Vibrations and Spatial Patterns Change Effective Wetting Properties of Superhydrophobic and Regular Membranes
Abstract
:1. Introduction
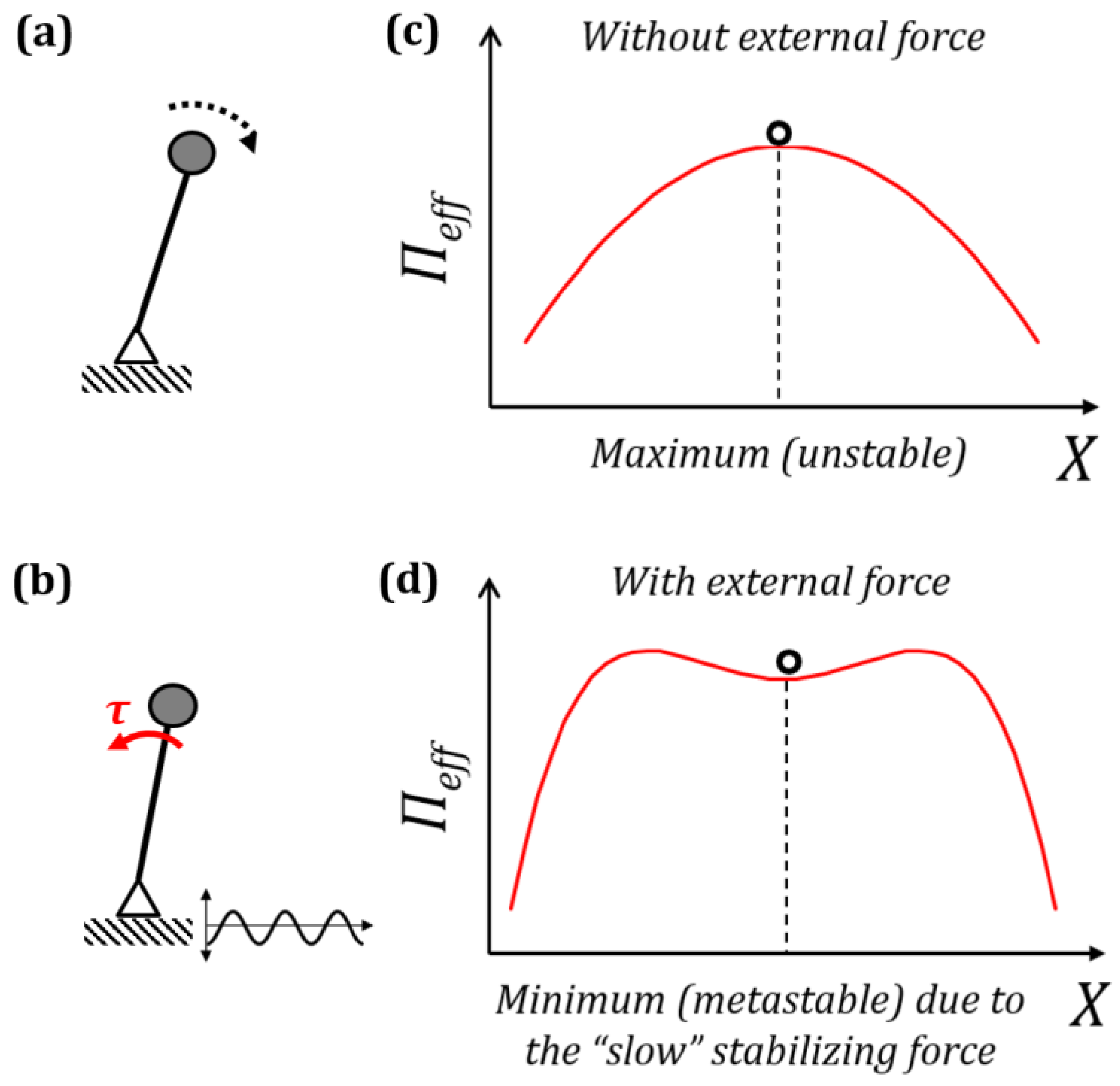
2. Effect of Small Fast Vibrations and Surface Patterns
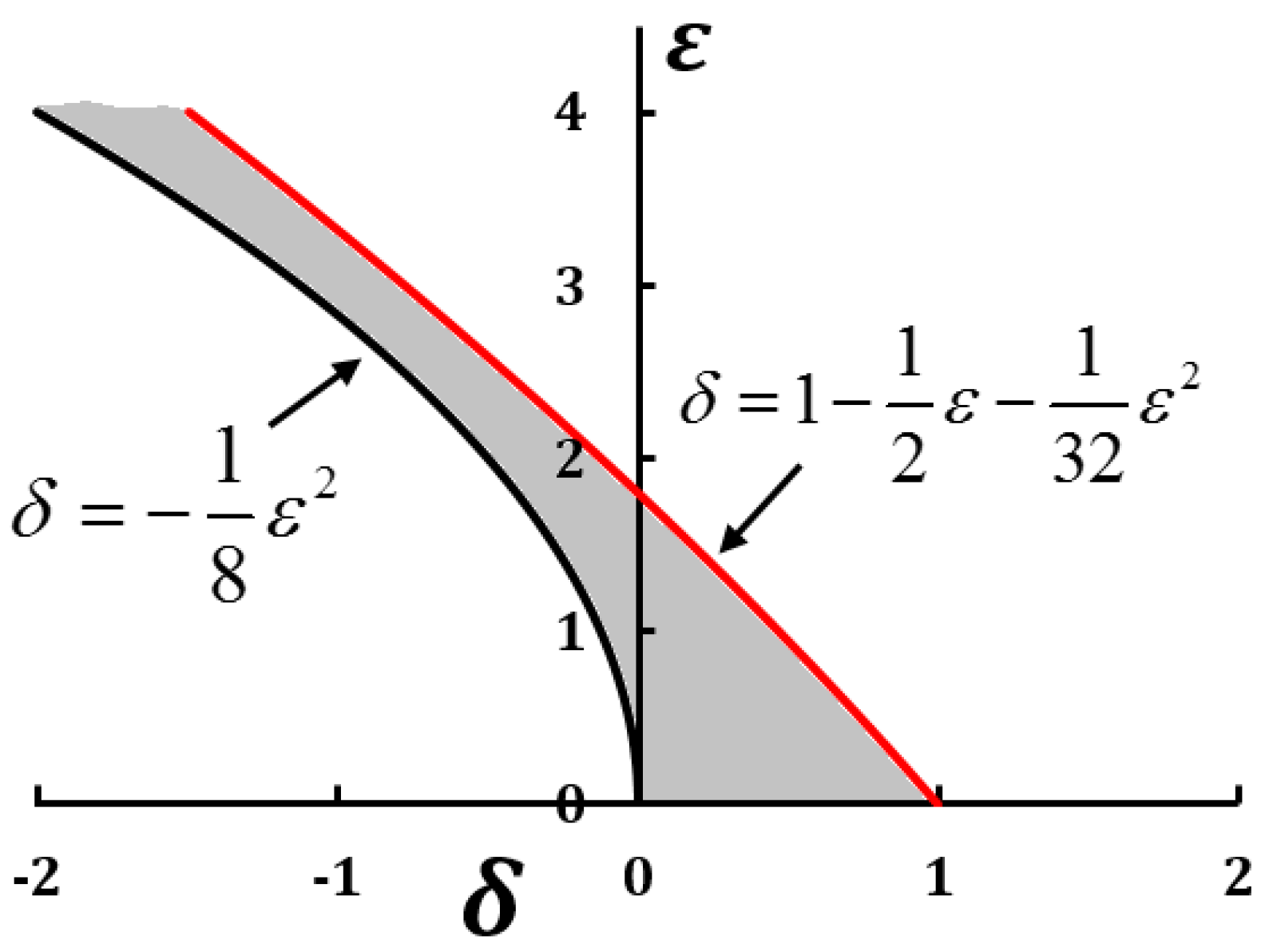
2.1. Separation of Motion and Effective Forces
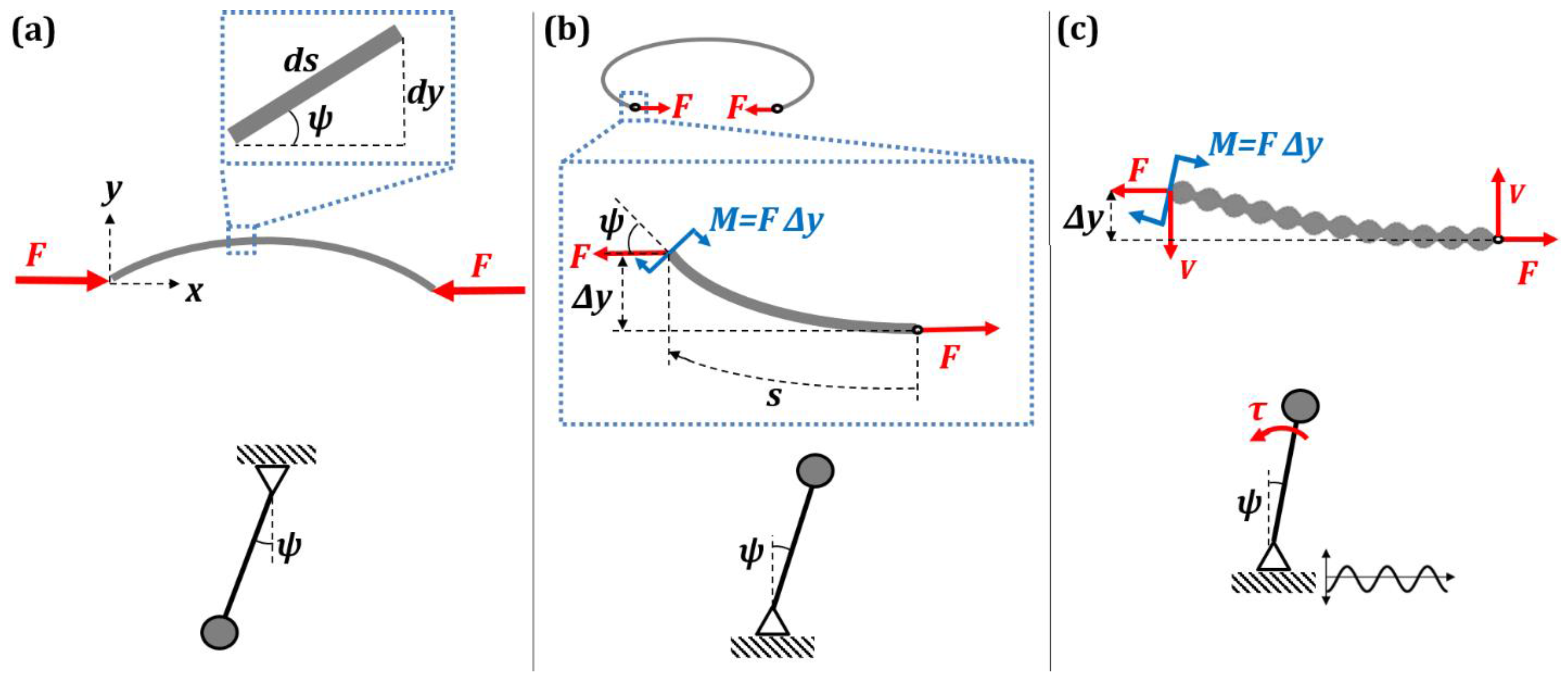
2.2. Kirchhoff’s Analogy between Spatial and Temporal Patterns
3. Wetting and Membranes
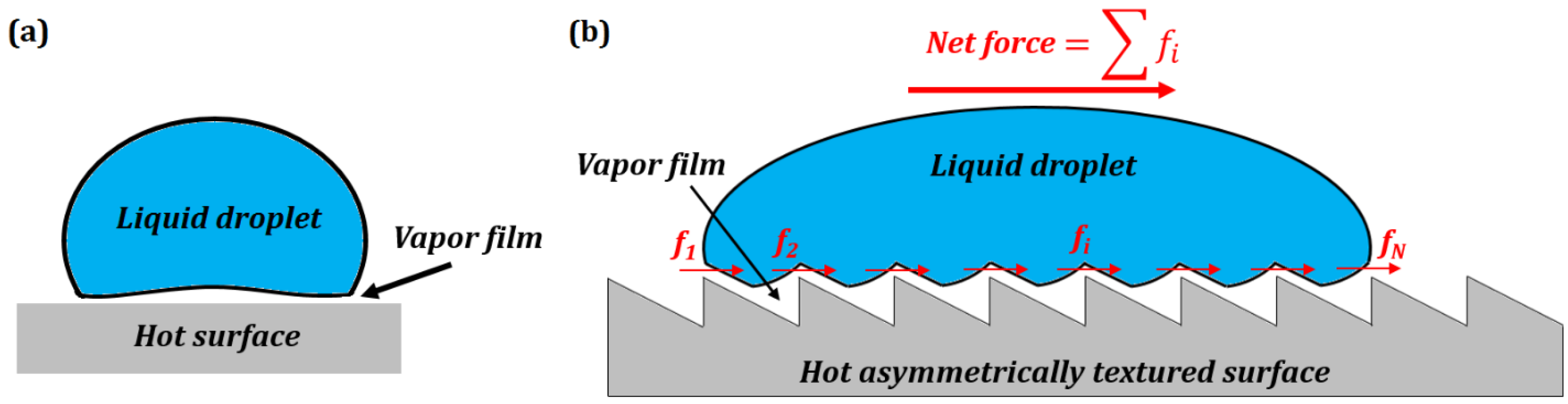
3.1. Superhydrophobicity: How Surface Patterns Change Wetting and Phase State
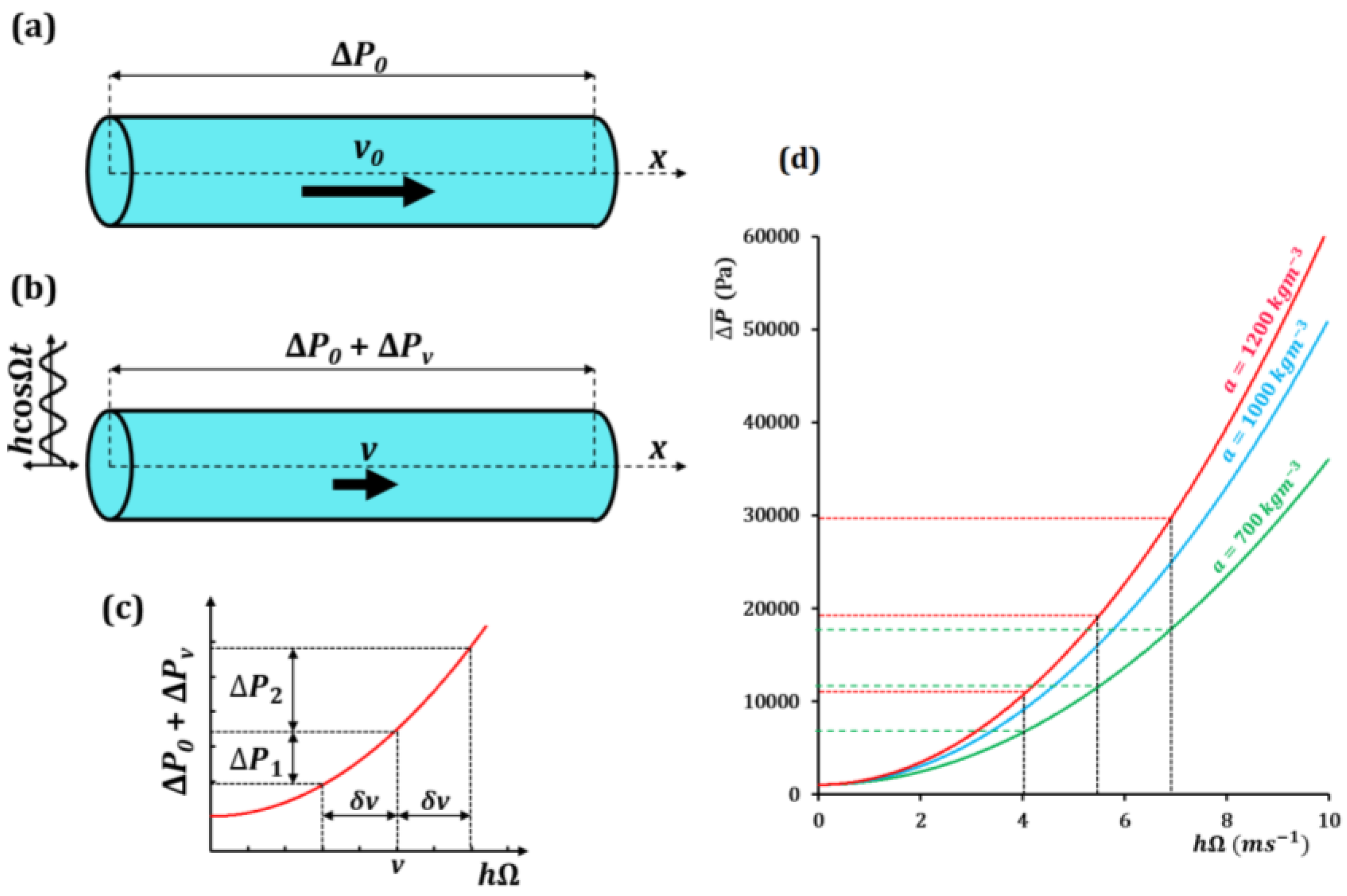
3.2. Water Flow through a Vibrating Pipe with Hysteresis
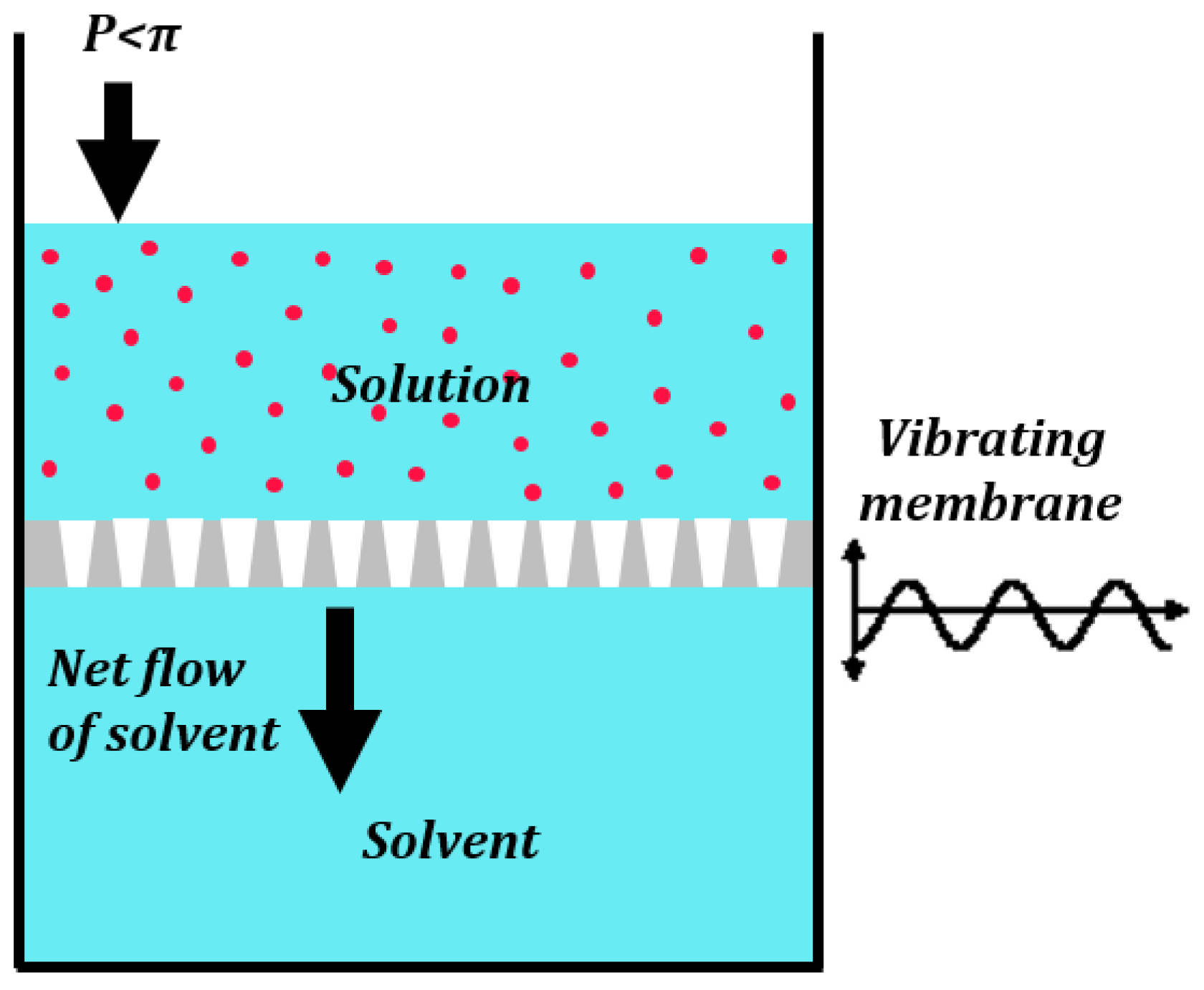
3.3. Liquid Penetration through Pores in Vibrating or Patterned Membranes
4. Conclusions
Conflicts of Interest
References
- Stephenson, A. On a new type of dynamical stability. Mem. Proc. Manch. Lit. Philos. Soc. 1908, 52, 1–10. [Google Scholar]
- Stephenson, A. On induced stability. Philos. Mag. 1908, 15, 233–236. [Google Scholar] [CrossRef]
- Kapitza, P.L. Pendulum with a vibrating suspension. Usp. Fiz. Nauk 1951, 44, 7–15. [Google Scholar]
- Stephenson, A. On induced stability. Philos. Mag. 1909, 17, 765–766. [Google Scholar] [CrossRef]
- Champneys, A.R.; Fraser, W.B. The ‘Indian rope trick’ for a parametrically excited flexible rod: Linearized analysis. Proc. R. Soc. A 2000, 456, 553–570. [Google Scholar] [CrossRef]
- Ramachandran, R.; Maani, N.; Rayz, V.L.; Nosonovsky, M. Vibrations and spatial patterns in biomimetic surfaces: Using the shark-skin effect to control blood clotting. Philos. Trans. R. Soc. A 2016, 374, 20160133. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Nosonovsky, M. Vibro-levitation and inverted pendulum: Parametric resonance in vibrating droplets and soft materials. Soft Matter 2014, 10, 4633–4639. [Google Scholar] [CrossRef] [PubMed]
- Blekhman, I.I. Vibrational Mechanics; World Scientific: Singapore, 2000. [Google Scholar]
- Lurie, K.A. An Introduction to the Mathematical Theory of Dynamic Materials; Springer: Berlin, Germany, 2007. [Google Scholar]
- Nosonovsky, M.; Bhushan, B. Superhydrophobic Surfaces and emerging applications: Non-adhesion, energy, green engineering. Curr. Opin. Colloid Interface Sci. 2009, 14, 270–280. [Google Scholar] [CrossRef]
- Wong, T.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, V.; Sobolev, K.; Nosonovsky, M. From superhydrophobicity to icephobicity: Forces and interaction analysis. Sci. Rep. 2013, 3, 2194. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, Z.Y.; Mai, Z.H.; Ma, Y.M.; Liu, B.Q.; Jiang, L.; Zhu, D.B. A Super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water. Angew. Chem. Int. Ed. 2004, 43, 2012–2014. [Google Scholar] [CrossRef] [PubMed]
- Vakarelski, I.U.; Patankar, N.A.; Marston, J.O.; Chan, D.Y.C.; Thoroddsen, S.T. Stabilization of Leidenfrost vapour layer by textured superhydrophobic surfaces. Nature 2012, 489, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Nosonovsky, M. Non-wetting, stabilization, and phase transitions induced by vibrations and spatial patterns. In Non-Wettable Surfaces: Theory, Preparation and Applications; Ras, R., Marmur, A., Eds.; RSC: Cambridge, UK, 2017. (in press) [Google Scholar]
- Landau, L.D.; Lifshitz, E.M. Mechanics Volume 1 of Course of Theoretical Physics, 2nd ed.; Pergamon Press: Oxford, UK, 1969. [Google Scholar]
- Shi, Y.M.; Hearst, J.E. The Kirchhoff elastic rod, the nonlinear Schrodinger-equation, and DNA supercoiling. J. Chem. Phys. 1994, 101, 5186–5200. [Google Scholar] [CrossRef]
- Starostin, E.L. Symmetric equilibria of a thin elastic rod with self-contacts. Philos. Trans. R. Soc. A 2004, 362, 1317–1334. [Google Scholar] [CrossRef] [PubMed]
- Timoshenko, S.; Gere, J.M. Theory of Elastic Stability, 2nd ed.; McGraw-Hill: New York, NY, USA, 1961. [Google Scholar]
- Meirovitch, L. Fundamentals of Vibrations; McGraw-Hill: Boston, MA, USA, 2001. [Google Scholar]
- Nayfeh, A.H. Perturbation Methods; John Wiley: New York, NY, USA, 1973. [Google Scholar]
- Dai, Q.; Huang, W.; Wang, X. Surface roughness and orientation effects on the thermo-capillary migration of a droplet of paraffin oil. Exp. Therm. Fluid Sci. 2014, 57, 200–206. [Google Scholar] [CrossRef]
- Cho, K.L.; Wu, A.H.; Liaw, I.I.; Cookson, D.; Lamb, R.N. Wetting transitions on hierarchical surfaces. J. Phys. Chem. C 2012, 116, 26810–26815. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Marmur, A. Underwater superhydrophobicity: Theoretical feasibility. Langmuir 2006, 22, 1400–1402. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Kirn, A.; Rich, D.; Elliot, A.; Patankar, N.A. Controlling phase change: Drying-up under water or staying wet during boiling. In Bulletin of the American Physical Society; APS: San Francisco, CA, USA, 2014. [Google Scholar]
- Patankar, N.A. Thermodynamics of sustaining gases in the roughness of submerged superhydrophobic surfaces. Langmuir 2016, 32, 7023–7028. [Google Scholar] [CrossRef] [PubMed]
- Leidenfrost, J.G. On the fixation of water in diverse fire. Int. J. Heat Mass Transf. 1966, 9, 1153–1166. [Google Scholar] [CrossRef]
- Wasnik, P.S.; N’guessan, H.E.; Tadmor, R. Controlling arbitrary humidity without convection. J. Colloid Interface Sci. 2015, 455, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Linke, H.; Aleman, B.J.; Melling, L.D.; Taormina, M.J.; Francis, M.J.; Dow-Hygelund, C.C.; Narayanan, V.; Taylor, R.P.; Stout, A. Self-propelled Leidenfrost droplets. Phys. Rev. Lett. 2006, 96, 154502. [Google Scholar] [CrossRef] [PubMed]
- Quere, D. Leidenfrost dynamics. Annu. Rev. Fluid Mech. 2013, 45, 197–215. [Google Scholar] [CrossRef]
- Dupeux, G.; Le Merrer, M.; Lagubeau, G.; Clanet, C.; Hardt, S.; Quere, D. Viscous mechanism for Leidenfrost propulsion on a ratchet. EPL 2011, 96, 58001. [Google Scholar] [CrossRef]
- Lagubeau, G.; Le Merrer, M.; Clanet, C.; Quere, D. Leidenfrost on a ratchet. Nat. Phys. 2011, 7, 395–398. [Google Scholar] [CrossRef]
- Marin, A.G.; del Cerro, D.A.; Romer, G.R.B.E.; Pathiraj, B.; in ’t Veld, A.H.; Lohse, D. Capillary droplets on Leidenfrost micro-ratchets. Phys. Fluids 2012, 24, 122001. [Google Scholar] [CrossRef]
- Wells, G.G.; Ledesma-Aguilar, R.; McHale, G.; Sefiane, K. A sublimation heat engine. Nat. Commun. 2015, 6, 6390. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liang, W.; Guo, Z.; Liu, W. Biomimetic super-lyophobic and super-lyophilic materials applied for oil/water separation: A new strategy beyond nature. Chem. Soc. Rev. 2015, 44, 336–361. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, V.; Nosonovsky, M. Wetting transitions in two-, three-, and four-phase systems. Langmuir 2012, 28, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.; Huang, X. In situ oil/water separation using hydrophobic-oleophilic fibrous wall: A lab-scale feasibility study for groundwater cleanup. J. Hazard. Mater. 2006, 137, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, M.; Lu, Q. Filter paper with selective absorption and separation of liquids that differ in surface tension. ACS Appl. Mater. Interfaces 2010, 2, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and superoleophilic PVDF membranes for effective separation of water-in-oil emulsions with high flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tao, S.; An, Y. A reverse membrane emulsification process based on a hierarchically porous monolith for high efficiency water-oil separation. J. Mater. Chem. A 2013, 1, 1701–1708. [Google Scholar] [CrossRef]
- Wang, C.; Lin, S. Robust superhydrophobic/superoleophilic sponge for effective continuous absorption and expulsion of oil pollutants from water. ACS Appl. Mater. Interfaces 2013, 5, 8861–8864. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, F.; Wang, D.; Pei, X.F.; Zhang, W.; Jin, J. A novel zwitterionic polyelectrolyte grafted PVDF membrane for thoroughly separating oil from water with ultrahigh efficiency. J. Mater. Chem. A 2013, 1, 5758–5765. [Google Scholar] [CrossRef]
- Xue, Z.; Wang, S.; Lin, L.; Chen, L.; Liu, M.; Feng, L.; Jiang, L. A novel superhydrophilic and underwater superoleophobic hydrogel-coated mesh for oil/water separation. Adv. Mater. 2011, 23, 4270–4273. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Baik, S. Vertically-aligned carbon nano-tube membrane filters with superhydrophobicity and superoleophilicity. Carbon 2010, 48, 2192–2197. [Google Scholar] [CrossRef]
- Wang, C.; Yao, T.; Wu, J.; Ma, C.; Fan, Z.; Wang, Z.; Cheng, Y.; Lin, Q.; Yang, B. Facile approach in fabricating superhydrophobic and superoleophilic surface for water and oil mixture separation. ACS Appl. Mater. Interfaces 2009, 1, 2613–2617. [Google Scholar] [CrossRef] [PubMed]
- La, D.; Nguyen, T.A.; Lee, S.; Kim, J.W.; Kim, Y.S. A stable superhydrophobic and superoleophilic Cu mesh based on copper hydroxide nanoneedle arrays. Appl. Surf. Sci. 2011, 257, 5705–5710. [Google Scholar] [CrossRef]
- Wang, C.; Tzeng, F.; Chen, H.; Chang, C. Ultraviolet-durable superhydrophobic zinc oxide-coated mesh films for surface and underwater-oil capture and transportation. Langmuir 2012, 28, 10015–10019. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Zhang, X.; Wang, X.; Zhai, J.; Jiang, L. Micro/nanoscale hierarchical structured ZnO mesh film for separation of water and oil. Phys. Chem. Chem. Phys. 2011, 13, 14606–14610. [Google Scholar] [CrossRef] [PubMed]

| Porous Material | Surface Texturing and Treatment | Wetting Property | Separates |
|---|---|---|---|
| Kapok plant fiber [39] | None | Hydrophobic, oleophilic | Diesel from water |
| Stainless steel mesh [45] | Polyacrylamide hydrogel polymerization | Superhydrophilic, underwater superoleophobic | Vegetable oil, gasoline, diesel, crude oil, n-hexane, and petroleum ether from water with 99% efficiency |
| Stainless steel mesh [46] | Vertically-aligned multi-walled carbon nanotubes | Superhydrophobic, superoleophilic | Diesel from water |
| Copper mesh [47] | Etching followed by immersion in 1-hexadecanethiol | Superhydrophobic, superoleophilic | Diesel from water |
| Filter paper [40] | Hydrophobic silica + polystyrene | Superhydrophobic, oleophilic | Diesel from water with 96% efficiency |
| Stainless steel mesh [13] | Spray coating an emulsion of PTFE, polyvinyl acetate, polyvinyl alcohol and sodium dodecylbenzenesulfonate in water | Superhydrophobic, superoleophilic | Diesel from water |
| Copper mesh [48] | Copper hydroxide needles grown electrochemically and coated with silane | Superhydrophobic, superoleophilic | n-hexane |
| Stainless steel mesh [49] | Zinc oxide nano rod coating followed by immersion in stearic acid | Superhydrophobic, superoleophilic | Toluene from water |
| Stainless steel mesh [50] | Hexagonal ZnO nanorods | Superhydrophobic, superoleophilic | Paraffin oil from water |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramachandran, R.; Nosonovsky, M. Vibrations and Spatial Patterns Change Effective Wetting Properties of Superhydrophobic and Regular Membranes. Biomimetics 2016, 1, 4. https://doi.org/10.3390/biomimetics1010004
Ramachandran R, Nosonovsky M. Vibrations and Spatial Patterns Change Effective Wetting Properties of Superhydrophobic and Regular Membranes. Biomimetics. 2016; 1(1):4. https://doi.org/10.3390/biomimetics1010004
Chicago/Turabian StyleRamachandran, Rahul, and Michael Nosonovsky. 2016. "Vibrations and Spatial Patterns Change Effective Wetting Properties of Superhydrophobic and Regular Membranes" Biomimetics 1, no. 1: 4. https://doi.org/10.3390/biomimetics1010004
APA StyleRamachandran, R., & Nosonovsky, M. (2016). Vibrations and Spatial Patterns Change Effective Wetting Properties of Superhydrophobic and Regular Membranes. Biomimetics, 1(1), 4. https://doi.org/10.3390/biomimetics1010004








