. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R.; Deveci, H. Comparison of different reductants in leaching of spent lithium ion batteries. JOM 2016, 68, 2613–2623. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Moscardini, E.; Granata, G.; Cerbelli, S.; Agosta, L.; Fieramosca, A.; Toro, L. Acid reducing leaching of cathodic powder from spent lithium ion batteries: Glucose oxidative pathways and particle area evolution. J. Ind. Eng. Chem. 2014, 20, 3201–3207. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Manjanna, J.; Pai, K.V.; Vadavi, R.; Keny, S.J.; Tripathi, V.S. Recovery of valuable metal ions from the spent lithium-ion battery using aqueous mixture of mild organic acids as alternative to mineral acids. Hydrometallurgy 2015, 151, 73–77. [Google Scholar] [CrossRef]
- Eckberg, C.; Petranikova, M. Lithium Battery Recycling. In Lithium Process Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 245–248. [Google Scholar]
- Pudas, J.; Erkkila, A.; Viljamaa, J. Battery Recycling Method. WIPO Patent WO2011113860A1, 22 September 2011. [Google Scholar]
- Freitas, M.B.J.G.; Garcia, E.M. Electrochemical recycling of cobalt from cathodes of spent lithium-ion batteries. J. Power Sources 2007, 171, 953–959. [Google Scholar] [CrossRef]
- Zhu, S.; He, W.; Li, G.; Zhou, X.; Zhang, X.; Huang, J. Recovery of Co and Li from spent lithium-ion batteries by combination method of acid leaching and chemical precipitation. Trans. Nonferrous Met. Soc. China 2012, 22, 2274–2281. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Chen, R.; Wu, F.; Chen, S.; Zhang, X. Environmental friendly leaching reagent for cobalt and lithium recovery from spent lithium-ion batteries. Waste Manag. 2010, 30, 2615–2621. [Google Scholar] [CrossRef] [PubMed]
- Saeki, S.; Lee, J.; Zhang, Q.; Saito, F. Co-grinding LiCoO2 with PVC and water leaching of metal chlorides formed in ground product. Int. J. Miner. Process. 2004, 74, 373–378. [Google Scholar] [CrossRef]
- Granata, G.; Moscardini, E.; Pagnanelli, F.; Trabucco, F.; Toro, L. Product recovery from Li-ion battery wastes coming from an industrial pre-treatment plant: Lab scale tests and process simulations. J. Power Sources 2012, 206, 393–401. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Ren, Y.; Zhang, X.X.; Chen, R.J.; Wu, F.; Amine, K. Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries. J. Power Sources 2012, 218, 21–27. [Google Scholar] [CrossRef]
- Creutz, C. The Complexities of Ascorbate as a Reducing Agent. Inorg. Chem. 1981, 20, 4449–4452. [Google Scholar] [CrossRef]
- Davies, M.B. Reactions of L-Ascorbic Acid with Transition Metal Complexes. Polyhedron 1992, 11, 285–321. [Google Scholar] [CrossRef]
- Maniyar, S.A.; Jargar, J.G.; Das, S.N.; Dhundasi, S.A.; Das, K.K. Alteration of chemical behavior of L-ascorbic acid in combination with nickel sulfate at different pH solutions in vitro. Asian Pac. J. Trop. Biomed. 2012, 2, 220–222. [Google Scholar] [CrossRef]

| Li | Co | Cu | Ni | Mn | Al | Fe | |
|---|---|---|---|---|---|---|---|
| Batch 1 | 2.9–3.4 * | 20.5–23.4 * | 2.8–2.9 * | 2.5–3.2 * | 2.7–2.8 * | 1.8–2.0 * | 0.8 |
| Batch 2 | 3.7 | 23.6 | 5.2 | 3.7 | N | 2.8 | 1.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aaltonen, M.; Peng, C.; Wilson, B.P.; Lundström, M. Leaching of Metals from Spent Lithium-Ion Batteries. Recycling 2017, 2, 20. https://doi.org/10.3390/recycling2040020
Aaltonen M, Peng C, Wilson BP, Lundström M. Leaching of Metals from Spent Lithium-Ion Batteries. Recycling. 2017; 2(4):20. https://doi.org/10.3390/recycling2040020
Chicago/Turabian StyleAaltonen, Miamari, Chao Peng, Benjamin P. Wilson, and Mari Lundström. 2017. "Leaching of Metals from Spent Lithium-Ion Batteries" Recycling 2, no. 4: 20. https://doi.org/10.3390/recycling2040020





