Intra-Subject and Inter-Subject Movement Variability Quantified with Muscle Synergies in Upper-Limb Reaching Movements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
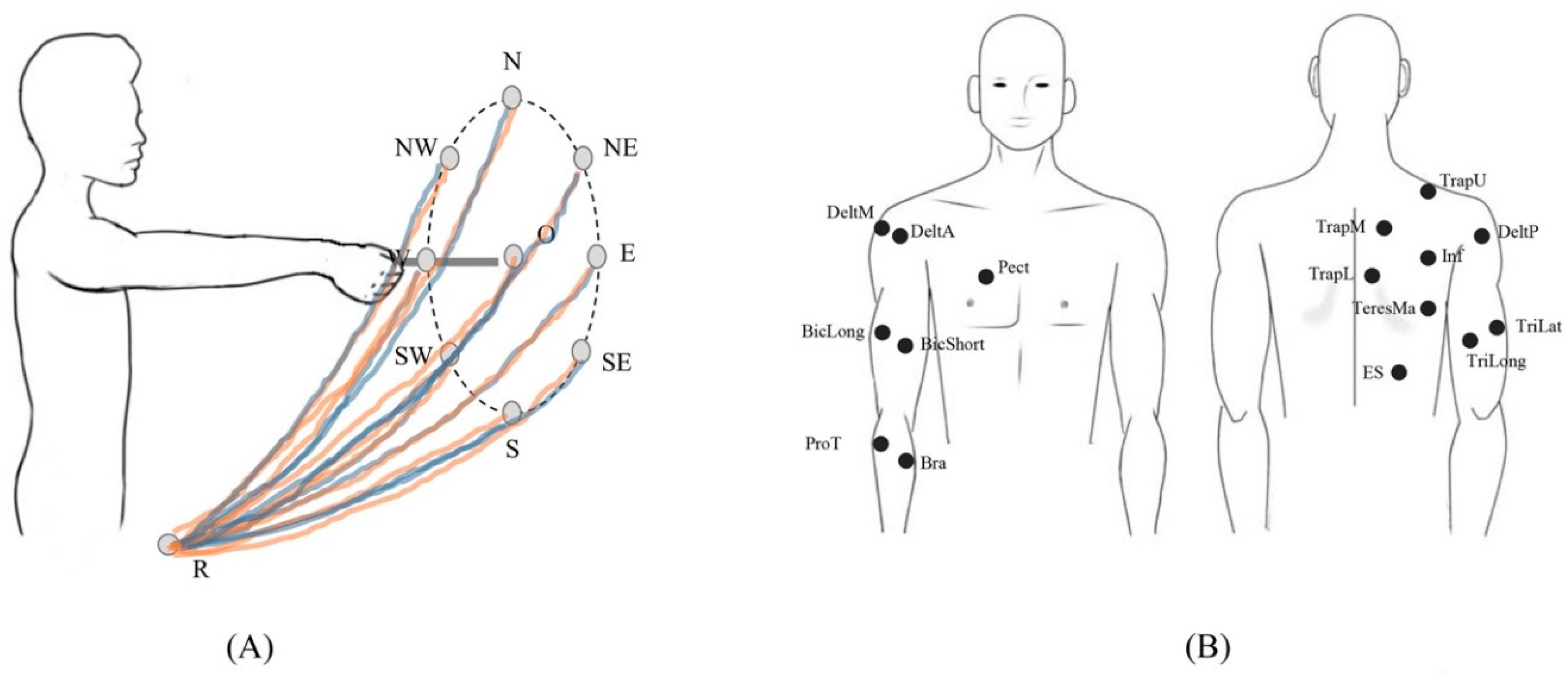
2.2. Experimental Set-Up
2.3. Data Analysis
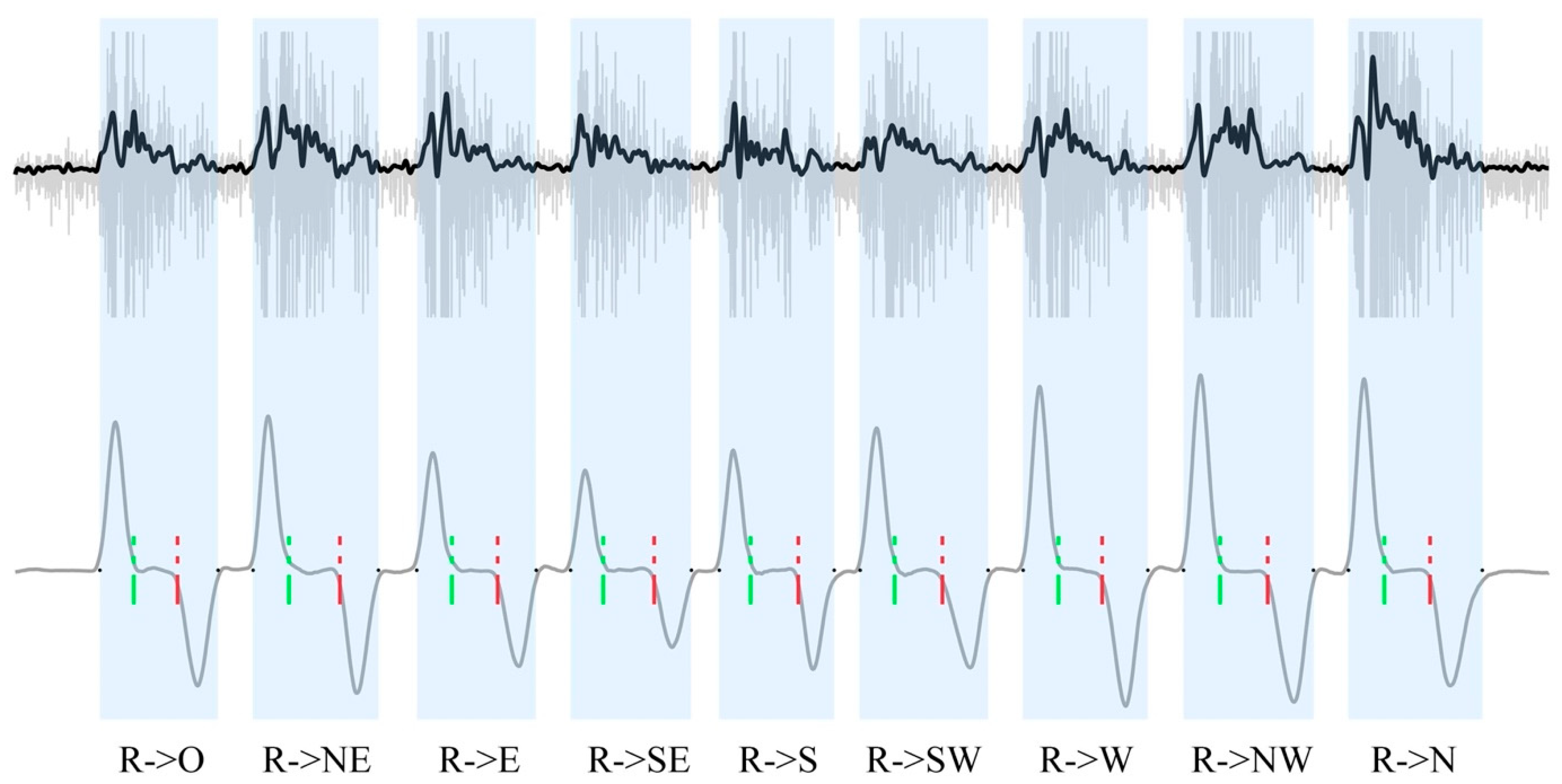
2.3.1. Signal Processing
2.3.2. Synergy Extraction
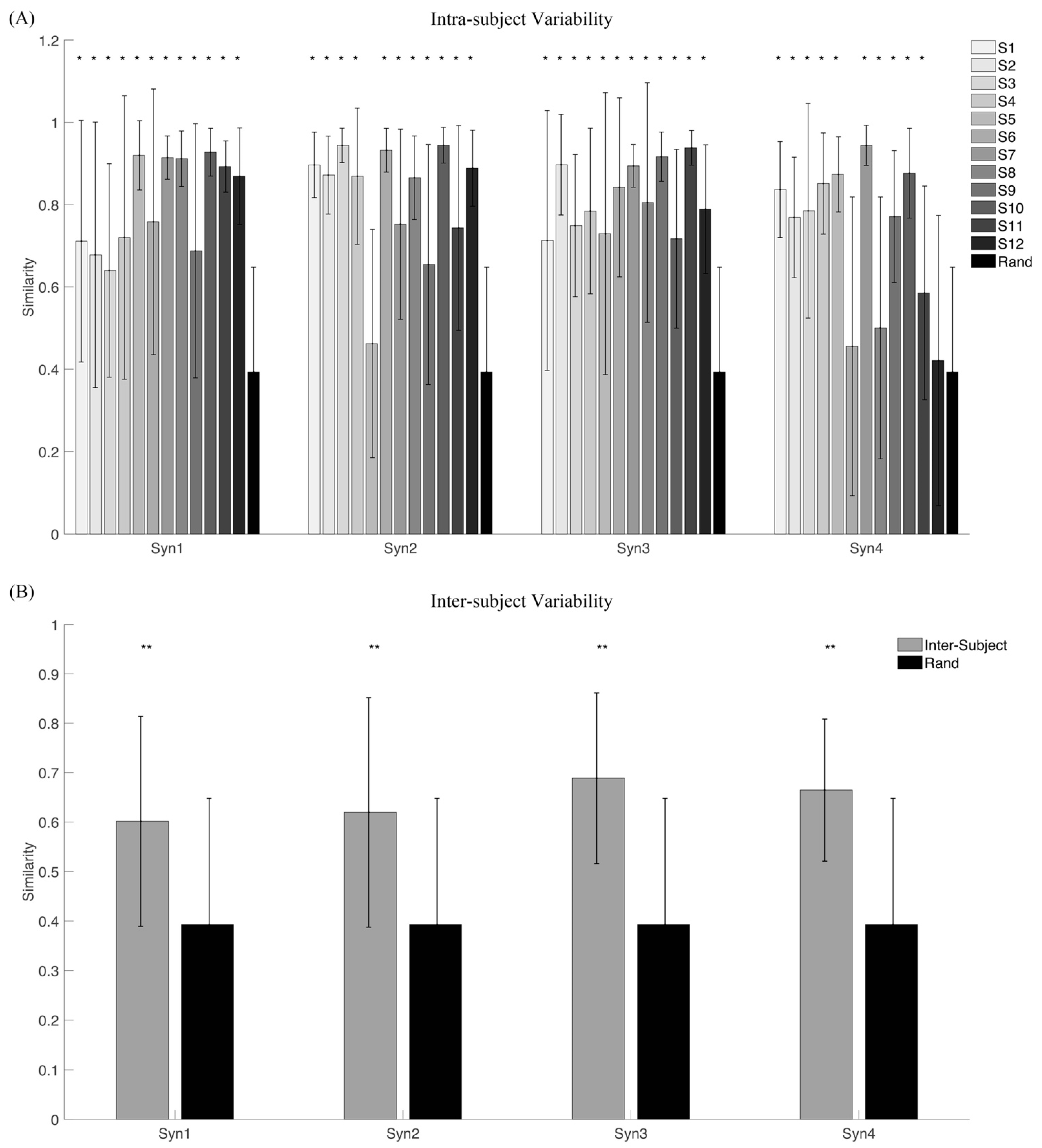
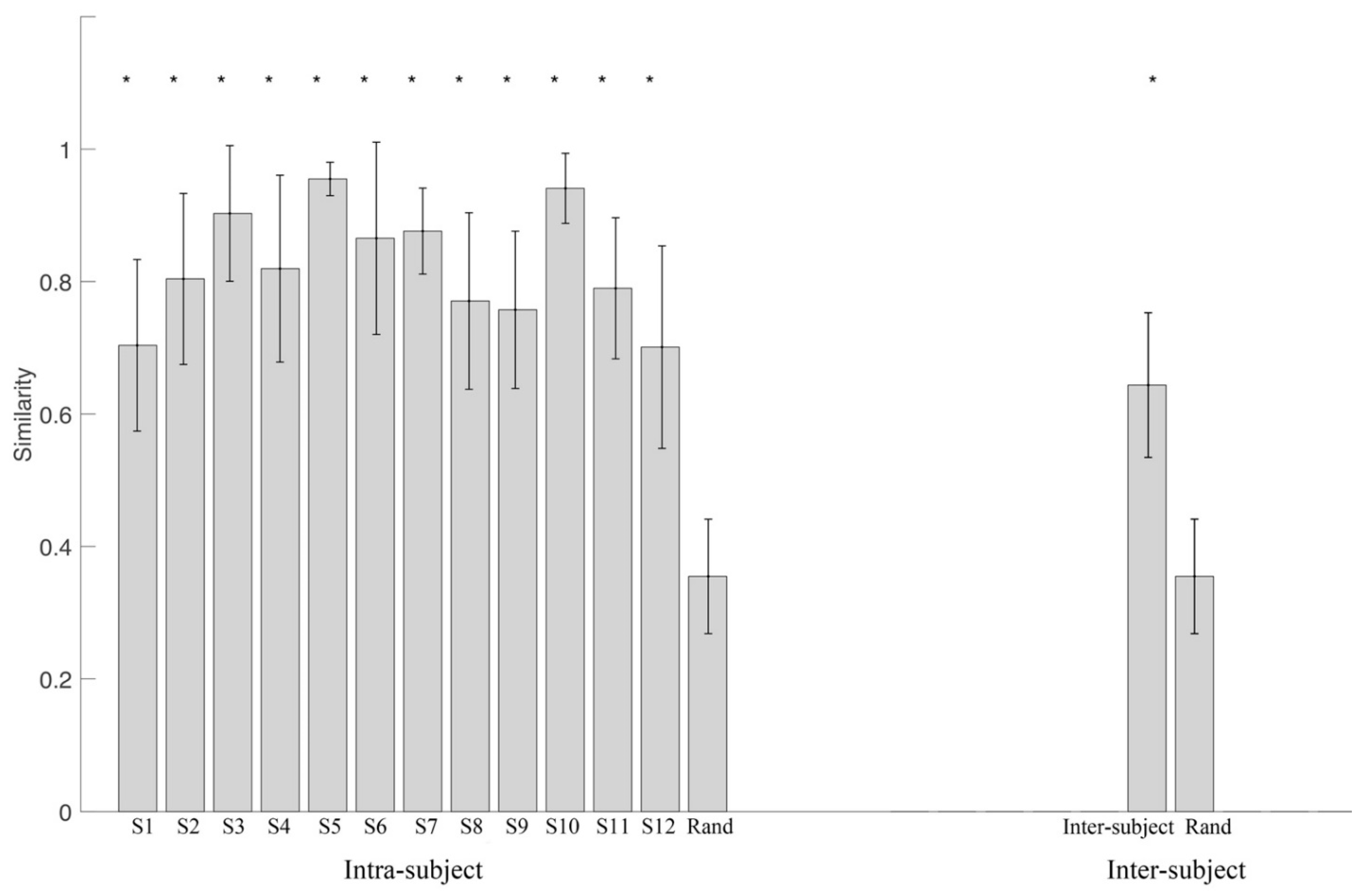
2.3.3. Variability Analysis
2.3.4. Statistical Analysis
3. Results
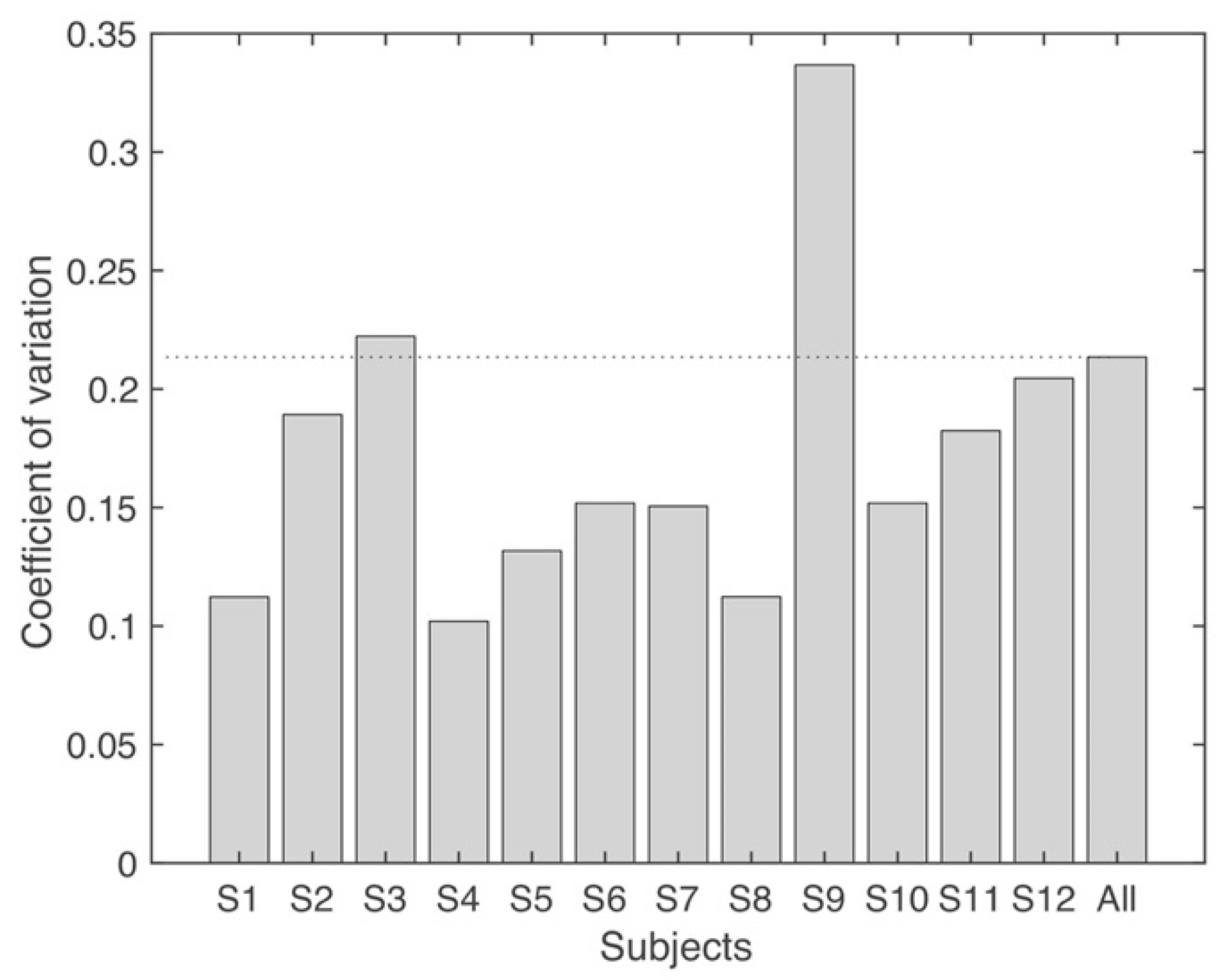
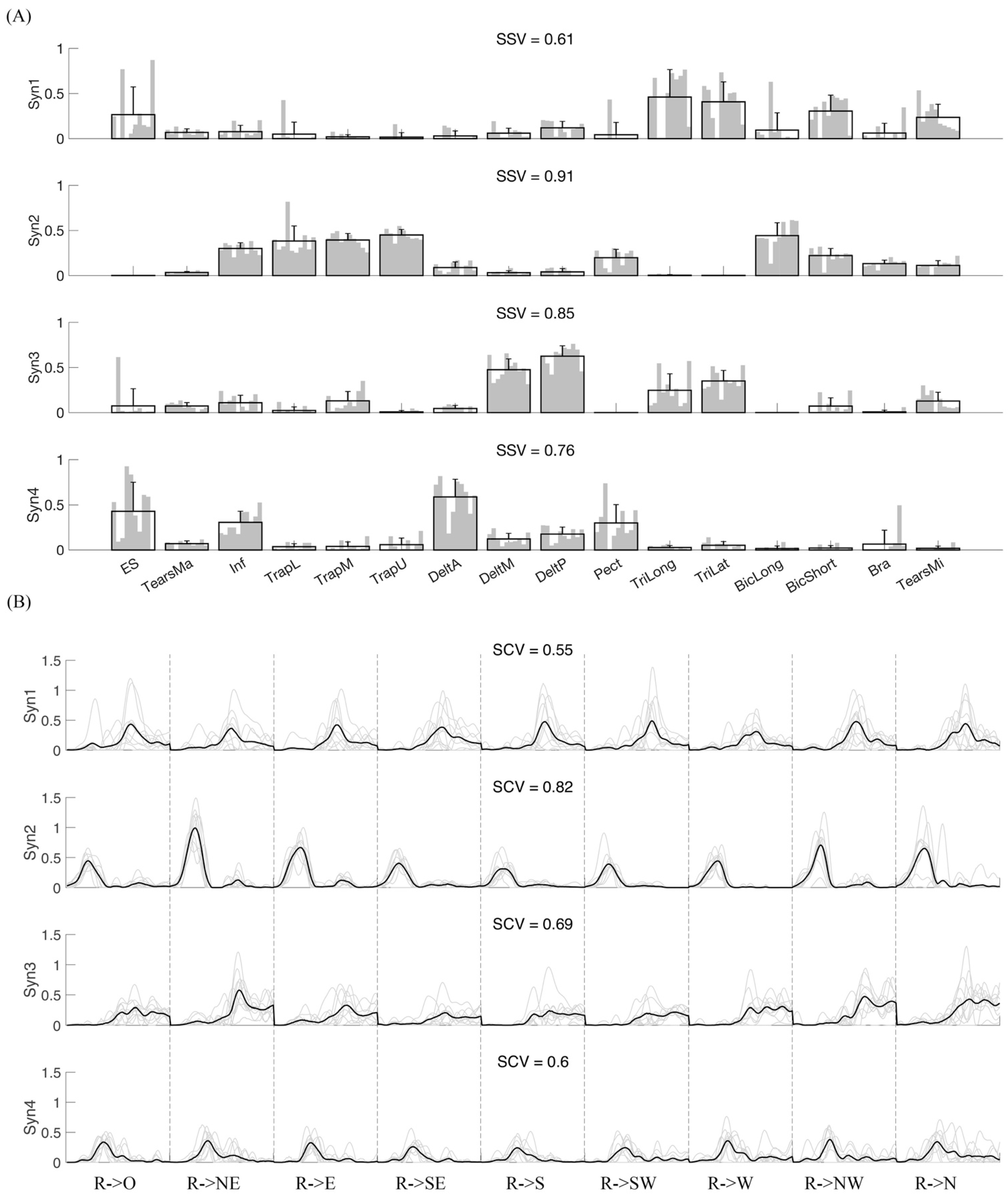
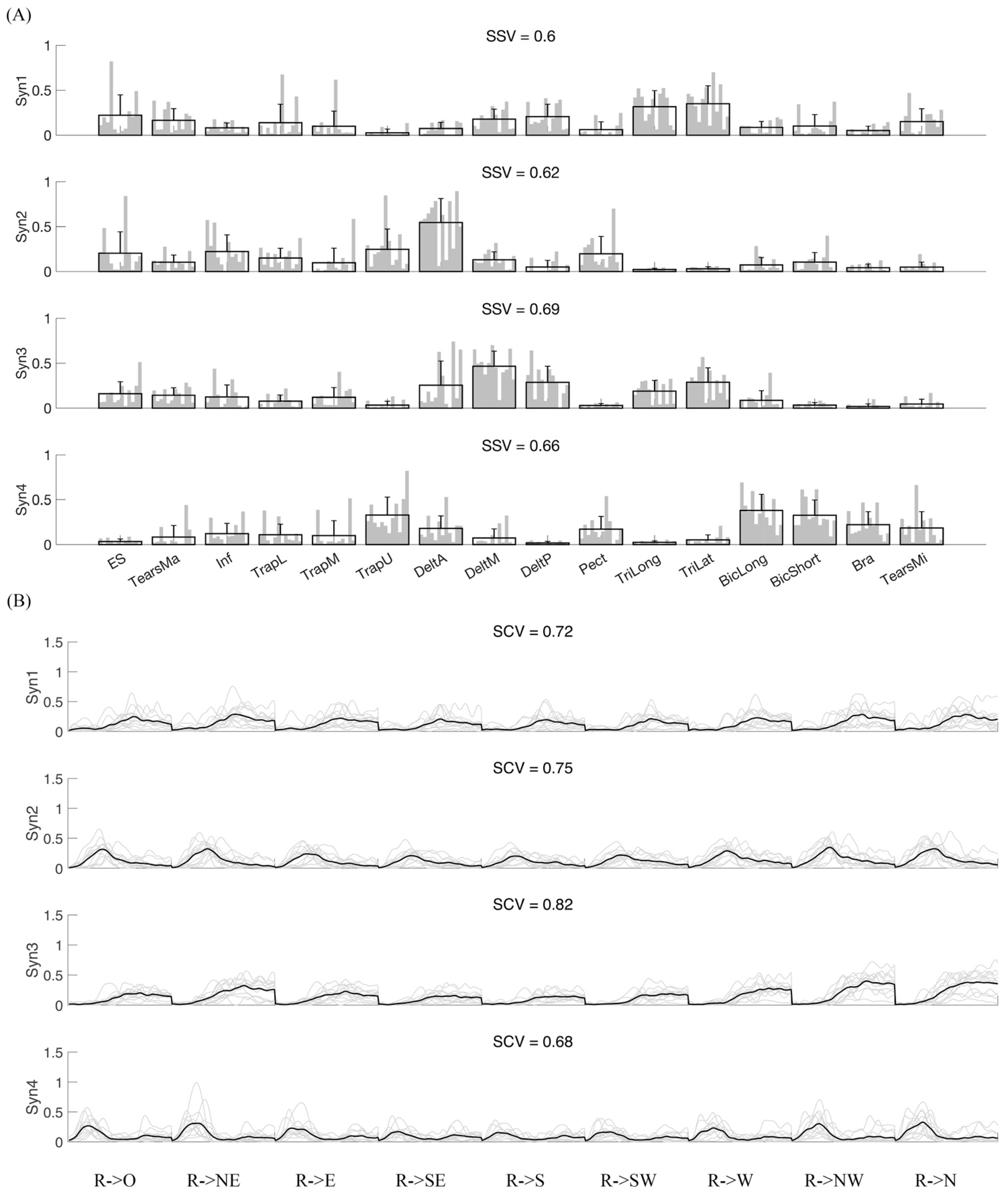
3.1. Variability of Synergy Modules
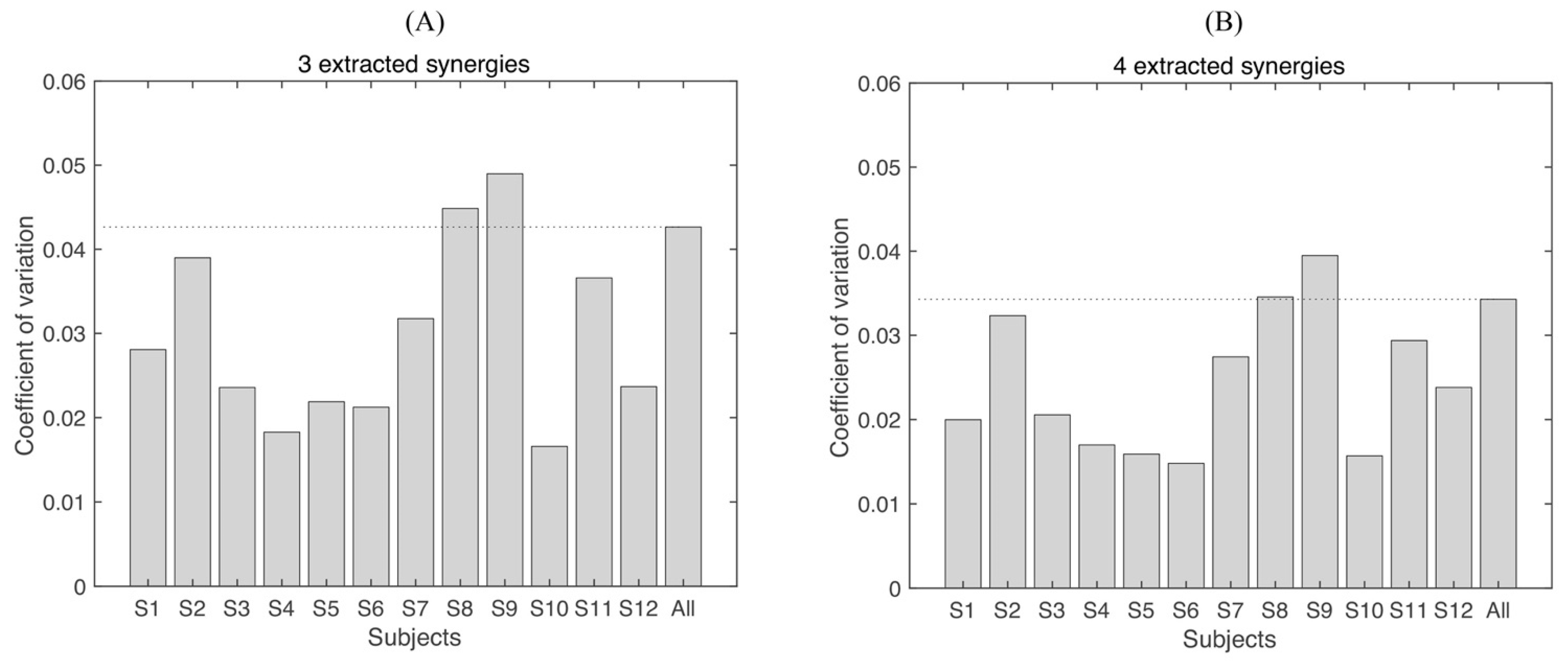
3.2. Synergy Variability
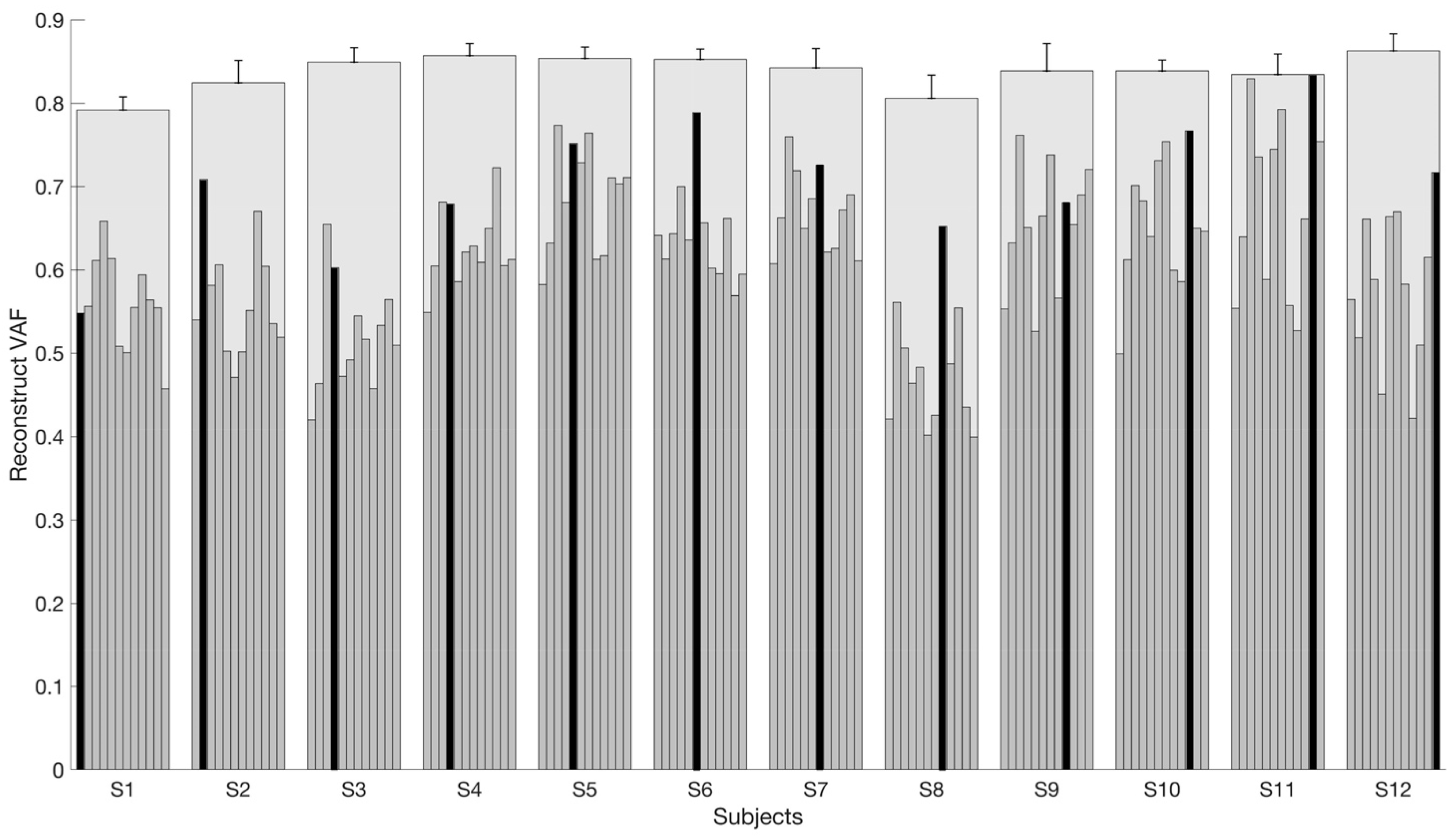
3.3. Reconstruction VAF
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rimini, D.; Agostini, V.; Knaflitz, M. Intra-Subject Consistency during Locomotion: Similarity in Shared and Subject-Specific Muscle Synergies. Front. Hum. Neurosci. 2017, 11, 586. [Google Scholar] [CrossRef] [Green Version]
- De Marchis, C.; Schmid, M.; Bibbo, D.; Bernabucci, I.; Conforto, S. Inter-Individual Variability of Forces and Modular Muscle Coordination in Cycling: A Study on Untrained Subjects. Hum. Mov. Sci. 2013, 32, 1480–1494. [Google Scholar] [CrossRef]
- Frère, J.; Hug, F. Between-Subject Variability of Muscle Synergies during a Complex Motor Skill. Front. Comput. Neurosci. 2012, 6, 99. [Google Scholar] [CrossRef] [Green Version]
- Zunino, A.; Cavazza, J.; Volpi, R.; Morerio, P.; Cavallo, A.; Becchio, C.; Murino, V. Predicting Intentions from Motion: The Subject-Adversarial Adaptation Approach. Int. J. Comput. Vis. 2020, 128, 220–239. [Google Scholar] [CrossRef] [Green Version]
- Di Nardo, F.; Morbidoni, C.; Mascia, G.; Verdini, F.; Fioretti, S. Intra-Subject Approach for Gait-Event Prediction by Neural Network Interpretation of EMG Signals. BioMed. Eng. OnLine 2020, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Pale, U.; Atzori, M.; Müller, H.; Scano, A. Variability of Muscle Synergies in Hand Grasps: Analysis of Intra- and Inter-Session Data. Sensors 2020, 20, 4297. [Google Scholar] [CrossRef] [PubMed]
- Cheung, V.C.K.; Turolla, A.; Agostini, M.; Silvoni, S.; Bennis, C.; Kasi, P.; Paganoni, S.; Bonato, P.; Bizzi, E. Muscle Synergy Patterns as Physiological Markers of Motor Cortical Damage. Proc. Natl. Acad. Sci. USA 2012, 109, 14652–14656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostini, V.; Ghislieri, M.; Rosati, S.; Balestra, G.; Knaflitz, M. Surface Electromyography Applied to Gait Analysis: How to Improve Its Impact in Clinics? Front. Neurol. 2020, 11, 994. [Google Scholar] [CrossRef]
- Scano, A.; Chiavenna, A.; Malosio, M.; Molinari Tosatti, L. Kinect V2 Performance Assessment in Daily-Life Gestures: Cohort Study on Healthy Subjects for a Reference Database for Automated Instrumental Evaluations on Neurological Patients. Appl. Bionics Biomech. 2017, 2017, 8567084. [Google Scholar] [CrossRef] [Green Version]
- Rosati, S.; Agostini, V.; Knaflitz, M.; Balestra, G. Muscle Activation Patterns during Gait: A Hierarchical Clustering Analysis. Biomed. Signal Process. Control 2017, 31, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Valk, T.A.; Mouton, L.J.; Otten, E.; Bongers, R.M. Fixed Muscle Synergies and Their Potential to Improve the Intuitive Control of Myoelectric Assistive Technology for Upper Extremities. J. Neuroeng. Rehabil. 2019, 16, 6. [Google Scholar] [CrossRef]
- Ramos-Murguialday, A.; García-Cossio, E.; Walter, A.; Cho, W.; Broetz, D.; Bogdan, M.; Cohen, L.G.; Birbaumer, N. Decoding Upper Limb Residual Muscle Activity in Severe Chronic Stroke. Ann. Clin. Transl. Neurol. 2015, 2, 1–11. [Google Scholar] [CrossRef]
- Gracia-Ibáñez, V.; Sancho-Bru, J.L.; Vergara, M.; Jarque-Bou, N.J.; Roda-Sales, A. Sharing of Hand Kinematic Synergies across Subjects in Daily Living Activities. Sci. Rep. 2020, 10, 6116. [Google Scholar] [CrossRef]
- Jarque-Bou, N.J.; Vergara, M.; Sancho-Bru, J.L.; Gracia-Ibanez, V.; Roda-Sales, A. Hand Kinematics Characterization While Performing Activities of Daily Living Through Kinematics Reduction. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 1556–1565. [Google Scholar] [CrossRef]
- Alibeji, N.A.; Molazadeh, V.; Moore-Clingenpeel, F.; Sharma, N. A Muscle Synergy-Inspired Control Design to Coordinate Functional Electrical Stimulation and a Powered Exoskeleton: Artificial Generation of Synergies to Reduce Input Dimensionality. IEEE Control Syst. 2018, 38, 35–60. [Google Scholar] [CrossRef]
- Furui, A.; Eto, S.; Nakagaki, K.; Shimada, K.; Nakamura, G.; Masuda, A.; Chin, T.; Tsuji, T. A Myoelectric Prosthetic Hand with Muscle Synergy–Based Motion Determination and Impedance Model–Based Biomimetic Control. Sci. Robot. 2019, 4, eaaw6339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atzori, M.; Gijsberts, A.; Castellini, C.; Caputo, B.; Hager, A.-G.M.; Elsig, S.; Giatsidis, G.; Bassetto, F.; Müller, H. Electromyography Data for Non-Invasive Naturally-Controlled Robotic Hand Prostheses. Sci. Data 2014, 1, 140053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarque-Bou, N.J.; Atzori, M.; Müller, H. A Large Calibrated Database of Hand Movements and Grasps Kinematics. Sci. Data 2020, 7, 12. [Google Scholar] [CrossRef]
- Liu, J.; Kang, S.H.; Xu, D.; Ren, Y.; Lee, S.J.; Zhang, L.-Q. EMG-Based Continuous and Simultaneous Estimation of Arm Kinematics in Able-Bodied Individuals and Stroke Survivors. Front. Neurosci. 2017, 11, 480. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, Y.; Xu, D.; Kang, S.H.; Zhang, L.-Q. EMG-Based Real-Time Linear-Nonlinear Cascade Regression Decoding of Shoulder, Elbow and Wrist Movements in Able-Bodied Persons and Stroke Survivors. IEEE Trans. Biomed. Eng. 2019, 67, 1272–1281. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.-S.; Kim, J. Automated Evaluation of Upper-Limb Motor Function Impairment Using Fugl-Meyer Assessment. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 125–134. [Google Scholar] [CrossRef]
- Mesbah, S.; Gonnelli, F.; Angeli, C.A.; El-baz, A.; Harkema, S.J.; Rejc, E. Neurophysiological Markers Predicting Recovery of Standing in Humans with Chronic Motor Complete Spinal Cord Injury. Sci. Rep. 2019, 9, 14474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scano, A.; Dardari, L.; Molteni, F.; Giberti, H.; Tosatti, L.M.; d’Avella, A. A Comprehensive Spatial Mapping of Muscle Synergies in Highly Variable Upper-Limb Movements of Healthy Subjects. Front. Physiol. 2019, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhang, Z.; Wen, H.; Wang, Z.; Wu, J. Modular Organization of Muscle Synergies to Achieve Movement Behaviors. J. Healthc. Eng. 2019, 2019, 8130297. [Google Scholar] [CrossRef] [PubMed]
- Mira, R.M.; Molinari Tosatti, L.; Sacco, M.; Scano, A. Detailed Characterization of Physiological EMG Activations and Directional Tuning of Upper-Limb and Trunk Muscles in Point-to-Point Reaching Movements. Curr. Res. Physiol. 2021, 4, 60–72. [Google Scholar] [CrossRef]
- D’Avella, A.; Saltiel, P.; Bizzi, E. Combinations of Muscle Synergies in the Construction of a Natural Motor Behavior. Nat. Neurosci. 2003, 6, 300–308. [Google Scholar] [CrossRef]
- D’Avella, A.; Bizzi, E. Shared and Specific Muscle Synergies in Natural Motor Behaviors. Proc. Natl. Acad. Sci. USA 2005, 102, 3076–3081. [Google Scholar] [CrossRef] [Green Version]
- Ting, L.H.; Macpherson, J.M. A Limited Set of Muscle Synergies for Force Control During a Postural Task. J. Neurophysiol. 2005, 93, 609–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overduin, S.A.; d’Avella, A.; Carmena, J.M.; Bizzi, E. Microstimulation Activates a Handful of Muscle Synergies. Neuron 2012, 76, 1071–1077. [Google Scholar] [CrossRef] [Green Version]
- Torres-Oviedo, G.; Macpherson, J.M.; Ting, L.H. Muscle Synergy Organization Is Robust Across a Variety of Postural Perturbations. J. Neurophysiol. 2006, 96, 1530–1546. [Google Scholar] [CrossRef] [Green Version]
- d’Avella, A.; Portone, A.; Fernandez, L.; Lacquaniti, F. Control of Fast-Reaching Movements by Muscle Synergy Combinations. J. Neurosci. 2006, 26, 7791–7810. [Google Scholar] [CrossRef] [Green Version]
- Clark, D.J.; Ting, L.H.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A. Merging of Healthy Motor Modules Predicts Reduced Locomotor Performance and Muscle Coordination Complexity Post-Stroke. J. Neurophysiol. 2010, 103, 844–857. [Google Scholar] [CrossRef] [Green Version]
- Roh, J.; Cheung, V.C.K.; Bizzi, E. Modules in the Brain Stem and Spinal Cord Underlying Motor Behaviors. J. Neurophysiol. 2011, 106, 1363–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israely, S.; Leisman, G.; Machluf, C.C.; Carmeli, E. Muscle Synergies Control during Hand-Reaching Tasks in Multiple Directions Post-Stroke. Front. Comput. Neurosci. 2018, 12, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieliba, P.; Tropea, P.; Pirondini, E.; Coscia, M.; Micera, S.; Artoni, F. How Are Muscle Synergies Affected by Electromyography Pre-Processing? IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 882–893. [Google Scholar] [CrossRef] [Green Version]
- Saito, A.; Tomita, A.; Ando, R.; Watanabe, K.; Akima, H. Muscle Synergies Are Consistent across Level and Uphill Treadmill Running. Sci. Rep. 2018, 8, 5979. [Google Scholar] [CrossRef]
- Taborri, J.; Palermo, E.; Del Prete, Z.; Rossi, S. On the Reliability and Repeatability of Surface Electromyography Factorization by Muscle Synergies in Daily Life Activities. Appl. Bionics Biomech. 2018, 2018, 5852307. [Google Scholar] [CrossRef]
- Hug, F.; Turpin, N.A.; Guével, A.; Dorel, S. Is Interindividual Variability of EMG Patterns in Trained Cyclists Related to Different Muscle Synergies? J. Appl. Physiol. 2010, 108, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuman, B.; Goudriaan, M.; Bar-On, L.; Schwartz, M.H.; Desloovere, K.; Steele, K.M. Repeatability of Muscle Synergies within and between Days for Typically Developing Children and Children with Cerebral Palsy. Gait Posture 2016, 45, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Steele, K.M.; Munger, M.E.; Peters, K.M.; Shuman, B.R.; Schwartz, M.H. Repeatability of Electromyography Recordings and Muscle Synergies during Gait among Children with Cerebral Palsy. Gait Posture 2019, 67, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Bulea, T.C.; Damiano, D.L. Children with Cerebral Palsy Have Greater Stride-to-Stride Variability of Muscle Synergies During Gait Than Typically Developing Children: Implications for Motor Control Complexity. Neurorehabil. Neural Repair 2018, 32, 834–844. [Google Scholar] [CrossRef] [Green Version]
- Kostraba, B.; Wu, Y.-N.; Kao, P.-C.; Stark, C.; Yen, S.-C.; Roh, J. Muscle Activation Pattern during Self-Propelled Treadmill Walking. J. Phys. Ther. Sci. 2018, 30, 1069–1072. [Google Scholar] [CrossRef]
- Barroso, F.O.; Torricelli, D.; Moreno, J.C.; Taylor, J.; Gomez-Soriano, J.; Bravo-Esteban, E.; Piazza, S.; Santos, C.; Pons, J.L. Shared Muscle Synergies in Human Walking and Cycling. J. Neurophysiol. 2014, 112, 1984–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guigon, E.; Baraduc, P.; Desmurget, M. Computational Motor Control: Redundancy and Invariance. J. Neurophysiol. 2007, 97, 331–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.; Chen, L.; Barsotti, M.; Hu, L.; Li, Y.; Wu, X.; Bai, L.; Frisoli, A.; Hou, W. Kinematic Synergy of Multi-DoF Movement in Upper Limb and Its Application for Rehabilitation Exoskeleton Motion Planning. Front. Neurorobot. 2019, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Delis, I.; Berret, B.; Pozzo, T.; Panzeri, S. Quantitative Evaluation of Muscle Synergy Models: A Single-Trial Task Decoding Approach. Front. Comput. Neurosci. 2013, 7, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delis, I.; Berret, B.; Pozzo, T.; Panzeri, S. A Methodology for Assessing the Effect of Correlations among Muscle Synergy Activations on Task-Discriminating Information. Front. Comput. Neurosci. 2013, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of Recommendations for SEMG Sensors and Sensor Placement Procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Ngeo, J.G.; Tamei, T.; Shibata, T. Continuous and Simultaneous Estimation of Finger Kinematics Using Inputs from an EMG-to-Muscle Activation Model. J. Neuroeng. Rehabil. 2014, 11, 122. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.D.; Seung, H.S. Algorithms for Non-Negative Matrix Factorization. Adv. Neural Inf. Process. Syst. 2001, 13, 556–562. [Google Scholar]
- Israely, S.; Leisman, G.; Machluf, C.; Shnitzer, T.; Carmeli, E. Direction Modulation of Muscle Synergies in a Hand-Reaching Task. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 2427–2440. [Google Scholar] [CrossRef]
- Taborri, J.; Palermo, E.; Masiello, D.; Rossi, S. Factorization of EMG via Muscle Synergies in Walking Task: Evaluation of Intra-Subject and Inter-Subject Variability. In Proceedings of the 2017 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Torino, Italy, 22–25 May 2017; pp. 1–6. [Google Scholar]
- Ebied, A.; Kinney-Lang, E.; Spyrou, L.; Escudero, J. Evaluation of Matrix Factorisation Approaches for Muscle Synergy Extraction. Med. Eng. Phys. 2018, 57, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Russo, M.; D’Andola, M.; Portone, A.; Lacquaniti, F.; d’Avella, A. Dimensionality of Joint Torques and Muscle Patterns for Reaching. Front. Comput. Neurosci. 2014, 8, 24. [Google Scholar] [CrossRef]
- Sun, L.; Pan, B.; Ye, S.; Huang, Z.; Wu, J. Modulation of Muscle Synergies with Direction and Distance during Reaching Movements. In Proceedings of the 2019 9th International IEEE/EMBS Conference on Neural Engineering (NER), San Francisco, CA, USA, 20–23 March 2019; pp. 1146–1150. [Google Scholar]
- Scano, A.; Chiavenna, A.; Malosio, M.; Molinari Tosatti, L.; Molteni, F. Muscle Synergies-Based Characterization and Clustering of Poststroke Patients in Reaching Movements. Front. Bioeng. Biotechnol. 2017, 5, 62. [Google Scholar] [CrossRef] [Green Version]
- Tresch, M.C.; Cheung, V.C.K.; d’Avella, A. Matrix Factorization Algorithms for the Identification of Muscle Synergies: Evaluation on Simulated and Experimental Data Sets. J. Neurophysiol. 2006, 95, 2199–2212. [Google Scholar] [CrossRef] [Green Version]
- There Is No Motor Redundancy in Human Movements. There Is Motor Abundance. Mot. Control 2000, 4, 259–261. [CrossRef]
- Profeta, V.L.S.; Turvey, M.T. Bernstein’s Levels of Movement Construction: A Contemporary Perspective. Hum. Mov. Sci. 2018, 57, 111–133. [Google Scholar] [CrossRef]
- Alnajjar, F.S.; Moreno, J.C.; Ozaki, K.; Kondo, I.; Shimoda, S. Motor Control System for Adaptation of Healthy Individuals and Recovery of Poststroke Patients: A Case Study on Muscle Synergies. Neural Plast. 2019, 2019, 8586416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latash, M.L. The Bliss (Not the Problem) of Motor Abundance (Not Redundancy). Exp. Brain Res. 2012, 217, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taborri, J.; Agostini, V.; Artemiadis, P.K.; Ghislieri, M.; Jacobs, D.A.; Roh, J.; Rossi, S. Feasibility of Muscle Synergy Outcomes in Clinics, Robotics, and Sports: A Systematic Review. Appl. Bionics Biomech. 2018, 2018, 3934698. [Google Scholar] [CrossRef]
- Cheung, V.C.K.; Piron, L.; Agostini, M.; Silvoni, S.; Turolla, A.; Bizzi, E. Stability of Muscle Synergies for Voluntary Actions after Cortical Stroke in Humans. Proc. Natl. Acad. Sci. USA 2009, 106, 19563–19568. [Google Scholar] [CrossRef] [Green Version]
- Roh, J.; Rymer, W.Z.; Perreault, E.J.; Yoo, S.B.; Beer, R.F. Alterations in Upper Limb Muscle Synergy Structure in Chronic Stroke Survivors. J. Neurophysiol. 2013, 109, 768–781. [Google Scholar] [CrossRef] [Green Version]
- Roh, J.; Rymer, W.Z.; Beer, R.F. Evidence for Altered Upper Extremity Muscle Synergies in Chronic Stroke Survivors with Mild and Moderate Impairment. Front. Hum. Neurosci. 2015, 9, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, J.L.; Kesar, T.M.; Ting, L.H. Motor Module Generalization across Balance and Walking Is Impaired after Stroke. J. Neurophysiol. 2019, 122, 277–289. [Google Scholar] [CrossRef]
- Brough, L.G.; Kautz, S.A.; Bowden, M.G.; Gregory, C.M.; Neptune, R.R. Merged Plantarflexor Muscle Activity Is Predictive of Poor Walking Performance in Post-Stroke Hemiparetic Subjects. J. Biomech. 2019, 82, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhuang, C.; Niu, C.M.; Bao, Y.; Xie, Q.; Lan, N. Evaluation of Functional Correlation of Task-Specific Muscle Synergies with Motor Performance in Patients Poststroke. Front. Neurol. 2017, 8, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, B.; Sun, Y.; Xie, B.; Huang, Z.; Wu, J.; Hou, J.; Liu, Y.; Huang, Z.; Zhang, Z. Alterations of Muscle Synergies during Voluntary Arm Reaching Movement in Subacute Stroke Survivors at Different Levels of Impairment. Front. Comput. Neurosci. 2018, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.M.; Bao, Y.; Zhuang, C.; Li, S.; Wang, T.; Cui, L.; Xie, Q.; Lan, N. Synergy-Based FES for Post-Stroke Rehabilitation of Upper-Limb Motor Functions. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-H.; Wang, T.; Sun, X.; Niu, C.M.; Hao, M.; Xie, Q.; Lan, N. Automated Functional Electrical Stimulation Training System for Upper-Limb Function Recovery in Poststroke Patients. Med. Eng. Phys. 2020, 84, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Steele, K.M.; Tresch, M.C.; Perreault, E.J. The Number and Choice of Muscles Impact the Results of Muscle Synergy Analyses. Front. Comput. Neurosci. 2013, 7, 105. [Google Scholar] [CrossRef] [Green Version]
- Barradas, V.R.; Kutch, J.J.; Kawase, T.; Koike, Y.; Schweighofer, N. When 90% of the Variance Is Not Enough: Residual EMG from Muscle Synergy Extraction Influences Task Performance. J. Neurophysiol. 2020, 123, 2180–2190. [Google Scholar] [CrossRef]
- Rabbi, M.F.; Pizzolato, C.; Lloyd, D.G.; Carty, C.P.; Devaprakash, D.; Diamond, L.E. Non-Negative Matrix Factorisation Is the Most Appropriate Method for Extraction of Muscle Synergies in Walking and Running. Sci. Rep. 2020, 10, 8266. [Google Scholar] [CrossRef] [PubMed]
- Kipp, K.; Pfeiffer, R.; Sabick, M.; Harris, C.; Sutter, J.; Kuhlman, S.; Shea, K. Muscle Synergies During a Single-Leg Drop-Landing in Boys and Girls. J. Appl. Biomech. 2014, 30, 262–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santuz, A.; Janshen, L.; Brüll, L.; Munoz-Martel, V.; Taborri, J.; Rossi, S.; Arampatzis, A. Sex-Specific Tuning of Modular Muscle Activation Patterns for Locomotion in Young and Older Adults. bioRxiv 2021. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Zhang, Z.; Wen, H.; Scano, A. Intra-Subject and Inter-Subject Movement Variability Quantified with Muscle Synergies in Upper-Limb Reaching Movements. Biomimetics 2021, 6, 63. https://doi.org/10.3390/biomimetics6040063
Zhao K, Zhang Z, Wen H, Scano A. Intra-Subject and Inter-Subject Movement Variability Quantified with Muscle Synergies in Upper-Limb Reaching Movements. Biomimetics. 2021; 6(4):63. https://doi.org/10.3390/biomimetics6040063
Chicago/Turabian StyleZhao, Kunkun, Zhisheng Zhang, Haiying Wen, and Alessandro Scano. 2021. "Intra-Subject and Inter-Subject Movement Variability Quantified with Muscle Synergies in Upper-Limb Reaching Movements" Biomimetics 6, no. 4: 63. https://doi.org/10.3390/biomimetics6040063





