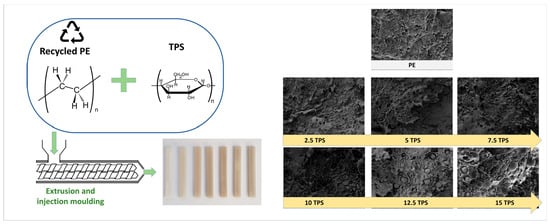
Evaluation of Thermoplastic Starch Contamination in the Mechanical Recycling of High-Density Polyethylene
Abstract
:1. Introduction
2. Results and Discussion
2.1. Miscibility and Microstructural Characterization
2.2. Mechanical Properties
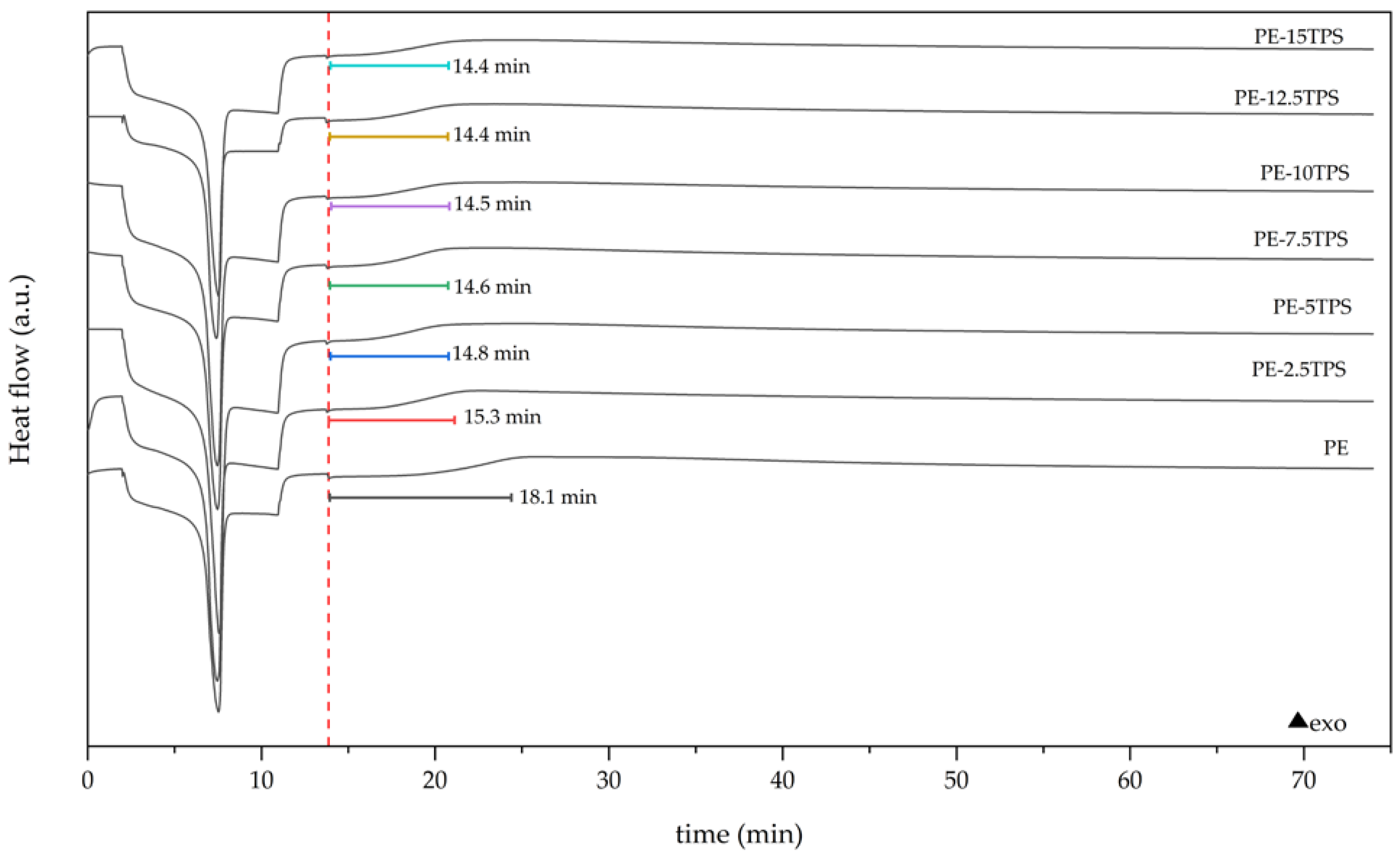
2.3. Thermal Characterization
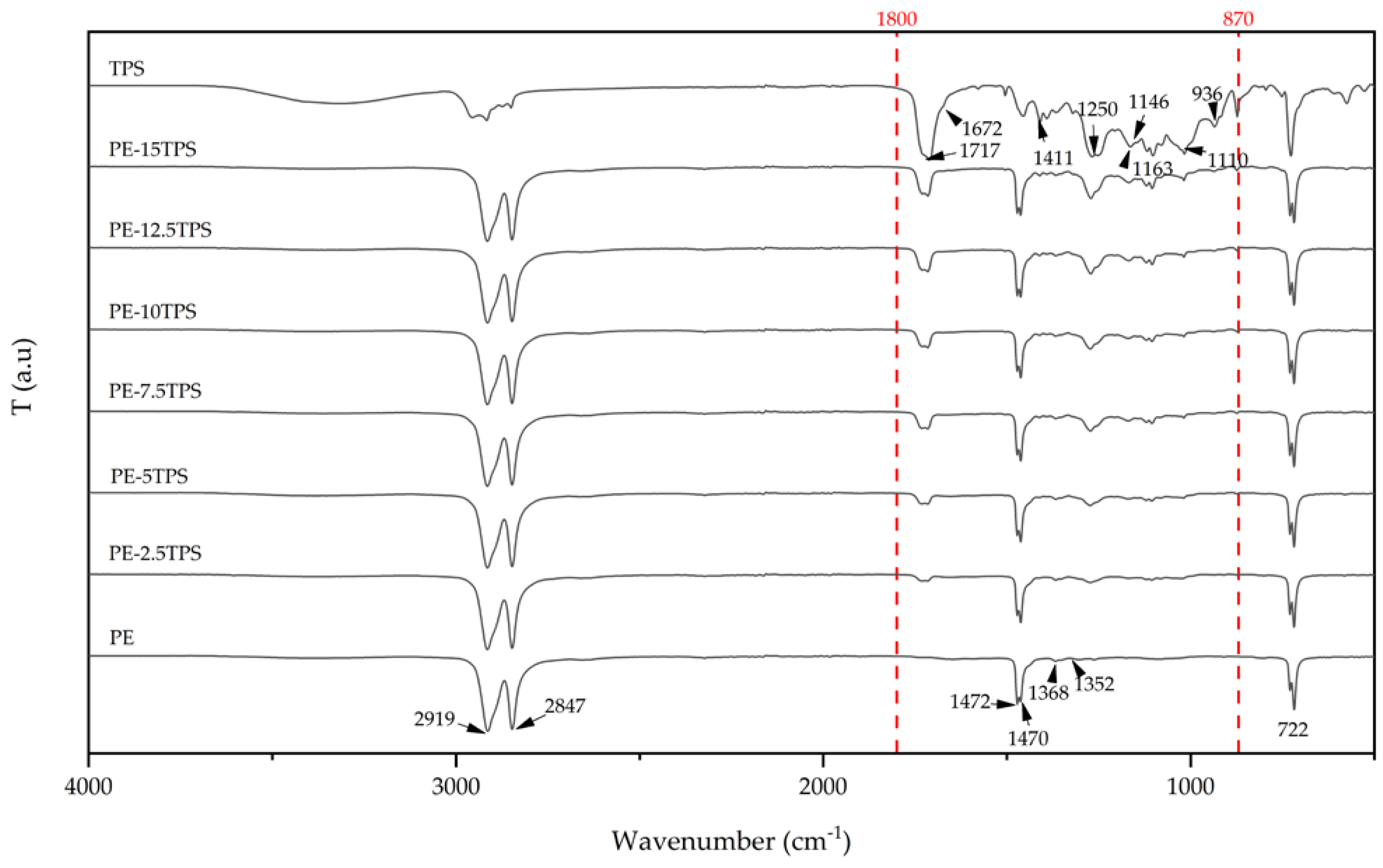
2.4. Fourier Transformed Infrared Spectroscopy
2.5. Visual Appearance
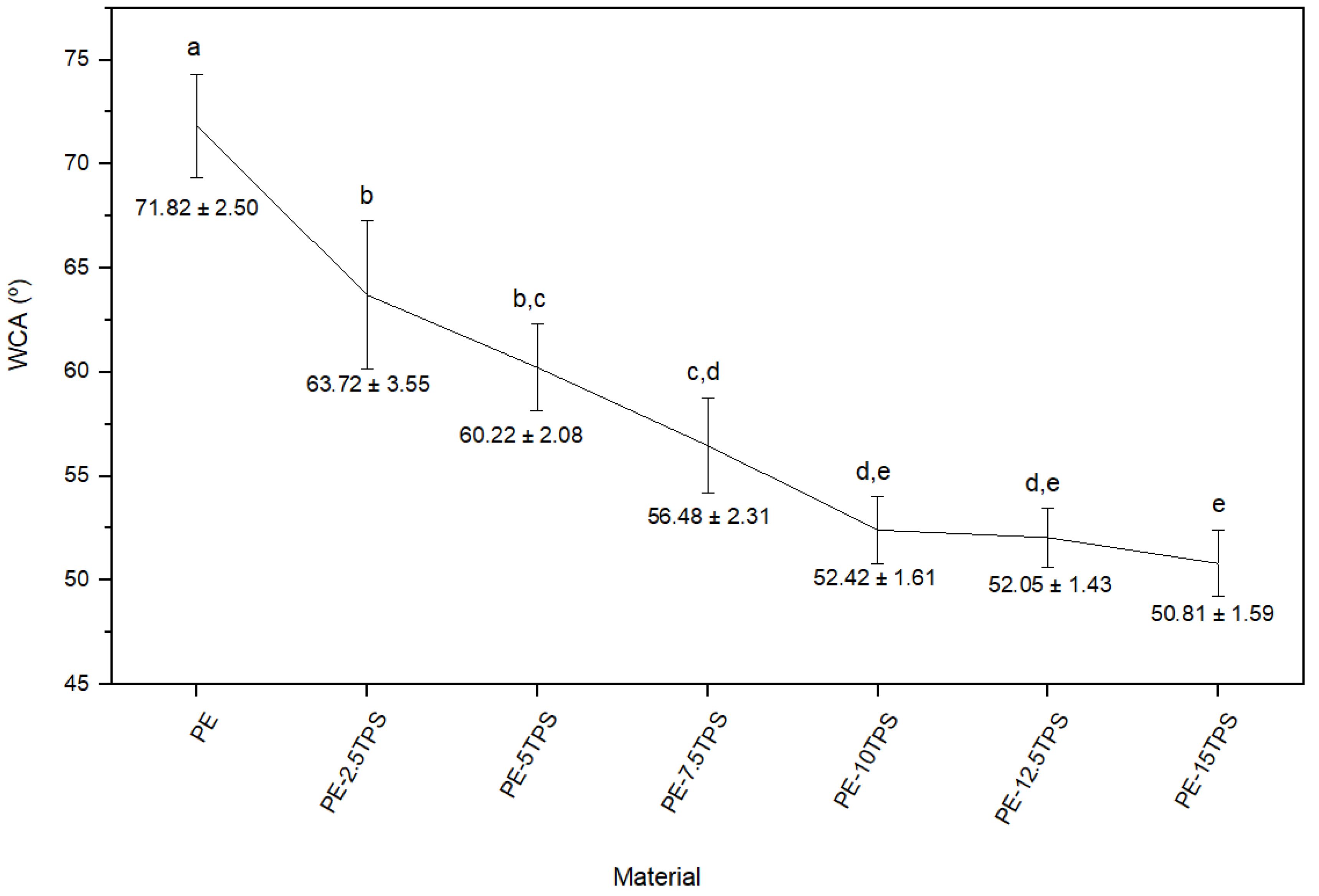
2.6. Wettability
3. Materials and Methods
3.1. Materials
3.2. Miscibility and Microstructural Characterization
3.3. Mechanical Characterization
3.4. Thermal Characterization
3.5. Fourier Transformed Infrared Spectroscopy
3.6. Visual Appearance
3.7. Wettability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plastics Europe Market Research Group (PEMRG). Plastics-the Facts 2022, An Analysis of European Plastics Production, Demand and Waste Data; Plastics Europe AISBL: Brussels, Belgium, 2022. [Google Scholar]
- EMF (Ellen MacArthur Foundation). The New Plastic Economy—Rethinking Thefutureofplastics. Available online: https://emf.thirdlight.com/file/24/_A-BkCs_skP18I_Am1g_JWxFrX/The%20New%20Plastics%20Economy%3A%20Rethinking%20the%20future%20of%20plastics.pdf (accessed on 26 May 2023).
- European Commission. A European Strategy for Plastics in a Circular Economy; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- ISO 15270:2008; Plastics—Guidelines for the Recovery and Recycling of Plastics Waste. International Standards Organization: Geneva, Switzerland, 2008.
- Ragaert, K.; Ragot, C.; Van Geem, K.M.; Kersten, S.; Shiran, Y.; De Meester, S. Clarifying European Terminology in Plastics Recycling. Curr. Opin. Green Sustain. Chem. 2023, 44, 100871. [Google Scholar] [CrossRef]
- Ignatyev, I.A.; Thielemans, W.; Vander Beke, B. Recycling of Polymers: A Review. ChemSusChem 2014, 7, 1579–1593. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, F.; Wei, X.; Yang, Y.; Xu, S.; Deng, D.; Wang, Y.Z. From Trash to Treasure: Chemical Recycling and Upcycling of Commodity Plastic Waste to Fuels, High-Valued Chemicals and Advanced Materials. J. Energy Chem. 2022, 69, 369–388. [Google Scholar] [CrossRef]
- Eriksen, M.K.; Christiansen, J.D.; Daugaard, A.E.; Astrup, T.F. Closing the Loop for PET, PE and PP Waste from Households: Influence of Material Properties and Product Design for Plastic Recycling. Waste Manag. 2019, 96, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, G.; Uchida, N.; Tuyen, L.H.; Tanaka, K.; Matsukami, H.; Kunisue, T.; Takahashi, S.; Viet, P.H.; Kuramochi, H.; Osako, M. Mechanical Recycling of Plastic Waste as a Point Source of Microplastic Pollution. Environ. Pollut. 2022, 303, 119114. [Google Scholar] [CrossRef] [PubMed]
- Dobry, A.; Boyer-Kawenoki, F. Phase Separation in Polymer Solution. J. Polym. Sci. 1947, 2, 90–100. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics Recycling: Challenges and Opportunities. Philos. Trans. R. Soc. B: Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Pavon, C.; Aldas, M.; Bertomeu, D.; de la Rosa-Ramírez, H.; Samper, M.D.; López-Martínez, J. Influence of the Presence of Poly(Butylene Succinate) in the Poly(Ethylene Terephthalate) Recycling Process. Clean Technol. 2023, 5, 190–202. [Google Scholar] [CrossRef]
- Pavon, C.; Aldas, M.; Ferri, J.M.; Bertomeu, D.; Pawlak, F.; Samper, M.D. Identification of Biodegradable Polymers as Contaminants in the Thermoplastics Recycling Process. Dyna 2021, 96, 415–421. [Google Scholar] [CrossRef]
- Lim, B.K.H.; Thian, E.S. Biodegradation of Polymers in Managing Plastic Waste—A Review. Sci. Total Environ. 2022, 813, 151880. [Google Scholar] [CrossRef]
- European Bioplastics Bioplastics Market Development. Available online: http://www.european-bioplastics.org/news/publications/ (accessed on 2 February 2022).
- Bioplastics, E. What Are Bioplastics?-Material Types, Terminology, and Labels—An Introduction; European Bioplastics: Berlin, Germany, 2016. [Google Scholar]
- Alaerts, L.; Augustinus, M.; Van Acker, K. Impact of Bio-Based Plastics on Current Recycling of Plastics. Sustainability 2018, 10, 1487. [Google Scholar] [CrossRef]
- Åkesson, D.; Kuzhanthaivelu, G.; Bohlén, M. Effect of a Small Amount of Thermoplastic Starch Blend on the Mechanical Recycling of Conventional Plastics. J. Polym. Environ. 2021, 29, 985–991. [Google Scholar] [CrossRef]
- Samper, M.D.; Arrieta, M.P.; Ferrándiz, S.; López-Martínez, J. Influence of Biodegradable Materials in the Recycled Polystyrene. J. Appl. Polym. Sci. 2014, 131, 41161–41168. [Google Scholar] [CrossRef]
- Samper, M.D.; Bertomeu, D.; Arrieta, M.P.; Ferri, J.M.; López-Martínez, J.; Bartomeu, D.; Arrieta, M.P.; Ferri, J.M.; López-Martínez, J. Interference of Biodegradable Plastics in the Polypropylene Recycling Process. Materials 2018, 11, 1886. [Google Scholar] [CrossRef] [PubMed]
- Aldas, M.; Pavon, C.; De La Rosa-Ramírez, H.; Ferri, J.M.; Bertomeu, D.; Samper, M.D.; López-Martínez, J. The Impact of Biodegradable Plastics in the Properties of Recycled Polyethylene Terephthalate. J. Polym. Environ. 2021, 29, 2686–2700. [Google Scholar] [CrossRef]
- Aldas, M.; Pavon, C.; López-Martínez, J.; Arrieta, M.P.P. Pine Resin Derivatives as Sustainable Additives to Improve the Mechanical and Thermal Properties of Injected Moulded Thermoplastic Starch. Appl. Sci. 2020, 10, 2561. [Google Scholar] [CrossRef]
- Baum, B. The Mechanism of Polyethylene Oxidation. J. Appl. Polym. Sci. 1959, 2, 281–288. [Google Scholar] [CrossRef]
- Odelius, K.; Ohlson, M.; Höglund, A.; Albertsson, A.C. Polyesters with Small Structural Variations Improve the Mechanical Properties of Polylactide. J. Appl. Polym. Sci. 2013, 127, 27–33. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Dirk, W.; Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 0080915108. [Google Scholar]
- Chemical Retrieval on the Web (CROW) Plastic Library. Available online: https://polymerdatabase.com/polymer%20classes/Intro.html (accessed on 3 August 2022).
- Ghodgaonkar, P.G.; Sundararaj, U. Prediction of Dispersed Phase Drop Diameter in Polymer Blends: The Effect of Elasticity. Polym. Eng. Sci. 1996, 36, 1656–1665. [Google Scholar] [CrossRef]
- Sundararaj, U.; Macosko, C.W. Drop Breakup and Coalescence in Polymer Blends: The Effects of Concentration and Compatibilizationt. Macromolecules 1995, 28, 2647–2657. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A.; Jhurry, D. Biodegradable Polymer Blends: Miscibility, Physicochemical Properties and Biological Response of Scaffolds. Polym. Int. 2015, 64, 1289–1302. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Botta, L.; Morreale, M.; Scaffaro, R. Effect of Small Amounts of Poly(Lactic Acid) on the Recycling of Poly(Ethylene Terephthalate) Bottles. Polym. Degrad. Stab. 2012, 97, 21–24. [Google Scholar] [CrossRef]
- Fekete, E.; Földes, E.; Pukánszky, B. Effect of Molecular Interactions on the Miscibility and Structure of Polymer Blends. Eur. Polym. J. 2005, 41, 727–736. [Google Scholar] [CrossRef]
- Titone, V.; Botta, L.; Mistretta, M.C.; La Mantia, F.P. Influence of a Biodegradable Contaminant on the Mechanical Recycling of a Low-Density Polyethylene Sample. Polym. Eng. Sci. 2024, 64, 845–851. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montava-Jordà, S.; Boronat, T.; Sammon, C.; Balart, R.; Torres-Giner, S. On the Use of Gallic Acid as a Potential Natural Antioxidant and Ultraviolet Light Stabilizer in Cast-Extruded Bio-Based High-Density Polyethylene Films. Polymers 2020, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Lei, H.; Xie, C.S. The Effect of Nucleating Agent on the Crystalline Morphology of Polypropylene (PP). Mater. Des. 2003, 24, 227–230. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Rayón, E.; López, J.; Arrieta, M.P.; Hernández-Fernández, J.; Rayón, E.; López, J.; Arrieta, M.P. Enhancing the Thermal Stability of Polypropylene by Blending with Low Amounts of Natural Antioxidants. Macromol. Mater. Eng. 2019, 304, 1900379. [Google Scholar] [CrossRef]
- Novák, I.; Popelka, A.; Krupa, I.; Chodák, I.; Janigová, I.; Nedelčev, T.; Špírková, M.; Kleinová, A. High-Density Polyethylene Functionalized by Cold Plasma and Silanes. Vacuum 2012, 86, 2089–2094. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradation of Maleated Linear Low-Density Polyethylene and Starch Blends. Polym. Degrad. Stab. 1997, 56, 185–202. [Google Scholar] [CrossRef]
- Dang, K.M.; Yoksan, R. Development of Thermoplastic Starch Blown Film by Incorporating Plasticized Chitosan. Carbohydr. Polym 2015, 115, 575–581. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of Irradiated Starches by Using FT-Raman and FTIR Spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Agüero, A.; Morcillo, M.d.C.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Lascano, D.; Torres-Giner, S.; Fenollar, O. Study of the Influence of the Reprocessing Cycles on the Final Properties of Polylactide Pieces Obtained by Injection Molding. Polymers 2019, 11, 1908. [Google Scholar] [CrossRef] [PubMed]
- Pavon, C.; Aldas, M.; López-Martínez, J.; Hernández-Fernández, J.; Patricia Arrieta, M. Films Based on Thermoplastic Starch Blended with Pine Resin Derivatives for Food Packaging. Foods 2021, 10, 1171. [Google Scholar] [CrossRef] [PubMed]
- Lazrak, C.; Kabouchi, B.; Hammi, M.; Famiri, A.; Ziani, M. Structural Study of Maritime Pine Wood and Recycled High-Density Polyethylene (HDPEr) Plastic Composite Using Infrared-ATR Spectroscopy, X-Ray Diffraction, SEM and Contact Angle Measurements. Case Stud. Constr. Mater. 2019, 10, e00227. [Google Scholar] [CrossRef]
- David, D.J.; Sincock, T.F. Estimation of Miscibility of Polymer Blends Using the Solubility Parameter Concept. Polymers (Guildf) 1992, 33, 4505–4514. [Google Scholar] [CrossRef]
- Small, P.A. Some Factors Affecting the Solubility of Polymers. J. Appl. Chem. 1953, 3, 71–80. [Google Scholar] [CrossRef]
- Carraher, C.E. Polymer Chemistry, 6th ed.; Marcel Dekker, Inc.: New York, NY, USA, 2003; ISBN 0824708067. [Google Scholar]
- ISO 527-1:2012; Plastics—Determination of Tensile Properties—Part 1: General Principles. International Standards Organization: Geneva, Switzerland; AENOR: Madrid, Spain, 2012.
- ISO 179-1:2010; Plastics—Determination of Charpy Impact Properties/Part 1: Non-Instrumented Impact Test. International Standards Organization: Geneva, Switzerland, 2010; Volume 22.
- ISO 11357-6:2018; Differential Scanning Calorimetry (DSC) Part 6: Determination of Oxidation Induction Time (Isothermal OIT) and Oxidation Induction Temperature (Dynamic OIT). International Standards Organization: Geneva, Switzerland, 2018.



| Polymer | Structure | (MPa1/2) Calculated | δ(MPa1/2) Literature [26] |
|---|---|---|---|
| PE |  | 16.7 | 14.8–19.9 |
| TPS |  | 8.4 | - |
| Material | σmax (MPa) | Etensile (MPa) | εb (%) | Charpy Impact (kJ/m2) |
|---|---|---|---|---|
| PE | 20.2 ± 2.6 a | 1013 ± 64 a | 1253 ± 24 a | 5.6 ± 0.2 a |
| PE-2.5TPS | 17.8 ± 1.2 b | 857 ± 68 b | 1204 ± 13 b | 5.3 ± 0.2 b |
| PE-5TPS | 17.8 ± 3.2 b | 816 ± 54 b | 1121 ± 26 c | 4.8 ± 0.2 c |
| PE-7.5TPS | 17.5 ± 5.3 b | 679 ± 91 c | 815 ± 144 d | 4.7 ± 0.3 c |
| PE-10TPS | 17.3 ± 1.6 b | 563 ± 41 d | 952 ± 17 e | 4.6 ± 0.2 c |
| PE-12.5TPS | 12.9 ± 0.8 c | 560 ± 54 d | 537 ± 163 f | 4.5 ± 0.3 c,d |
| PE-15TPS | 12.5 ± 0.7 c | 450 ± 66 e | 275 ± 72 g | 4.2 ± 0.3 c,d |
| Material | Tm (°C) | ΔHm (J/g) | χc (%) | OOT (°C) | OIT (min) |
|---|---|---|---|---|---|
| PE | 130.2 | 147.83 | 50.5 | 219.7 | 18.1 |
| PE-2.5TPS | 131.6 | 139.25 | 48.7 | 218.1 | 15.3 |
| PE-5TPS | 131.6 | 129.83 | 46.6 | 217.7 | 14.8 |
| PE-7.5TPS | 131.0 | 127.78 | 47.1 | 219.8 | 14.6 |
| PE-10TPS | 130.4 | 119.95 | 45.5 | 220.3 | 14.5 |
| PE-12.5TPS | 131.2 | 117.36 | 45.8 | 220.1 | 14.4 |
| PE-15TPS | 130.1 | 115.19 | 46.3 | 218.0 | 14.4 |
| TPS | 123.0 | 23.95 | - | - | - |
| Material | L* | a* | b* | ΔEab* |
|---|---|---|---|---|
| PE | 62.89 ± 0.47 a | −1.92 ± 0.25 a | −3.81 ± 0.3 a | - |
| PE-2.5TPS | 61.91 ± 0.35 b | −1.83 ± 0.03 a | −2.38 ± 0.09 b | 1.75 ± 0.3 a |
| PE-5TPS | 59.52 ± 0.27 c | −1.31 ± 0.09 b | −1.29 ± 0.18 c | 4.25 ± 0.3 b |
| PE-7.5TPS | 59.06 ± 0.43 c,d | −1.28 ± 0.06 b | −0.95 ± 0.09 d | 4.81 ± 0.4 b |
| PE-10TPS | 58.59 ± 0.39 d | −1.38 ± 0.05 b | −0.32 ± 0.12 e | 5.57 ± 0.3 c |
| PE-12.5TPS | 55.52 ± 0.52 e | −0.87 ± 0.06 c | −0.22 ± 0.07 e | 8.27 ± 0.5 d |
| PE-15TPS | 51.54 ± 0.76 f | −0.58 ± 0.07 c | −0.34 ± 0.09 e | 11.09 ± 0.7 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cascales, A.; Pavon, C.; Ferrandiz, S.; López-Martínez, J. Evaluation of Thermoplastic Starch Contamination in the Mechanical Recycling of High-Density Polyethylene. Recycling 2024, 9, 33. https://doi.org/10.3390/recycling9030033
Cascales A, Pavon C, Ferrandiz S, López-Martínez J. Evaluation of Thermoplastic Starch Contamination in the Mechanical Recycling of High-Density Polyethylene. Recycling. 2024; 9(3):33. https://doi.org/10.3390/recycling9030033
Chicago/Turabian StyleCascales, Antonio, Cristina Pavon, Santiago Ferrandiz, and Juan López-Martínez. 2024. "Evaluation of Thermoplastic Starch Contamination in the Mechanical Recycling of High-Density Polyethylene" Recycling 9, no. 3: 33. https://doi.org/10.3390/recycling9030033