1. Introduction
The growing need for sustainable technologies is resulting in an increasing emphasis on the recycling of materials. This development is particularly important in the case of rare earth elements (REEs). These rare earth-based magnets—especially Neodymium-iron-boron (NdFeB) magnets—possess the highest energy product of all permanent magnets, which makes them highly efficient and suitable for lightweight mobile applications [
1]. Therefore, they are widely used in computer hard drives (HDDs), loudspeakers, medical imaging, household electrical appliances, hybrid and electric vehicles (HEVs and EVs), wind turbines, and many other small consumer electronic devices. About 48% of total NdFeB magnets produced in 2012 were used in the production of motors, generators, HDD, CDs and DVDs. The amount being used varies between a few grams (e.g., loudspeakers) to tonnes of materials (e.g., wind turbines) [
2].
In recent years, the increasing popularity of hybrid and electric cars and wind turbines has caused an increase in the demand for rare earth. China is currently producing more than 95% of these rare earth elements [
3]. Growing demand for REEs and restrictions on the supply from the China have already triggered a rare earth crisis in the past. The reason being that the supply of these rare earths is dependent on single source, i.e., China, and this supply risk can be felt outside China. In the report on critical raw materials for the EU published in 2014, the European Commission considers rare earth elements as the most critical raw materials group, with the highest supply risk [
4]. Moreover, it is anticipated that the demand for neodymium and dysprosium for production of magnets could rise by 700% and 2600%, respectively, over the next 25 years [
5].
Previous studies have shown that hydrogen can efficiently be used to separate NdFeB magnets from HDD scrap [
6,
7]. NdFeB magnets become demagnetised when reacted with hydrogen, thus allowing the powder to be separated much more readily. During this hydrogen decrepitation process, the NdFeB magnets absorb hydrogen and break down into an interstitial matrix phase hydride (Nd
2Fe
14BH
x) and a grain boundary phase hydride. The average particles size of grain boundary phase is <2 μm [
8], whereas the matrix phase breaks down in particle sizes ranging from 10 to 500 μm, depending on hydrogen cycling [
9]. It should be noted that the HD powder is very friable due to the presence of microcracks in the particles [
10]. Therefore, this HD powder can easily be milled down (using a jet mill) to the particle size required for sintering.
During sintering of NdFeB magnets, the RE-rich GBP melts down, resulting in liquid phase sintering. When the recycled HD powder is used to re-manufacture sintered NdFeB magnets, the GBP has a higher oxygen content and therefore it does not fully melt during the re-sintering process, which results in a lower-density magnet and reduction in magnetic properties. This liquid phase sintering process in recycled magnets can be improved by adding fresh neodymium hydride to the recycled material [
8,
11,
12]. The aim of this work is, therefore, to separate the grain boundary phase (<2 μm) with higher oxygen content from the matrix phase using a hydrocyclone, and optimise the process to achieve optimum separation.
Hydrocyclones have been used in the chemical and mineral industries for many years. Their usage is very wide in the mineral, chemical and bio-industries due to the simple design, operational flexibility and low operation and maintenance costs. The devices use centrifugal forces to separate two products of different densities or sizes [
13,
14]. Despite their simplicity and low cost, they are very efficient for solid-liquid separations [
15].
3. Results and Discussion
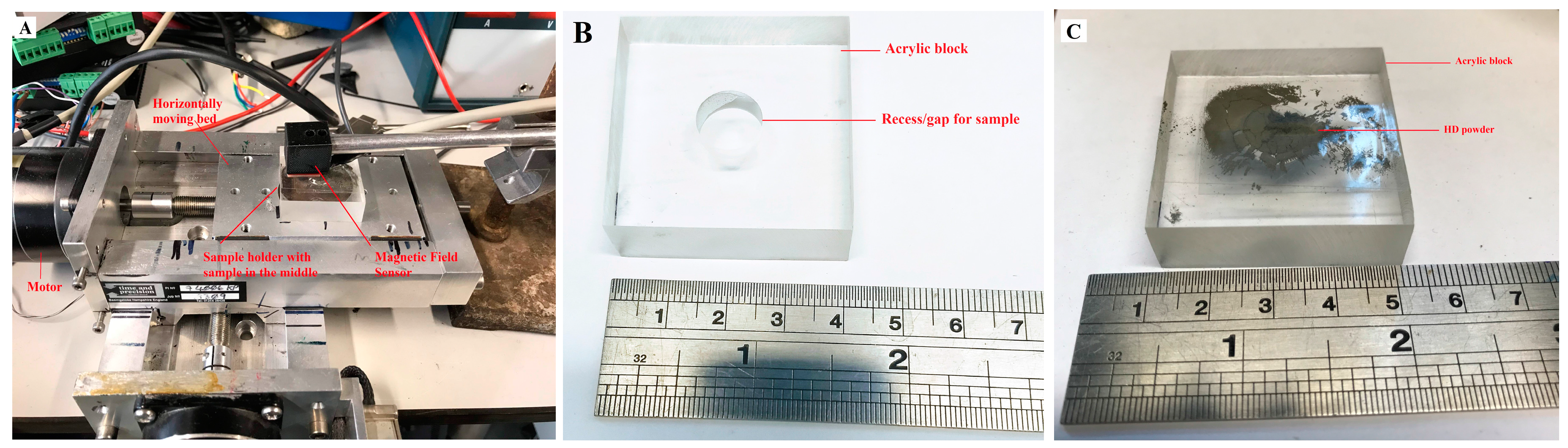
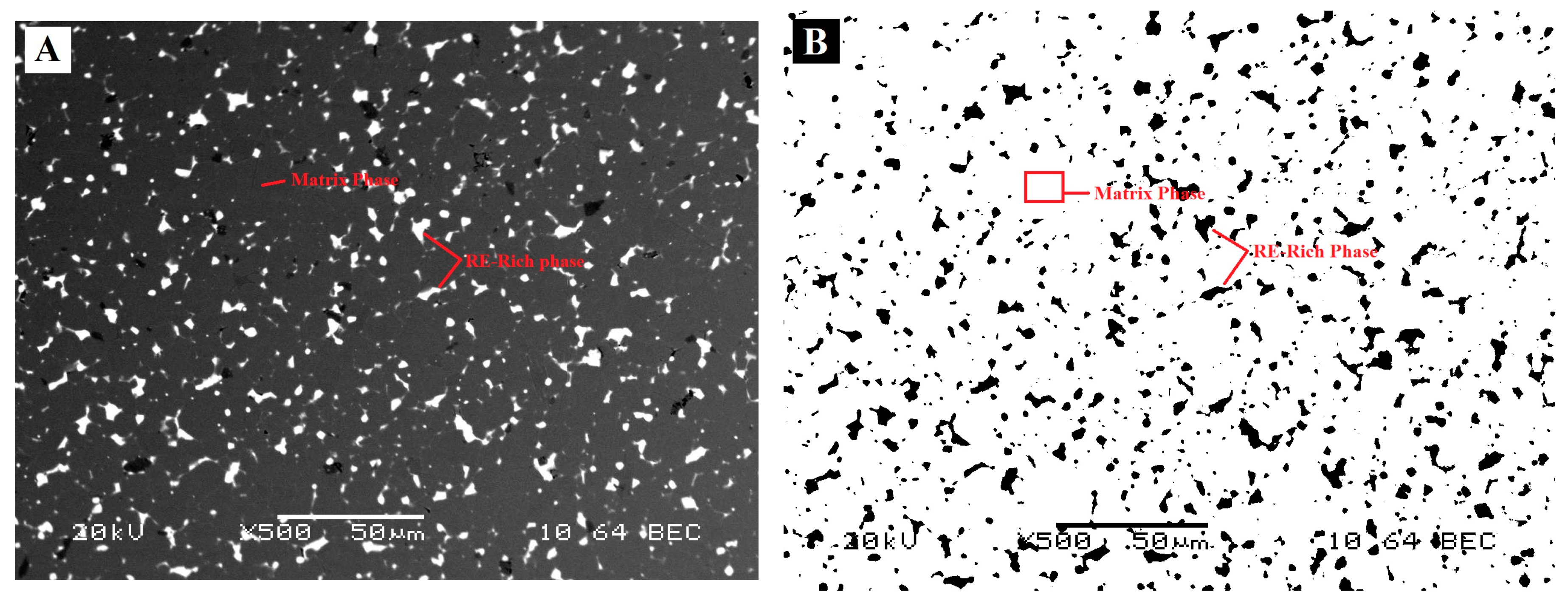
A VCM magnet was mounted in conductive bakelite, and the microstructures were analysed using SEM. The theoretical maximum amount of RE-rich (as shown in
Figure 3A,B) present in the magnets was estimated by computing the total area of RE-rich in an SEM image. Image J was used for this estimation and the area fraction was then converted into mass by multiplying with density. It should be noted that the theoretical maximum of RE-rich phase was an estimation, and it was assumed that the intergranular RE-rich phase was uniformly distributed, and that the volume of RE-rich phase was the same as the area fraction. To minimise the error, and taking into account the uneven distribution of RE-rich phase, 10 different SEM images were taken at different locations. After setting the threshold in Image J, these images were then converted into a binary images, and the area fraction was calculated. The maximum and minimum value of area fraction was 9.7% and 6.6%, respectively, with a standard deviation of 1.14. The SEM and binary images used are presented in
Figure 3. The average of these areas was then used for calculation. The theoretical maximum was estimated to be 8% by weight. Thus, in 1 kg of starting material, it was expected that there would be a maximum of 80 g of RE-rich phase.
Results from hydrocyclone separation are presented in
Table 1 below. There was a very small number of particles separated as overflow in the first three hydrocyclone passes, although this increased in last three passes due to the action of the pump.
Underflows and overflows collected from all of these passes (after drying) were then sieved again through different mesh sizes to classify the collected material based on particle sizes.
Table 2 gives the percentage of underflow samples collected at each sieve. It should be noted that a sample size of 40 g was used for sieving, as it is representative of the material collected as underflow during each pass.
It is clear from
Table 2 that about 20% (by weight) of the HD material used in this study was comprised of particles larger than 125 µm, and more than half of the material had particles less than 38 µm in size. As the material was passed through the hydrocyclone, the particle size distribution was changed. The HD material, being very friable, broke down inside the rotary pump and hydrocyclone, continuously generating smaller particles. These particles were then directed to the overflow with the very fine particles.
Sieving data of samples collected as overflow is presented in
Table 3, below. In this case, a sample size of 10 g was used for all overflows. It can be seen that, for the first two passes, the majority of the particles were less than 45 µm, and no material with a particle size larger than 63 µm was collected. As we move on to the third pass, larger particles started to appear on higher mesh size sieves. It can be noted that particle less than 38 µm in size were removed by the hydrocyclone as overflow up to the third pass. No considerable amount of particles less than 38 µm was collected after the third pass.
Table 4 presents the results obtained from XRF and oxygen analysis of the feed and underflow fractions. The term ∑REE represents the sum of rare earth elements in the material (Nd + Dy). The hydrided matrix phase (Nd
2Fe
14BH
x), being large-sized particles, should report to the underflow; and grain boundary phase, being smaller, should report to the overflow. As both of these particles have Nd in them, the number of passes required for optimum separation is based on total rare earth elements and concentration of iron. It should be noted that XRF data is normalised to 100% and the amount of oxygen was determined using different equipment at a later stage.
It can be seen from the
Table 4 that the concentration of iron increased with each hydrocyclone pass, whereas the amount of neodymium (Nd) decreased as expected. The slight variation in the values of Nd and Dy could be associated with the precision of the equipment.
The XRF and oxygen analysis of the overflow fractions are presented in
Table 5. The overflows were found to be rich in Neodymium. The presence of Fe in the overflow indicates that some of the large particles (matrix phase) were present in overflow alongside small particles (GBP). Moreover, Dy was also present in the GBP, which means that the Dy and Nd detected in the overflow originated in both the matrix phase and the GBP. From the fourth pass, the amount of Fe suddenly started to increase in the overflow, meaning that, after the third pass, there was more matrix phase in the overflow than the grain boundary phase. Sieving data of overflows (
Table 3) also agrees with this hypothesis. After the third pass, the continuous production of smaller particles affected the separation process, and no particles smaller than 38 µm were collected on the sieves, or the remaining small particles adhered to the larger particles by triboelectric charges, and could not be further separated [
16].
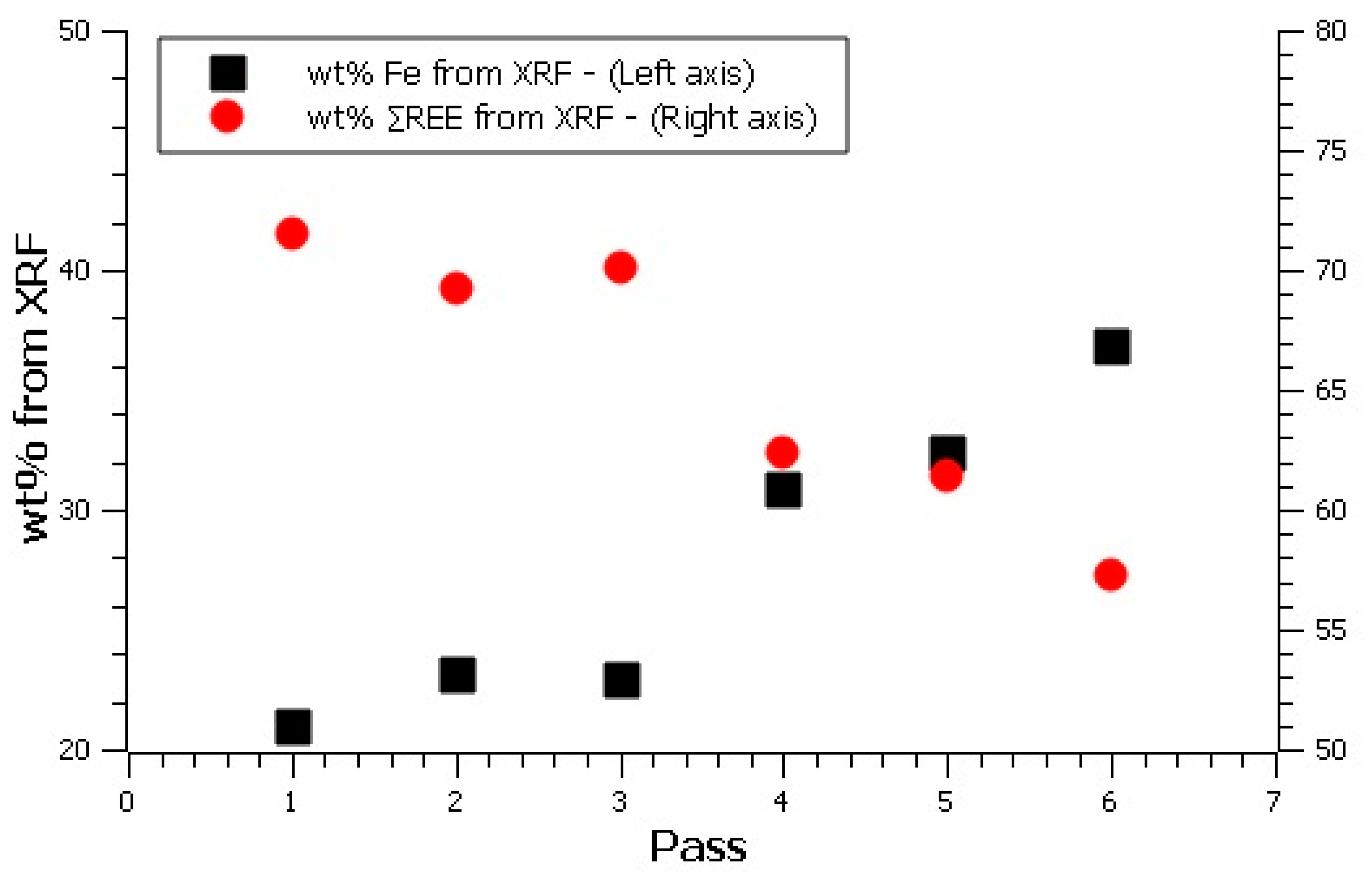
The plot of ∑REE and Fe (
Figure 4) from XRF shows that the REE-rich decreased and the Fe content increased after the 3rd pass. From this, we can assume that the overflows of first three passes were mainly RE-rich particles, and so can assume a separation efficiency close to 45% after three passes.
The oxygen content in all underflow samples (
Table 4) was slightly higher than the starting material (feed), which is likely to be due to the neodymium reacting with water to form neodymium hydroxide [
8,
19]. Formation of Nd(OH)
3 increases the amount of oxygen present in the starting material. On the other hand, there was a considerably higher amount of oxygen present in overflow streams (
Table 5). This means that the smaller particles, which were mainly neodymium oxide and hydroxide, were successfully separated from the matrix phase by this separation process.
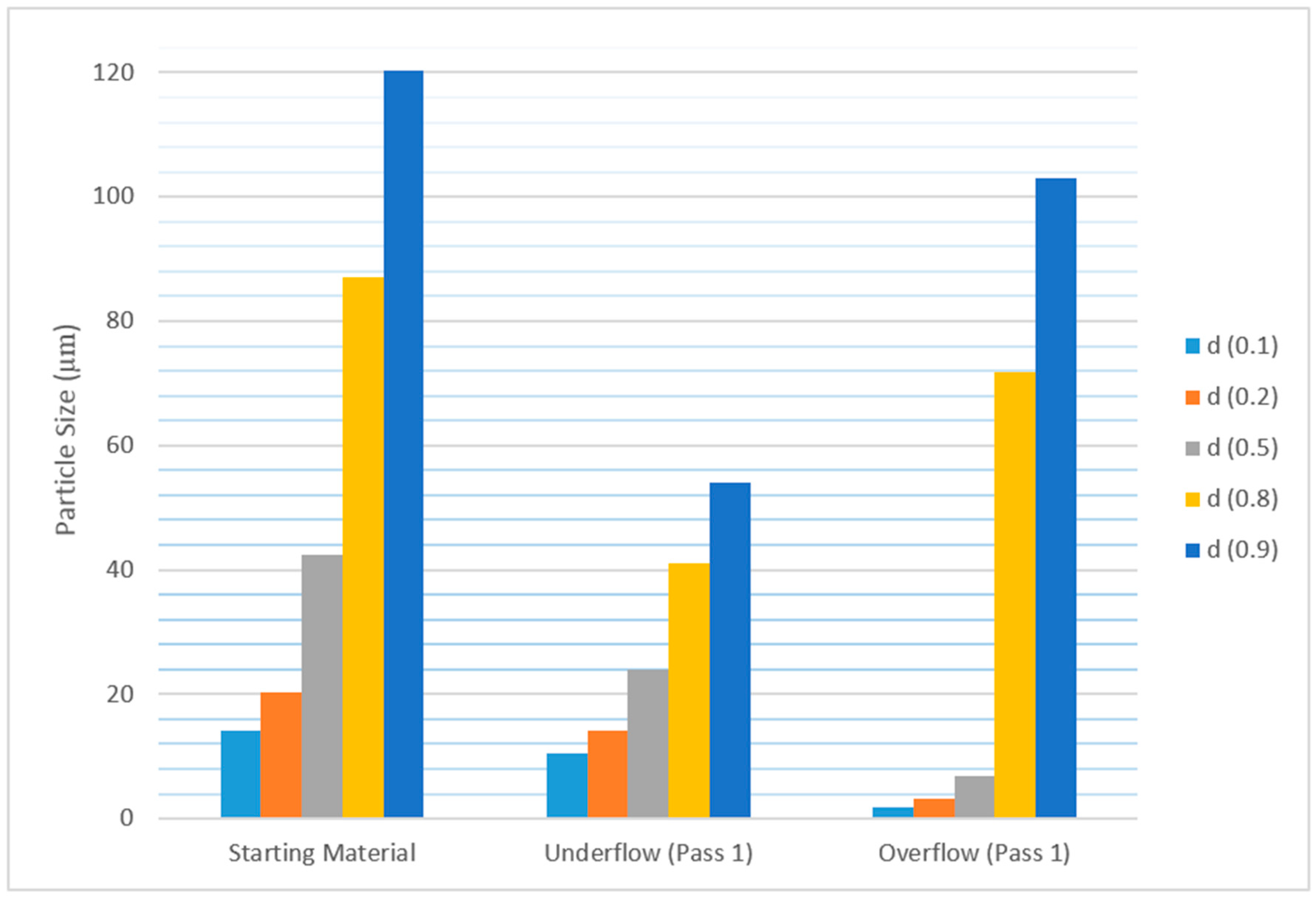
The particle size distribution results from the Mastersizer are shown in
Figure 5, below. The figure shows that the particles in the starting material (feed) were distributed between 1.5 µm and 120 µm, and 10% of total particles were smaller than 14 µm.
It can be seen from the above figure that the particle size in the underflow obtained after the first pass had reduced considerably. This confirms that the larger particles present in the feed keep breaking down into smaller particles as they pass through the hydrocyclone system.
The overflow stream contains mainly smaller particles (GBP), along with some larger particles (matrix phase). Evidence of the presence of matrix phase in overflow was also found in the chemical analysis (
Table 5) and sieving data (
Table 3).
Figure 5 also shows that particle size distribution (D50) had reduced from 42 µm (starting material) to 24 µm in underflow just after the first pass.
The measurement of particle size distribution was then repeated using Sympatec Qicpic 119D, and it was noticed that the none of the samples were free-flowing material.
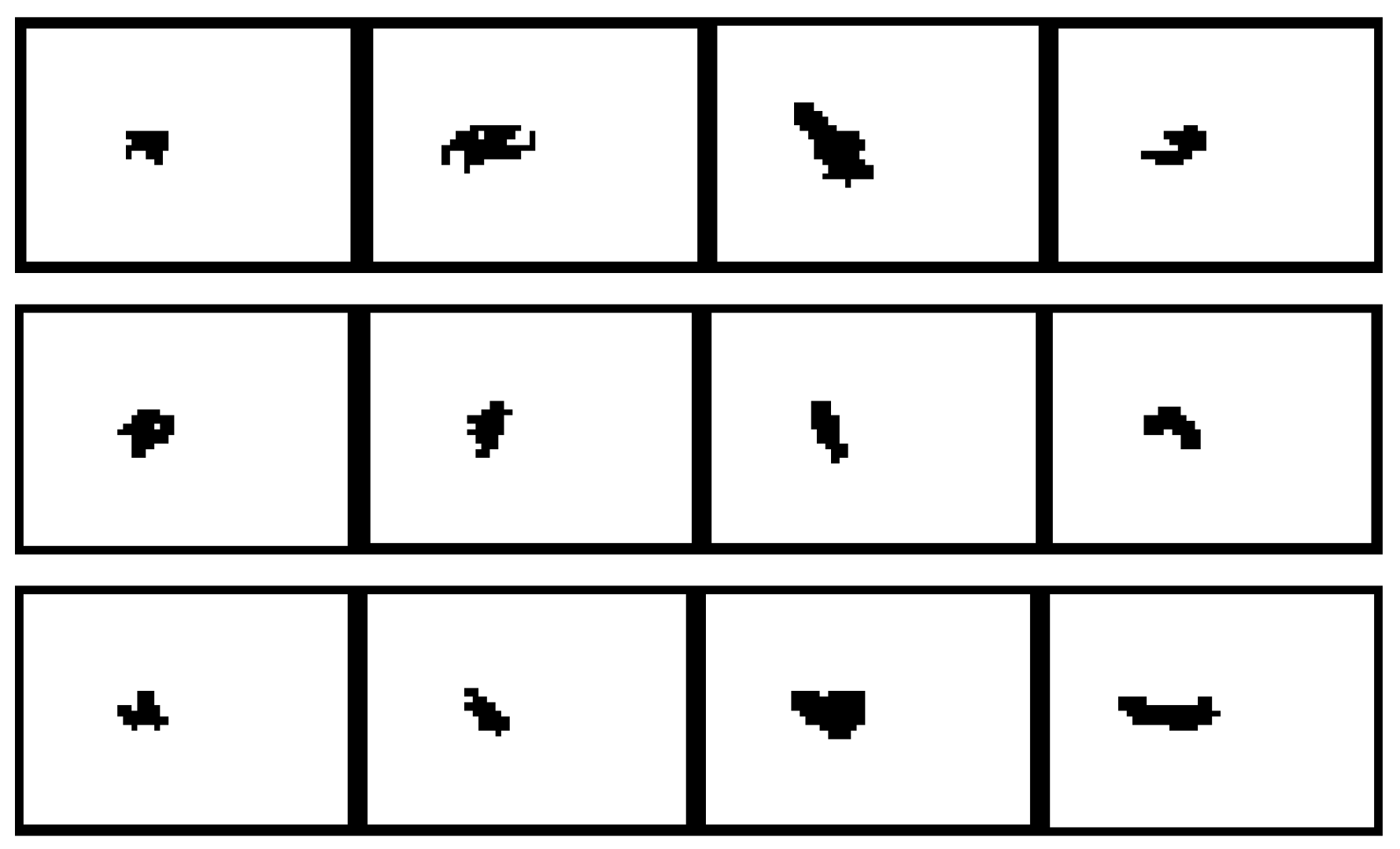
Figure 6 shows the images of some of the particles larger than 100 µm in underflow 1 taken by the Qicpic camera. From these images, it can be seen that larger particles are random in shape, and a clear void can also be noticed in some of the particles. These images suggest that these are not individual particles, but more than one particle clumped together. Thus, accurate measurement of particle size distribution in any of the samples is not possible using the optical or laser-based equipment. For all these techniques, for particle analysis to work properly, the particles must be deagglomerated.
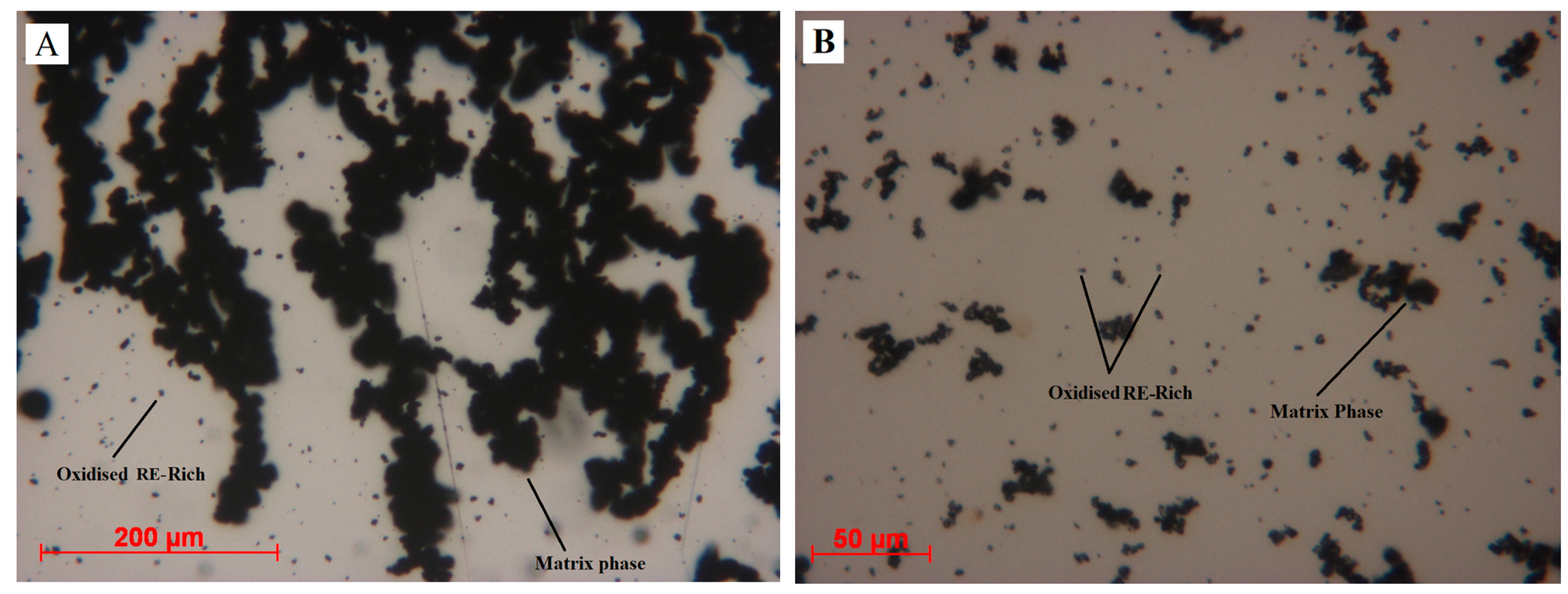
To investigate this agglomeration, underflow 1 and overflow 1 were mixed with water and observed under the Leitz optical microscope. To start with, a clear visual difference between underflow and overflow was noticed. The overflow was light coloured and the underflow was grey. Two drops of each suspension were dropped onto a mirror and spread evenly using a glass slide. The images of underflow and overflow are presented as
Figure 7A,B respectively.
Matrix phase and RE-rich particles are highlighted in
Figure 7. Matrix phase particles are agglomerated forming a chain network. On the other hand, oxidised RE-rich particles can be seen individually on the surface of the mirror. When a magnet was brought close to these particles, the matrix phase responded to the movement of the magnet, whereas no movement was observed in oxidised RE-rich particles. Based on this information, it was suspected that the HD particles were not fully demagnetized. Rather, they attract each other with very weak magnetic force to form agglomerations.
Figure 7A shows the powder forming chain-like structures; from this, we suspected that the particles from the matrix phase still had remanent magnetization and were interacting with each other, forming chain-like agglomerations. To confirm this observation, these samples were analysed for their magnetic field using MagScan.
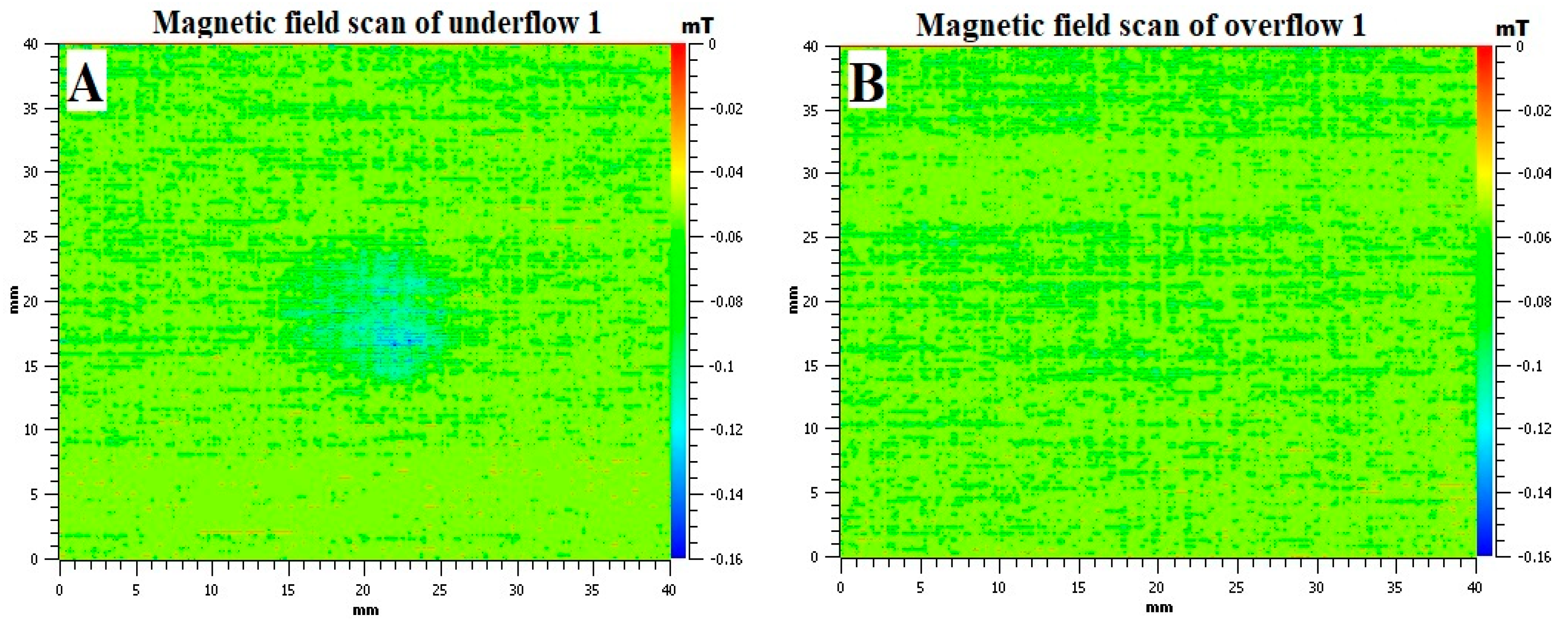
The contrasting colour images produced by MagScan for underflow 1 and overflow 1 are shown in
Figure 8. It can be seen that the underflow has a slight magnetic field in the area where the sample was placed, as shown in
Figure 2B. On the other hand, the overflow does not show any magnetic field. This confirms the observation made in
Figure 6 that the matrix phase particles are still magnetic, even after exposure to water.
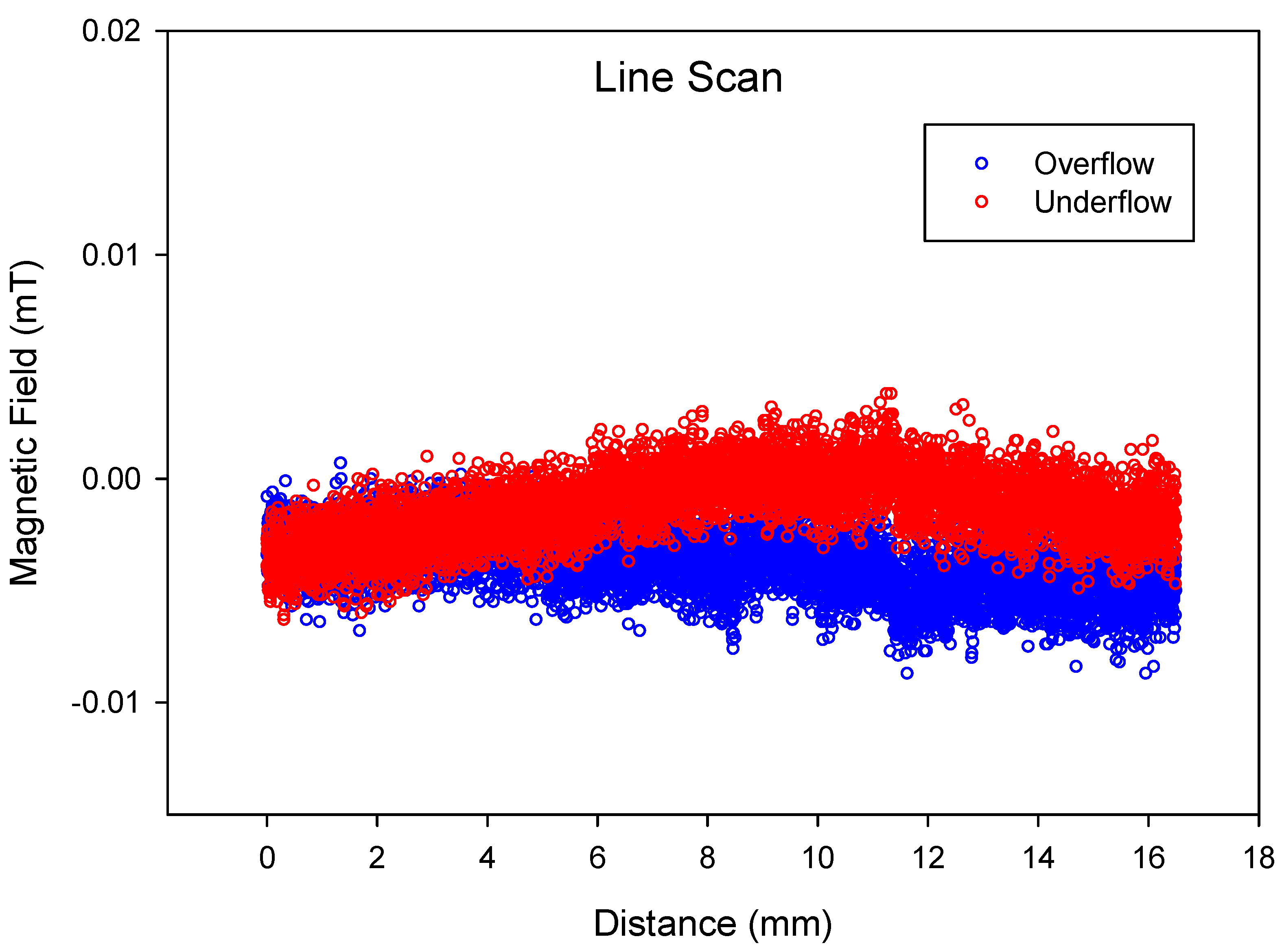
This was also confirmed by a more sensitive in-house-built MagScan (
Figure 2A), and the Line Scan of underflow and overflow is presented in
Figure 9. It can be concluded from the figure that both samples are slightly magnetic, but that the underflow has more magnetic field than the overflow. The reason for this difference in the magnetic field is the amount of matrix phase and oxidised RE-rich present in each sample. The overflow contains mostly oxidised RE-rich, with some matrix phase, as confirmed by
Figure 7B.