Updated Aspects of Safety Regulations for Biomedical Applications of Aerogel Compounds—Compendia-Like Evaluation
Abstract
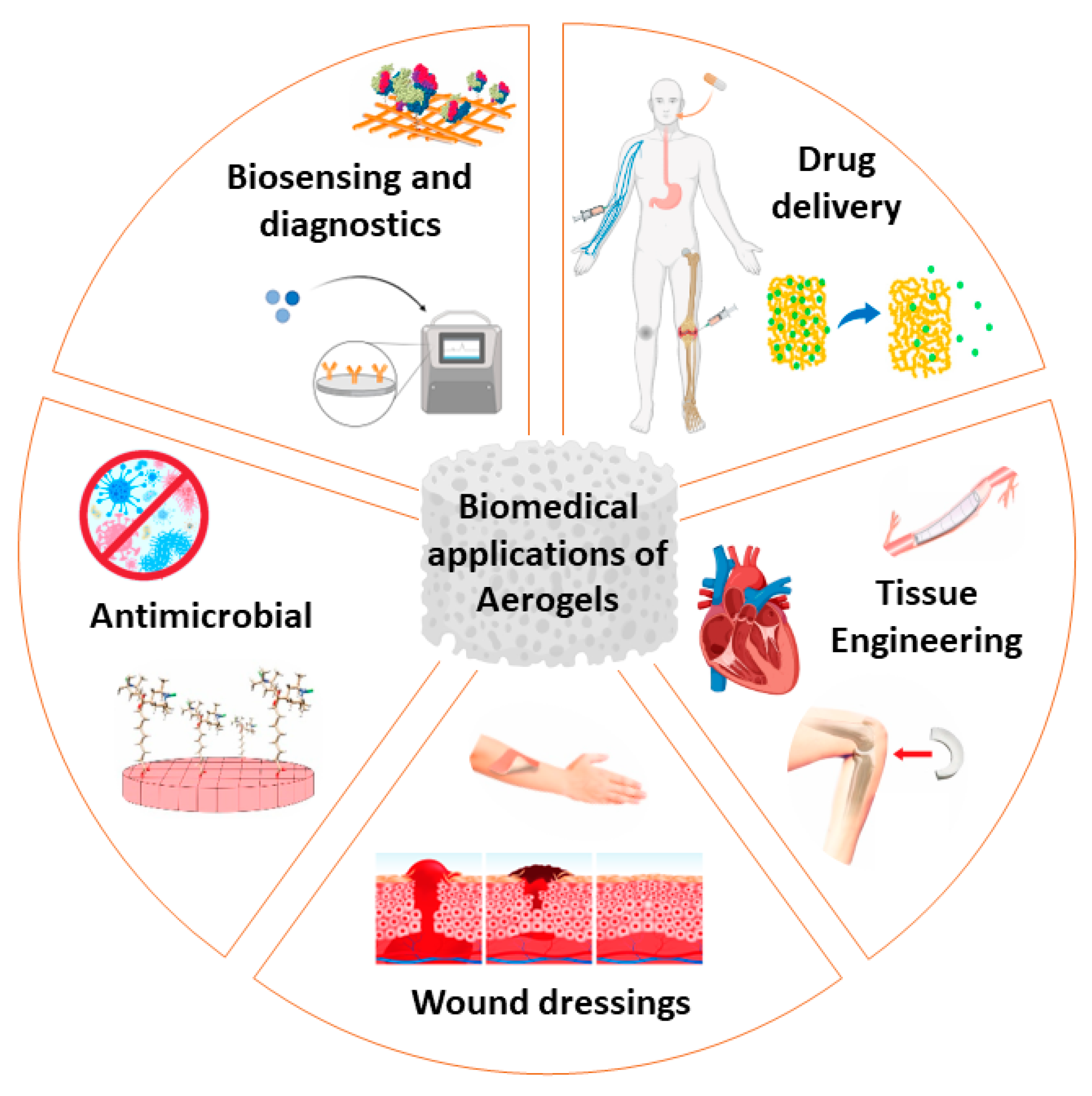
:1. Introduction
2. Aerogels’ Particularities as Innovative Materials
3. Safety Regulation in Aerogels Testing
3.1. Level 1
3.1.1. Testing the Oxidative Stress in Abiotic Conditions
3.1.2. Testing the Dissolution Characteristics in Biological Fluids in Acellular Conditions
3.1.3. Stretch-Stress Tests
3.2. Level 2—In Vitro Cellular Models for Testing Aerogels
3.3. Level 3—In Vivo Testing of Aerogel
4. Health Risk Outlines
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selmer, I.; Kleemann, C.; Kulozik, U.; Heinrich, S.; Smirnova, I. Development of egg white protein aerogels as new matrix material for microencapsulation in food. J. Supercrit. Fluids 2015, 106, 42–49. [Google Scholar] [CrossRef]
- García-González, C.A.; Jin, M.; Gerth, J.; Alvarez-Lorenzo, C.; Smirnova, I. Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohydr. Polym 2015, 117, 797–806. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Ulker, Z.; Erkey, C. An emerging platform for drug delivery: Aerogel-based systems. J. Control. Release 2014, 177, 51–63. [Google Scholar] [CrossRef] [PubMed]
- European Commission Recommendation on the Definition of Nanomaterial. Definition of Nanomaterial—European Observatory for Nanomaterials. 2011. Available online: europa.eu (accessed on 26 August 2023).
- Mech, A.; Wohlleben, W.; Ghanem, A.; Hodoroaba, V.D.; Weigel, S.; Babick, F.; Brüngel, R.; Friedrich, C.M.; Rasmussen, K.; Rauscher, H. Nano or Not Nano? A Structured Approach for Identifying Nanomaterials According to the European Commission’s Definition. Small 2020, 16, e2002228. [Google Scholar] [CrossRef] [PubMed]
- Wigger, H.; Wohlleben, W.; Nowack, B. Redefining environmental nanomaterial lows: Consequences of the regulatory nanomaterial definition on the results of environmental exposure models. Environ. Sci. Nano 2018, 5, 1372–1385. [Google Scholar] [CrossRef]
- Rauscher, H.; Kestens, V.; Rasmussen, K.; Linsinger, T.; Stefaniak, E.; European Commission; Joint Research Centre. Guidance on the Implementation of the Commission Recommendation 2022/C 229/01 on the Definition of Nanomaterial; Publications Office of the European Union: Luxembourg, 2023; ISSN 1831-9424. Available online: https://data.europa.eu/doi/10.2760/143118 (accessed on 23 August 2023).
- Steinhauser, K.G.; Sayre, P.G. Reliability of methods and data for regulatory assessment of nanomaterial risks. NanoImpact 2017, 7 (Suppl. C), 66–74. [Google Scholar] [CrossRef]
- Donaldson, K.; Poland, C.A. Nanotoxicity: Challenging the myth of nano-specific toxicity. Curr. Opin. Biotechnol. 2013, 24, 724–734. [Google Scholar] [CrossRef]
- Keller, J.G.; Wiemann, M.; Groters, S.; Werle, K.; Vennemann, A.; Landsiedela, R.; Wohlleben, W. Aerogels are not regulated as nanomaterials, but can be assessed by tiered testing and grouping strategies for nanomaterials. Nanoscale Adv. 2021, 3, 3881–3893. [Google Scholar] [CrossRef]
- Oberdorster, G.; Kuhlbusch, T.A.J. In vivo effects: Methodologies and biokinetics of inhaled nanomaterials. NanoImpact 2018, 10 (Suppl. C), 38–60. [Google Scholar] [CrossRef]
- Charalabidis, A.; Sfouni, M.; Bergstrom, C.; Macheras, P. The Biopharmaceutics Classification System (BCS) and the Biopharmaceutics Drug Disposition Classification System (BDDCS): Beyond guidelines. Int. J. Pharm. 2019, 566, 264–281. [Google Scholar] [CrossRef] [PubMed]
- Hua, S. Advances in oral drug delivery for regional targeting in the gastrointestinal tract—Influence of physiological, pathophysiological and pharmaceutical factors. Front. Pharmacol. 2020, 11, 524–546. [Google Scholar] [CrossRef]
- Sellitto, M.R.; Amante, C.; Aquino, R.P.; Russo, P.; Rodríguez-Dorado, R.; Neagu, M.; García-González, C.A.; Adami, R.; Del Gaudio, P. Hollow Particles Obtained by Prilling and Supercritical Drying as a Potential Conformable Dressing for Chronic Wounds. Gels 2023, 9, 492–505. [Google Scholar] [CrossRef]
- García-González, C.A.; Sosnik, A.; Kalmár, J.; De Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in drug delivery: From design to application. J. Control. Release 2021, 332, 40–63. [Google Scholar] [CrossRef]
- Sunargulov, T. Changes in the respiratory organs in experimental dust inhalation with silica aerogel. Arkhiv Patol. 1966, 28, 15–20. [Google Scholar]
- Krishnakumari, M.K. Comparative acute oral toxicity of some mineral pesticides to albino rats. In Proceedings of the Symposium on Pesticides, Mysore, India; 1964; pp. 335–338. Available online: https://ir.cftri.res.in/3545/ (accessed on 23 August 2023).
- Cotton, R.T.; Frankenfeld, J.C. Silica Aerogel for Protecting Stored Seed or Milled Cereal Products from Insects. J. Econ. Entomol. 1949, 42, 553–561. [Google Scholar] [CrossRef]
- Gao, T.; Ihara, T.; Grynning, S.; Jelle, B.P.; Gunnarshaug Lien, A. Perspective of aerogel glazings in energy efficient buildings. Build. Environ. 2016, 95, 405–413. [Google Scholar] [CrossRef]
- García-González, C.A.; Budtova, T.; Durães, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An opinion paper on aerogels for biomedical and environmental applications. Molecules 2019, 24, 1815–1830. [Google Scholar] [CrossRef]
- Ferreira-Gonçalves, T.; Constantin, C.; Neagu, M.; Pinto Reis, C.; Sabri, F.; Simón-Vázquez, R. Safety and efficacy assessment of aerogels for biomedical applications. Biomed. Pharmacother. 2021, 144, 112356–112371. [Google Scholar] [CrossRef]
- Sabri, F.; Boughter, J.D., Jr.; Gerth, D.; Skalli, O.; Phung, T.C.; Tamula, G.R.; Leventis, N. Histological evaluation of the biocompatibility of polyurea crosslinked silica aerogel implants in a rat model: A pilot study. PLoS ONE 2012, 7, e50686. [Google Scholar] [CrossRef]
- Quraishi, S.; Martins, M.; Barros, A.A.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Novel non-cytotoxic alginate–lignin hybrid aerogels as scaffolds for tissue engineering. J. Supercrit. Fluids 2015, 105, 1–8. [Google Scholar] [CrossRef]
- Keil, C.; Hübner, C.; Richter, C.; Lier, S.; Barthel, L.; Meyer, V.; Subrahmanyam, R.; Gurikov, P.; Smirnova, I.; Haase, H. Ca-Zn-Ag alginate aerogels for wound healing applications: Swelling behavior in simulated human body fluids and effect on macrophages. Polymers 2020, 12, 2741–2758. [Google Scholar] [CrossRef] [PubMed]
- Alnaief, M.; Obaidat, R.M.; Alsmadi, M.T.M. Preparation of Hybrid Alginate-Chitosan Aerogel as Potential Carriers for Pulmonary Drug Delivery. Polymers 2020, 12, 2223–2240. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.X.; Zhang, Y.; Hu, T.; Li, W.X.; Li, Z.L.; Hu, H.J.; Zhu, S.R.; Chen, W.Z.; Zhou, C.S.; Jiang, G.B. Long-term antibacterial composite via alginate aerogel sustained release of antibiotics and Cu used for bone tissue bacteria infection. Int. J. Biol. Macromol. 2021, 167, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Liebner, F.; Pircher, N.; Schimper, C.; Haimer, E.; Rosenau, T. Aerogels: Cellulose-Based. In Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; Mishra, M., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 2015. [Google Scholar]
- Durães, L.; Maleki, H.; Vareda, J.P.; Lamy-Mendes, A.; Portugal, A. Exploring the Versatile Surface Chemistry of Silica Aerogels for Multipurpose Application. MRS Adv. 2017, 2, 3511–3519. [Google Scholar] [CrossRef]
- Smirnova, I.; Gurikov, P. Aerogels in chemical engineering: Strategies toward tailor-made aerogels. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 307–334. [Google Scholar] [CrossRef]
- Sahin, I.; Uzunlar, E.; Erkey, C. Investigation of kinetics of supercritical drying of alginate alcogel particles. J. Supercrit. Fluids 2019, 146, 78–88. [Google Scholar] [CrossRef]
- Vareda, J.P.; Lamy-Mendes, A.; Durães, L. A reconsideration on the definition of the term aerogel based on current drying trends. Microporous Mesoporous Mater. 2018, 258, 211–216. [Google Scholar] [CrossRef]
- García-González, C.A.; Camino-Rey, M.C.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2: Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar] [CrossRef]
- Budtova, T. Cellulose II aerogels: A review. Cellulose 2019, 26, 81–121. [Google Scholar] [CrossRef]
- Santos-Rosales, V.; Ardao, I.; Alvarez-Lorenzo, C.; Ribeiro, N.; Oliveira, L.A.; García-González, A.C. Sterile and Dual-Porous Aerogels Scaffolds Obtained through a Multistep Supercritical CO2-Based Approach. Molecules 2019, 24, 871–888. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.; Duan, G.; Jiang, S.; Zhang, K. Recent Progress on Nanocellulose Aerogels: Preparation, Modification, Composite Fabrication, Applications. Adv. Mater. 2021, 33, 2005569–2005603. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Markevicius, G.; Ladj, R.; Niemeyer, P.; Budtova, T.; Rigacci, A. Ambient-dried thermal superinsulating monolithic silica-based aerogels with short cellulosic fibers. J. Mater. Sci. 2017, 52, 2210–2221. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Silva, R.F.; Durães, L. Advances in carbon nanostructure–silica aerogel composites: A review. J. Mater. Chem. A 2018, 6, 1340–1369. [Google Scholar] [CrossRef]
- Ganesan, K.; Barowski, A.; Ratke, L.; Milow, B. Influence of hierarchical porous structures on the mechanical properties of cellulose aerogels. J. Sol-Gel Sci. Technol. 2019, 89, 156–165. [Google Scholar] [CrossRef]
- García-González, C.A.; López-Iglesias, C.; Concheiro, A.; Alvarez-Lorenzo, C. Chapter 16, Biomedical Applications of Polysaccharide and Protein Based Aerogels. In Biobased Aerogels: Polysaccharide and Protein-Based Materials; The Royal Society of Chemistry: Cambridge, UK, 2018; pp. 295–323. [Google Scholar]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the Production of Polysaccharide Aerogel Particles. Materials 2018, 11, 2144–2181. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Casielles, A.M.; Altay, A.; Bettini, R.; Alvarez-Lorenzo, C.; García-González, C.A. From the printer to the lungs: Inkjet-printed aerogel particles for pulmonary delivery. Chem. Eng. J. 2019, 357, 559–566. [Google Scholar] [CrossRef]
- Maleki, H. Recent advances in aerogels for environmental remediation applications: A review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Tucker, G.; DeSilva, B.; Dressman, J.; Ito, M.; Kumamoto, T.; Mager, D.; Mahler, H.-C.; Maitland-van der Zee, A.H.; Pauletti, G.M.; Sasaki, H.; et al. Current Challenges and Potential Opportunities for the Pharmaceutical Sciences to Make Global Impact: An FIP Perspective. J. Pharm. Sci. 2016, 105, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Fontenot, K.R.; Liebner, F.; Condon, B.D. Peptide-Cellulose Conjugates on Cotton-Based Materials Have Protease Sensor/Sequestrant Activity. Sensors 2018, 18, 2334–2350. [Google Scholar] [CrossRef] [PubMed]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Ågren, M. Wound Healing Biomaterials–Volume 2: Functional Biomaterials; Elsevier Science: Cambridge, UK, 2016. [Google Scholar]
- García-González, C.A.; Concheiro, A.; Alvarez-Lorenzo, C. Processing of Materials for Regenerative Medicine Using Supercritical Fluid Technology. Bioconjug. Chem. 2015, 26, 1159–1171. [Google Scholar] [CrossRef]
- Lu, T.H.; Li, Q.; Chen, W.S.; Yu, H.P. Composite aerogels based on dialdehyde nanocellulose and collagen for potential applications as wound dressing and tissue engineering scaffold. Compos. Sci. Technol. 2014, 94, 132–138. [Google Scholar] [CrossRef]
- Yin, W.; Rubenstein, D. Biomedical applications of aerogels. In Aerogels Handbook; Advances in sol–gel derived materials and, technologies; Aegerter, M.A., Leventis, N., Koebel, M.M., Eds.; Springer: New York, NY, USA, 2011; pp. 683–694. [Google Scholar]
- Power, M.; Hosticka, B.; Black, E.; Daitch, C.; Norris, P. Aerogels as biosensors: Viral particle detection by bacteria immobilized on large pore aerogel. J. Non-Cryst. Solids 2001, 285, 303–308. [Google Scholar] [CrossRef]
- Martins, M.; Barros, A.A.; Quraishi, S.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Preparation of macroporous alginate-based aerogels for biomedical applications. J. Supercrit. Fluids 2015, 106, 152–159. [Google Scholar] [CrossRef]
- Zhang, Y.; Nypelö, T.; Salas, C.; Arboleda, J.; Hoeger, I.C.; Rojas, O.J. Cellulose nanofibrils. J. Renew. Mater. 2013, 1, 195–211. [Google Scholar] [CrossRef]
- Cumana, S.; Ardao, I.; Zeng, A.-P.; Smirnova, I. Glucose-6-phosphate dehydrogenase encapsulated in silica-based hydrogels for operation in a microreactor. Eng. Life Sci. 2014, 14, 170–179. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Parikka, K.; Ghafar, A.; Tenkanen, M. Prospects of polysaccharide aerogels as modern advanced food materials. Trends Food Sci. Technol. 2013, 34, 124–136. [Google Scholar] [CrossRef]
- Smirnova, I.; García-González, C.A.; Gurikov, P. Pharmaceutical applications of aerogels. In Springer Handbook of Aerogels; Aegerter, M.A., Leventis, N., Koebel, M.M., Steiner III, S.A., Eds.; Springer: New York, NY, USA, 2023; pp. 1489–1504. [Google Scholar]
- Gaudio, P.D.; Auriemma, G.; Mencherini, T.; Porta, G.D.; Reverchon, E.; Aquino, R.P. Design of alginate-based aerogel for nonsteroidal anti-inflammatory drugs controlled delivery systems using prilling and supercritical-assisted drying. J. Pharm. Sci. 2013, 102, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Liebner, F.; Dunareanu, R.; Opietnik, M.; Haimer, E.; Wendland, M.; Werner, C.; Maitz, M.; Seib, P.; Neouze, M.-A.; Potthast, A.; et al. Shaped hemocompatible aerogels from cellulose phosphates: Preparation and properties. Holzforschung 2012, 66, 317–321. [Google Scholar] [CrossRef]
- Tseng, P.; Napier, B.; Zhao, S.; Mitropoulos, A.N.; Applegate, M.B.; Marelli, B.; Kaplan, D.L.; Omenetto, F.G. Directed assembly of bio-inspired hierarchical materials with controlled nanofibrillar architectures. Nat. Nanotechnol. 2017, 12, 474–480. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, L.; Zhang, T.; Qiu, F.; Yue, X.; Yang, D. Sustainable, Flexible, and Superhydrophobic Functionalized Cellulose Aerogel for Selective and Versatile Oil/Water Separation. ACS Sustain. Chem. Eng. 2019, 7, 9984–9994. [Google Scholar] [CrossRef]
- Takeshita, S.; Sadeghpour, A.; Malfait, W.J.; Konishi, A.; Otake, K.; Yoda, S. Formation of Nanofibrous Structure in Biopolymer Aerogel during Supercritical CO2 Processing: The Case of Chitosan Aerogel. Biomacromolecules 2019, 20, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Dorado, R.; López-Iglesias, C.; García-González, C.A.; Auriemma, G.; Aquino, R.P.; Del Gaudio, P. Design of Aerogels, Cryogels and Xerogels of Alginate: Effect of Molecular Weight, Gelation Conditions and Drying Method on Particles’ Micromeritics. Molecules 2019, 24, 1049–1061. [Google Scholar] [CrossRef]
- Feng, J.; Su, B.-L.; Xia, H.; Zhao, S.; Gao, C.; Wang, L.; Ogbeide, O.; Feng, J.; Hasan, T. Printed aerogels: Chemistry, processing, and applications (Review Article). Chem. Soc. Rev 2021, 50, 3842–3888. [Google Scholar] [CrossRef]
- Mohammadian, M.; Jafarzadeh Kashi, T.S.; Erfan, M.; Soorbaghi, F.P. Synthesis and characterization of silica aerogel as a promising drug carrier system. J. Drug Deliv. Sci. Technol. 2018, 44, 205–212. [Google Scholar] [CrossRef]
- Wu, K.C.W.; Yamauchi, Y.; Hong, C.Y.; Yang, Y.H.; Liang, Y.H.; Funatsu, T.; Tsunoda, M. Biocompatible, surface functionalized mesoporous titania nanoparticles for intracellular imaging and anticancer drug delivery. Chem. Commun. 2011, 47, 5232–5234. [Google Scholar] [CrossRef]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of chitosan aerogels as promising carriers for drug delivery: A review. Carbohydr. Polym. 2020, 231, 115744–115758. [Google Scholar] [CrossRef]
- Veronovski, A.; Tkalec, G.; Knez, Z.; Novak, Z. Characterisation of biodegradable pectin aerogels and their potential use as drug carriers. Carbohydr. Polym. 2014, 113, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Agostinho, D.A.S.; Paninho, A.I.; Cordeiro, T.; Nunes, A.V.M.; Fonseca, I.M.; Pereira, C.; Matias, A.; Ventura, M.G. Properties of κ-carrageenan aerogels prepared by using different dissolution media and its application as drug delivery systems. Mater. Chem. Phys. 2020, 253, 123290–123300. [Google Scholar] [CrossRef]
- Athamneh, T.; Amin, A.; Benke, E.; Ambrus, R.; Leopold, C.S.; Gurikov, P.; Smirnova, I. Alginate and hybrid alginate-hyaluronic acid aerogel microspheres as potential carrier for pulmonary drug delivery. J. Supercrit. Fluids 2019, 150, 49–55. [Google Scholar] [CrossRef]
- De Marco, I.; Iannone, R.; Miranda, S.; Riemma, S. An environmental study on starch aerogel for drug delivery applications: Effect of plant scale-up. Int. J. Life Cycle Assess. 2018, 23, 1228–1239. [Google Scholar] [CrossRef]
- Yue, J.; Xi, X.; Yu, C.; Liu, L.; Rui, Y.; Guoxin, S. Hierarchically structured cellulose aerogels with interconnected MXene networks and their enhanced microwave absorption properties. J. Mater. Chem. C 2018, 6, 8679–8687. [Google Scholar]
- Arts, H.; Hadi, M.; Keene, A.M.; Kreiling, R.; Lyon, D.; Monika Maier, M.; Michel, K.; Thomas Petry, T.; Sauer, U.G.; Warheit, D.; et al. A critical appraisal of existing concepts for the grouping of nanomaterials. Regul. Toxicol. Pharmacol. 2014, 70, 492–506. [Google Scholar] [CrossRef]
- Oomen, G.; Bleeker, E.A.; Bos, P.M.; van Broekhuizen, F.; Gottardo, S.; Groenewold, M.; Hristozov, D.; Hund-Rinke, K.; Irfan, M.A.; Marcomini, A.; et al. Grouping and read-across approaches for risk assessment of nanomaterials. Int. J. Environ. Res. Public Health 2015, 12, 13415–13434. [Google Scholar] [CrossRef]
- Oomen, G.; Steinhäuser, K.G.; Bleeker, E.A.J.; van Broekhuizen, F.; Sips, A.; Dekkers, S.; Wijnhoven, S.W.P.; Sayre, P.G. Risk assessment frameworks for nanomaterials: Scope, link to regulations, applicability, and outline for future directions in view of needed increase in efficiency. NanoImpact 2018, 9 (Suppl. C), 1–13. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Appendix for Nanoforms Applicable to the Guidance on Registration and Substance Identication, in ECHA-19-H-14-EN; ECHA: Helsinki, Finland, 2019; Available online: https://www.echa.europa.eu (accessed on 20 August 2023).
- Akhter, F.; Soomro, S.A.; Inglezakis, V.J. Silica aerogels; a review of synthesis, applications and fabrication of hybrid composites. J. Porous Mater. 2021, 28, 1387–1400. [Google Scholar] [CrossRef]
- Stone, V.; Gottardo, S.; Bleeker, E.A.J.; Braakhuis, H.; Dekkers, S.; Fernandes, T.; Haase, A.; Hunt, N.; Hristozov, D.; Jantunen, P.; et al. A framework for grouping and read-across of nanomaterials—Supporting innovation and risk assessment. Nano Today 2020, 35, 100941–100956. [Google Scholar] [CrossRef]
- Wohlleben, W.; Hellack, B.; Nickel, C.; Herrchen, M.; Hund-Rinke, K.; Kettler, K.; Riebeling, C.; Haase, A.; Funk, B.; Kühnel, D.; et al. The nanoGRAVUR framework to group (nano) materials for their occupational, consumer, environmental risks based on a harmonized set of material properties, applied to 34 case studies. Nanoscale 2019, 11, 17637–17654. [Google Scholar] [CrossRef]
- Arts, J.H.E.; Hadi, M.; Irfan, M.-A.; Keene, A.M.; Kreiling, R.; Lyon, D.; Maier, M.; Michel, K.; Petry, T.; Saueret, U.G.; et al. A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping). Regul. Toxicol. Pharmacol. 2015, 71, S1–S27. [Google Scholar] [CrossRef]
- Arts, J.H.E.; Hadi, M.; Irfan, M.-A.; Keene, A.M.; Kreiling, R.; Lyon, D.; Maier, M.; Michel, K.; Neubauer, N.; Petry, T.; et al. Case studies putting the decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping) into practice. Regul. Toxicol. Pharmacol. 2016, 76, 234–261. [Google Scholar] [CrossRef]
- Achawi, S.; Feneon, B.; Pourchez, J.; Forest, V. Assessing biological oxidative damage induced by graphene-based materials: An asset for grouping approaches using the FRAS assay. Regul. Toxicol. Pharmacol. 2021, 127, 105067–105095. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.-F.; Bello, D.; Schmidt, D.F.; Pal, A.K.; Stella, A.; Isaacs, J.A.; Rogers, E.J. Mapping the Biological Oxidative Damage of Engineered Nanomaterials. Small 2013, 9, 1853–1865. [Google Scholar] [CrossRef] [PubMed]
- Bahl, A.; Hellac, B.; Wiemannd, M.; Giustia, A.; Werlee, K.; Haase, A.; Wohlleben, W. Nanomaterial categorization by surface reactivity: A case study comparing 35 materials with four different test methods. NanoImpact 2020, 19, 100234–100252. [Google Scholar] [CrossRef]
- Hellack, B.; Nickel, C.; Albrecht, C.; Kuhlbusch, T.A.J.; Boland, S.; Baeza-Squiban, A.; Wendel Wohlleben, W.; Schins, R.P.F. Analytical methods to assess the oxidative potential of nanoparticles: A review. Environ. Sci. Nano 2017, 4, 1920–1934. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Appendix R.6-1 for Nanoforms Applicable to the Guidance on QSARs and Grouping of Chemicals, in ECHA-19-H-15-EN; ECHA: Helsinki, Finland, 2019. [Google Scholar]
- Magro, C.; Paz-Garcia, J.; Ottosen, L.; Mateus, E.; Ribeiro, A. Sustainability of construction materials: Electrodialytic technology as a tool for mortars production. J. Hazard. Mater. 2018, 2018, 421–427. [Google Scholar] [CrossRef]
- Gawron-Skarbek, A.; Kontarska-Krauza, M.; Dynowska, B.; Guligowska, A.; Prymont-Przymińska, A.; Nowak, D.; Kostka, T. Salivary and plasma native and non-urate total antioxidant capacity versus oral health status in older non-smoking adults. Arch. Oral Biol. 2019, 107, 104515–104530. [Google Scholar] [CrossRef]
- Cecchini, S.; Fazio, F. Assessment of Total Antioxidant Capacity in Serum of Healthy and Stressed Hens. Animals 2020, 10, 2019. [Google Scholar] [CrossRef]
- Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; Ricci, A.; et al. Guidance on risk assessment of the application of nanoscience and nano-technologies in the food and feed chain: Part 1, human and animal health. EFSA J. 2018, 16, e05327. [Google Scholar] [PubMed]
- Woignier, T.; Primera, J.; Alaoui, A.; Despetis, F.; Calas-Etienne, S.; Faivre, A.; Duffours, L.; Levelut, C.; Etienne, P. Techniques for characterizing the mechanical properties of aerogels. J. Sol-Gel Sci. Technol. 2020, 93, 6–27. [Google Scholar] [CrossRef]
- Wiemann, M.; Vennemann, A.; Sauer, U.G.; Wiench, K.; Ma-Hock, L.; Landsiedel, R. An in vitro alveolar macrophage assay for predicting the short-term inhalation toxicity of nanomaterials. J. Nanobiotechnol. 2016, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; Cokic, S.M.; Asbach, C.; Hoet, P.; Godderis, L.; Reichl, F.X.; Van Meerbeek, B.; Vennemann, A.; Wiemann, M. Interaction of rat alveolar macrophages with dental composite dust. Part. Fibre Toxicol. 2016, 13, 62–75. [Google Scholar] [CrossRef]
- Mularski, A.; Marie-Anaïs, F.; Mazzolini, J.; Niedergang, F. Observing Frustrated Phagocytosis and Phagosome Formation and Closure Using Total Internal Reflection Fluorescence Microscopy (TIRFM). Methods Mol. Biol. 2018, 1784, 165–175. [Google Scholar] [PubMed]
- Donaldson, K.; Seaton, A. A short history of the toxicology of inhaled particles. Part. Fibre Toxicol. 2012, 9, 13–25. [Google Scholar] [CrossRef]
- Cullen, R.T.; Miller, B.G.; Davis, J.M.; Brown, D.M.; Donaldson, K. Short-term inhalation and in vitro tests as predictors of fiber pathogenicity. Environ. Health Perspect. 1997, 105 (Suppl. S5), 1235–1240. [Google Scholar]
- Worle-Knirsch, J.M.; Pulskamp, K.; Krug, H.F. Oops they did it again! carbon nanotubes hoax scientists in viability assays. Nano Lett. 2006, 6, 1261–1268. [Google Scholar] [CrossRef]
- Wohlleben, W.; Kolle, S.N.; Hasenkamp, L.-C.; Böser, A.; Vogel, S.; von Vacano, B.; van Ravenzwaay, B.; Landsiedel, R. Artifacts by marker enzyme adsorption on nanomaterials in cytotoxicity assays with tissue cultures. J. Phys. Conf. Ser. 2011, 304, 012061–012072. [Google Scholar] [CrossRef]
- Kroll, A.; Dierker, C.; Rommel, C.; Hahn, D.; Wohlleben, W.; Schulze-Isfort, C.; Göbbert, C.; Voetz, M.; Hardinghaus, F.; Schnekenburger, J. Cytotoxicity screening of 23 engineered nanomaterials using a test matrix of ten cell lines and three different assays. Part. Fibre Toxicol. 2011, 8, 9–28. [Google Scholar] [CrossRef]
- Yin, W.; Venkitachalam, S.M.; Jarrett, E.; Staggs, S.; Leventis, N.; Lu, H.; Rubenstein, D.A. Biocompatibility of surfactant-templated polyurea–nanoencapsulated macroporous silica aerogels with plasma platelets and endothelial cells. J. Biomed. Mater. Res. Part A 2010, 92, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Király, G.; Veres, P.; Lázár, I.; Fábián, I.; Bánfalvi, G.; Juhász, I.; Kalmár, J. Controlled release of methotrexate from functionalized silica-gelatin aerogel microparticles applied against tumor cell growth. Int. J. Pharm. 2019, 558, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Burden, N.; Aschberger, K.; Chaudhry, Q.; Clift, M.J.D.; Doak, S.H.; Fowler, P.; Johnston, H.J.; Landsiedel, R.; Rowland, J.; Stone, V. The 3Rs as a framework to support a 21st century approach for nanosafety assessment. Nano Today 2017, 12, 10–13. [Google Scholar] [CrossRef]
- Nan, A.P.; Petit, A.; Yahia, L.; Huk, O.L.; Tabrizian, M. Cytotoxic reaction and TNF-a response of macrophages to polyurethane particles. J. Biomater. Sci. Polym. Ed. 2002, 13, 257–272. [Google Scholar] [CrossRef]
- Wiemann, M.; Vennemann, A.; Stintz, M.; Marín, R.R.M.; Babick, F.; Lindner, G.-G.; Schuster, T.B.; Brinkmann, U.; Krueger, N. Effects of ultrasonic dispersion energy on the preparation of amorphous SiO2 nanomaterials for in vitro toxicity testing. Nanomaterials 2019, 9, 11–43. [Google Scholar] [CrossRef]
- Moitra, S.; Tabrizi, A.F.; Machichi, K.I.; Kamravaei, S.; Miandashti, N.; Henderson, L.; Mukherjee, M.; Khadour, F.; Naseem, M.T.; Lacy, P.; et al. Non-Malignant Respiratory Illnesses in Association with Occupational Exposure to Asbestos and Other Insulating Materials: Findings from the Alberta Insulator Cohort. Int. J. Environ. Res. Public Health 2020, 17, 7085–7097. [Google Scholar] [CrossRef]
- Boccia, A.C.; Pulvirenti, A.; García-González, C.A.; Grisi, F.; Neagu, M. Compendium of Safety Regulatory for Safe Applications of Aerogels. Gels 2023, 9, 842. [Google Scholar] [CrossRef]
- Lovskaya, D.D.; Lebedev, A.E.; Menshutina, N.V. Aerogels as drug delivery systems: In vitro and in vivo evaluations. J. Supercrit. Fluids 2015, 106, 115–121. [Google Scholar] [CrossRef]
- Human Health and Environmental Exposure Assessment and Risk Characterization of Nanomaterials—Best Practice for REACH Registrants. In Proceedings of the Third GAARN Meeting, Helsinki, Finland, 30 September 2013; European Chemicals Agency: Helsinki, Finland, 2013. Available online: https://echa.europa.eu/documents/10162/788325/best_practices_human_health_environment_nano_3rd_en.pdf/a368f6df-fe40-4673-a041-83b63cc9f50b?t=1395828950292 (accessed on 8 November 2023).
- Kaur, A.; Singh, H. Nanomaterials in Environment: Sources, Risk Assessment, and Safety Aspect. In Advanced Functional Nanoparticles “Boon or Bane” for Environment Remediation Applications, 1st ed.; Environmental Contamination Remediation and, Management; Kumar, R., Kumar, R., Chaudhary, S., Eds.; Springer: Cham, Switzerland, 2023; pp. 75–93. [Google Scholar]
- DAMADEI. Design and Advanced Materials as a Driver of European Innovation. 2013. Available online: http://www.damadei.eu/ (accessed on 26 August 2023).
- MatSEEC. Knowledge and Technology Transfer in Materials Science and Engineering in Europe. 2015. Available online: www.esf.org/matseec (accessed on 26 August 2023).
- Zhao, S.; Siqueira, G.; Drdova, S.; Norris, D.; Ubert, C.; Bonnin, A.; Galmarini, S.; Ganobjak, M.; Pan, Z.; Brunner, S.; et al. Additive manufacturing of silica aerogels. Nature 2020, 584, 387–392. [Google Scholar] [CrossRef]
- Patterson, G.; Hsieh, Y.-L. Tunable dialdehyde/dicarboxylate nanocelluloses by stoichiometrically optimized sequential periodate–chlorite oxidation for tough and wet shape recoverable aerogels. Nanoscale Adv. 2020, 2, 5623–5634. [Google Scholar] [CrossRef]
- ISO/TR 22293:2021; Evaluation of Methods for Assessing the Release of Nanomaterials from Commercial, Nanomaterial-Containing Polymer Composites. International Organization for Standardization: Geneva, Switzerland, 2021. Available online: https://statnano.com/standard/iso/1406/ISOTR-22293-2021#ixzz89KDQnciS (accessed on 10 November 2023).
- Knight, D.J.; Deluyker, H.; Chaudhry, Q.; Vidal, J.M.; de Boer, A. A call for action on the development and implementation of new methodologies for safety assessment of chemical-based products in the EU—A short communication. Regul. Toxicol. Pharmacol. 2021, 119, 104837–104844. [Google Scholar] [CrossRef]
- Wohlleben, W.; Punckt, C.; Aghassi-Hagmann, J.; Siebers, F.; Menzel, F.; Esken, D.; Drexel, C.-P.; Zoz, H.; Benz, H.U.; Weier, A.; et al. Nanoenabled Products: Categories, Manufacture, and Applications: Protocols and Industrial Innovations. In Metrology and Standardization for Nanotechnology: Protocols and Industrial Innovations; Mansfield, E., Kaiser, D.L., Fujita, D., Van de Voorde, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 411–464. [Google Scholar]
- Westerhoff, P.; Alvarez, P.; Li, Q.; Gardea-Torresdey, J.; Zimmerman, J. Overcoming implementation barriers for nanotechnology in drinking water treatment. Environ. Sci. Nano 2016, 3, 1241–1253. [Google Scholar] [CrossRef]
- Westerhoff, P.; Atkinson, A.; Fortner, J.; Wong, M.S.; Zimmerman, J.; Gardea-Torresdey, J.; Ranville, J.; Herckes, P. Low risk posed by engineered and incidental nanoparticles in drinking water. Nat. Nanotechnol. 2018, 13, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shen, Q.; Kawabata, Y.; Segawa, J.; Cao, X.; Guan, K.; Istirokhatun, T.; Yoshioka, T.; Matsuyama, H. Graphene quantum dots (GQDs)-assembled membranes with intrinsic functionalized nanochannels for high-performance nanofiltration. Chem. Eng. J. 2020, 420, 127602–127615. [Google Scholar] [CrossRef]
- Camboni, M.; Floyd, P.; Hanlon, J.; Pérez García, R. A State of Play Study of the Market for So Called “Next Generation” Nanomaterials; ECHA-2019-R-14-EN; Catalogue number ED-02-19-746-EN-N; European Union Observatory for Nanomaterials Publisher: Helsinki, Finland, 2019; ISBN 978-92-9020-726-9. [Google Scholar] [CrossRef]


| Structure | Applications | References |
|---|---|---|
| Dialdehyde nanocellulose and collagen composite aerogels | Tissue engineering, wound dressing | [51] |
| Polyurea-nano-encapsulated macroporous silica aerogels, chitosan–silica hybrid aerogels | Tissue engineering, wound dressing | [23,52,53] |
| Alginate–starch aerogels | Tissue engineering, wound dressing | [15,54] |
| Alginate–lignin hybrid aerogels | Wound dressing | [24] |
| Ca-Zn-Ag alginate aerogels | Wound dressing | [25] |
| Macroporous silica aerogels | Biosensors | [55,56] |
| Cellulose nanofibrils | Biosensors and diagnostics | [4,55] |
| Silica aerogels | Biosensors and diagnostics | [3,4] |
| Polysaccharide aerogels | Food-related technologies | [4,56,57] |
| Alginate–chitosan aerogel | Drug delivery | [26] |
| Silica aerogels, surface-functionalized aerogels, composite aerogels | Drug delivery | [3,4,58] |
| Inorganic and organic aerogels | Drug delivery | [59] |
| Alginate-based aerogel | Drug delivery | [60] |
| Polysaccharide-based aerogels | Drug delivery | [3] |
| Alginate aerogel | Biocide | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neagu, M.; Grisi, F.; Pulvirenti, A.; Simón-Vázquez, R.; García-González, C.A.; Boccia, A.C. Updated Aspects of Safety Regulations for Biomedical Applications of Aerogel Compounds—Compendia-Like Evaluation. Safety 2023, 9, 80. https://doi.org/10.3390/safety9040080
Neagu M, Grisi F, Pulvirenti A, Simón-Vázquez R, García-González CA, Boccia AC. Updated Aspects of Safety Regulations for Biomedical Applications of Aerogel Compounds—Compendia-Like Evaluation. Safety. 2023; 9(4):80. https://doi.org/10.3390/safety9040080
Chicago/Turabian StyleNeagu, Monica, Fabia Grisi, Alfio Pulvirenti, Rosana Simón-Vázquez, Carlos A. García-González, and Antonella Caterina Boccia. 2023. "Updated Aspects of Safety Regulations for Biomedical Applications of Aerogel Compounds—Compendia-Like Evaluation" Safety 9, no. 4: 80. https://doi.org/10.3390/safety9040080







