Diverting the Use of Hand-Operated Tablet Press Machines to Bioassays: A Novel Protocol to Test ‘Waste’ Insoluble Shell Matrices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Samples
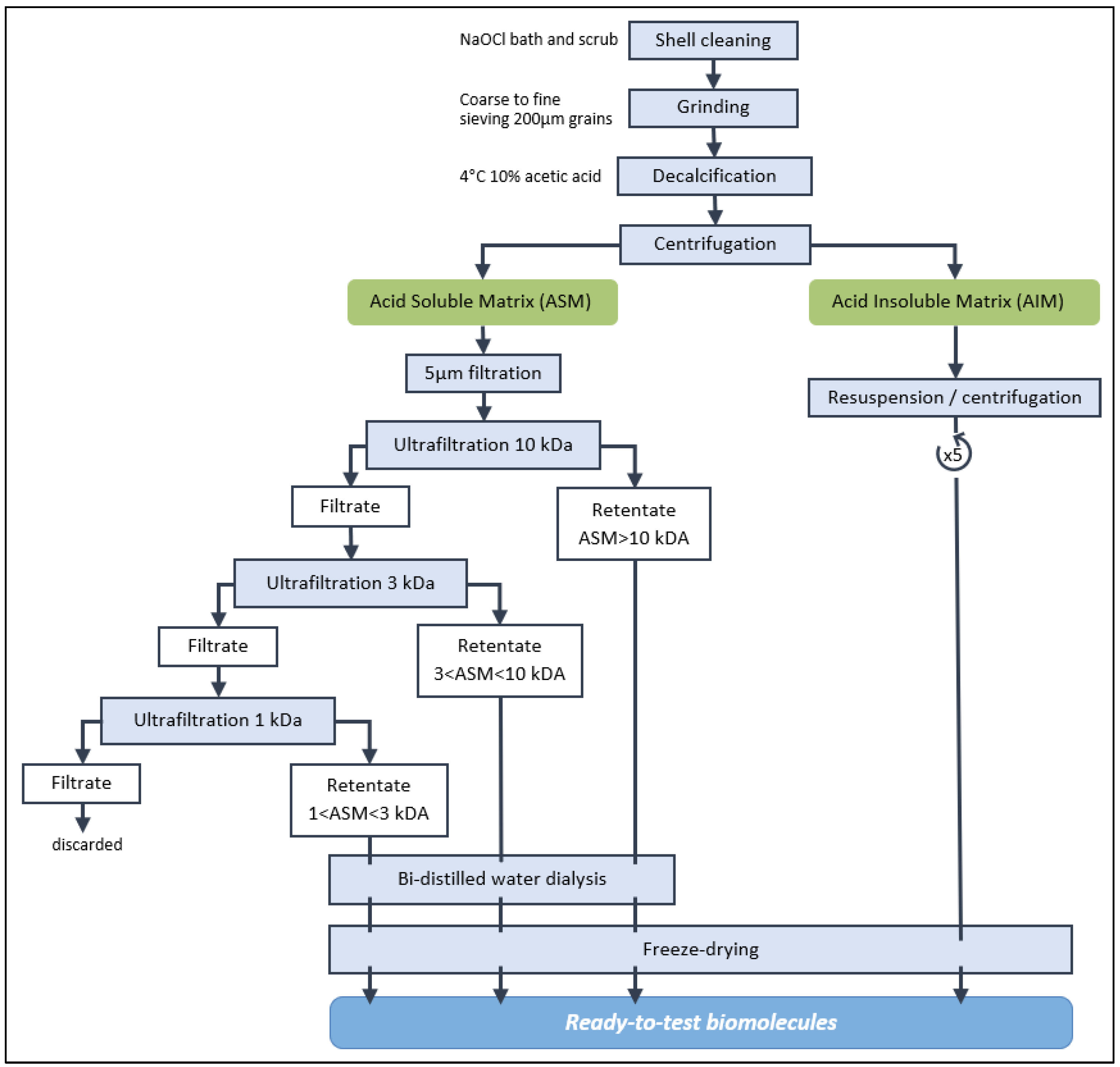
2.2. Shell Matrix Extraction
2.3. Hand-Operated Tablet Press for AIMs
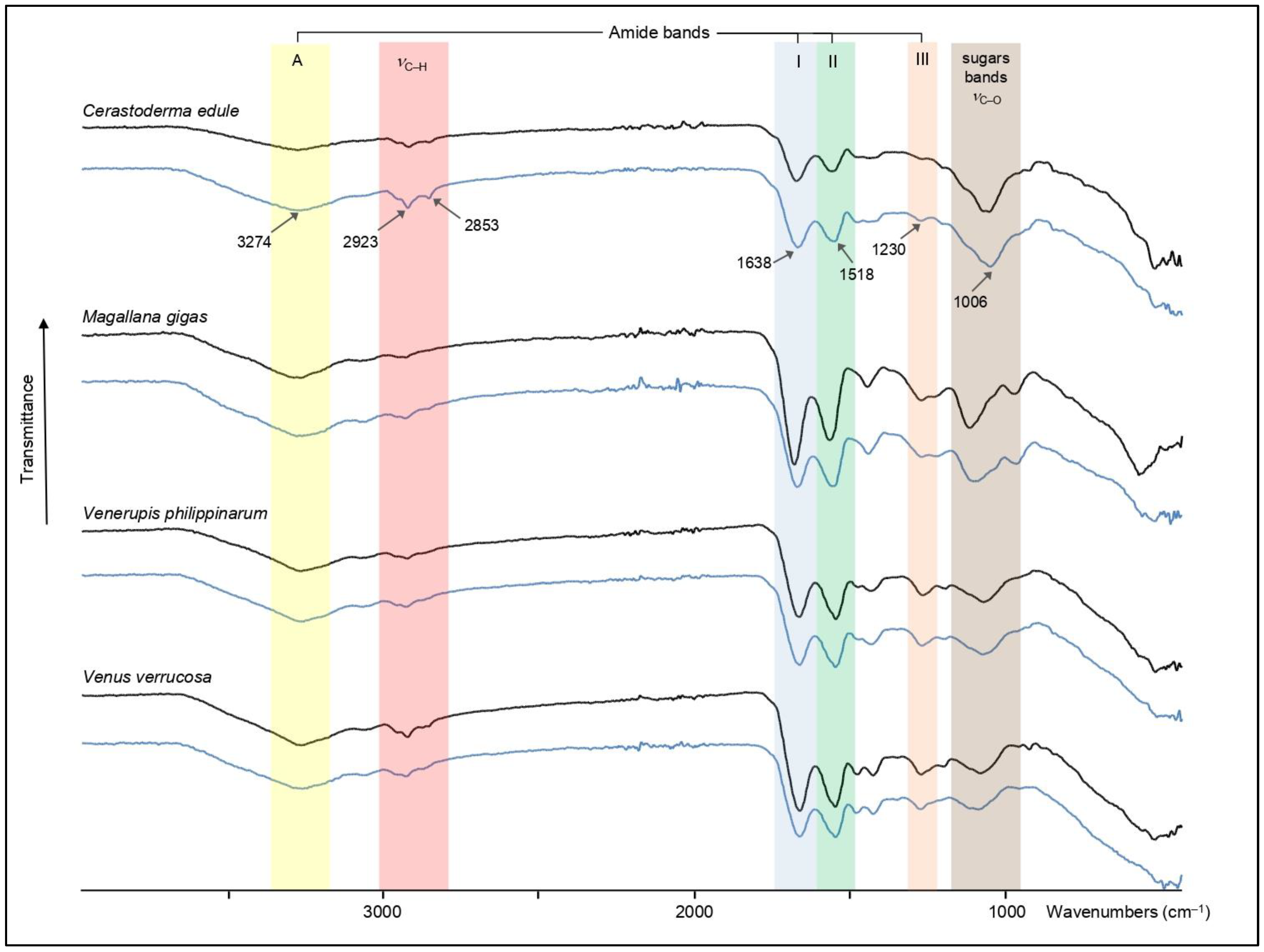
2.4. Fourier Transform Infra-Red Spectroscopy of AIMs
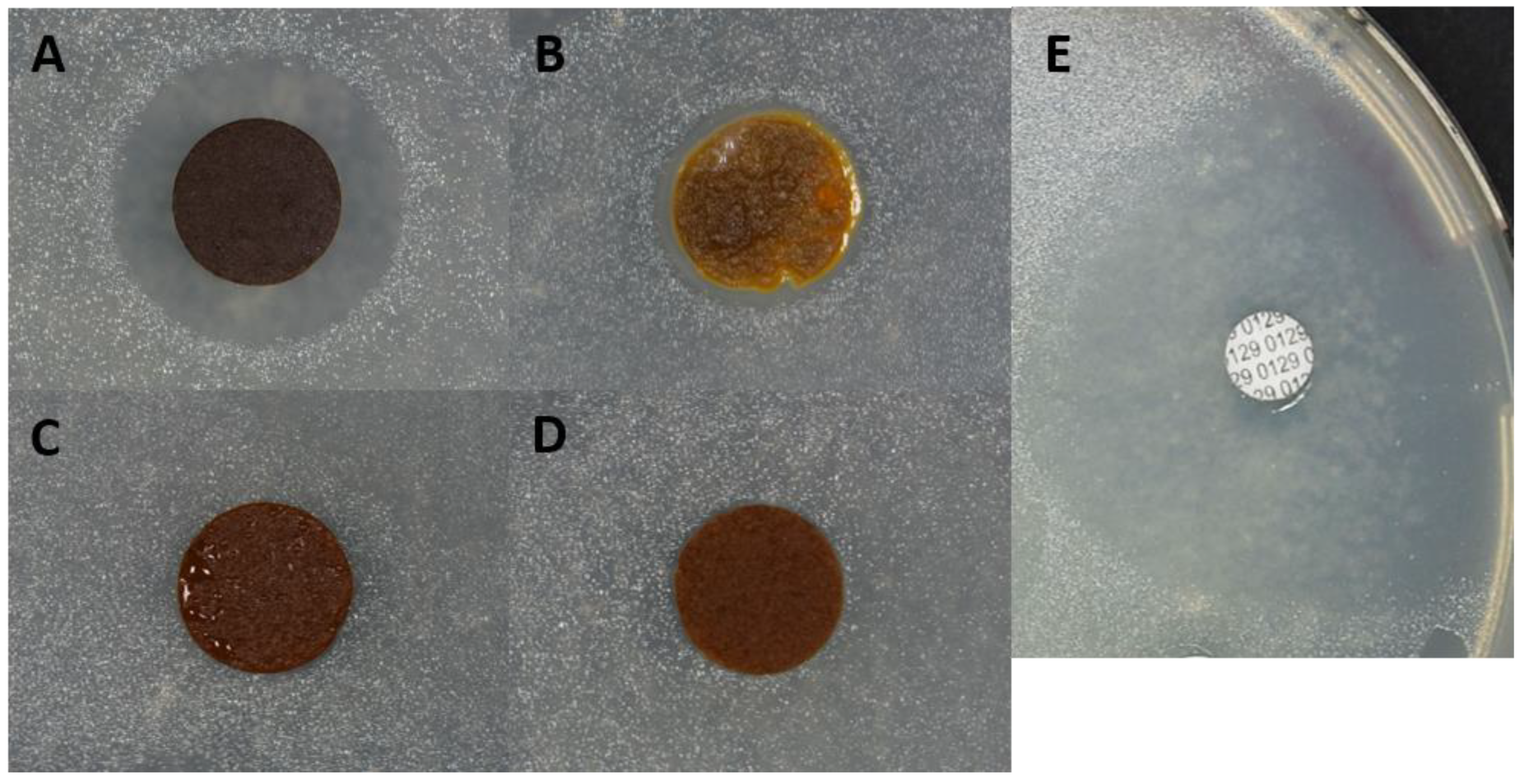
2.5. Antibacterial Assay
3. Results
3.1. Shell Matrix Extraction
3.2. Hand-Operated Tablet Press for Insoluble Matrix
3.3. Fourier Transform Infrared Spectroscopy
3.4. Antibacterial Assay on AIMs
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Summa, D.; Lanzoni, M.; Castaldelli, G.; Fano, E.A.; Tamburini, E. Trends and opportunities of bivalve shells’ waste valorization in a prospect of circular blue bioeconomy. Resources 2022, 11, 48. [Google Scholar] [CrossRef]
- Bourdot, A.; Martin-Cavaillé, C.; Vacher, M.; Honorio, T.; Sebaibi, N.; Bennacer, R. Microstructure and durability properties of concretes based on oyster shell co-products. In Proceedings of the 75th RILEM Annual Week 2021, Merida, Mexico, 29 August–3 September 2021; Volume 40, pp. 65–73. [Google Scholar]
- Alm, M.; Tauson, R.; Wall, H. Mussel shells as an environment enrichment and calcium source for floor-housed laying hens. J. Appl. Poult. Res. 2017, 26, 159–167. [Google Scholar] [CrossRef]
- Zeng, T.; Guo, J.; Li, Y.; Wang, G. Oyster shell amendment reduces cadmium and lead availability and uptake by rice in contaminated paddy soil. Environ. Sci. Pollut. Res. 2022, 29, 44582–44596. [Google Scholar] [CrossRef]
- Weerasooriyagedra, M.S.; Kumar, S.A. A review of utilization of Mollusca shell for the removal of contaminants in industrial waste water. Int. J. Sci. Res. Public 2018, 8, 282–287. [Google Scholar]
- Suzuki, M.; Nagasawa, H. Mollusk shell structures and their formation mechanism. Can. J. Zool. 2013, 91, 349–366. [Google Scholar] [CrossRef]
- Weiner, S.; Hood, L. Soluble proteins of the organic matrix of mollusc shells: A potential template for shell formation. Science 1975, 190, 987–989. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.P.; George, J.W.; Evans, C.A. Control of calcium carbonate nucleation and crystal growth by soluble matrix of oyster shell. Science 1981, 212, 1397–1398. [Google Scholar] [CrossRef]
- Marin, F. Mollusk shellomes: Past, present and future. J. Struct. Biol. 2020, 212, 107583. [Google Scholar] [CrossRef]
- Sleight, V.A.; Marie, B.; Jackson, D.J.; Dyrynda, E.A.; Marie, A.; Clark, M.S. An Antarctic molluscan biomineralisation tool-kit. Sci. Rep. 2016, 6, 36978. [Google Scholar] [CrossRef]
- Herlitze, I.; Marie, B.; Marin, F.; Jackson, D.J. Molecular modularity and asymmetry of the molluscan mantle revealed by a gene expression atlas. Gigascience 2018, 7, giy056. [Google Scholar] [CrossRef]
- Oudot, M.; Neige, P.; Ben Shir, I.; Schmidt, A.; Strugnell, J.M.; Plasseraud, L.; Broussard, C.; Hoffmann, R.; Lukeneder, A.; Marin, F. The shell matrix and microstructure of the Ram’s horn squid: Molecular and structural characterization. J. Struct. Biol. 2020, 211, 107507. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Negishi, L.; Ito, T.; Touma, S.; Matsumoto, T.; Awaji, M.; Kurumizaka, H.; Yoshitake, K.; Kinoshita, S.; Asakawa, S.; et al. Evolution of nacre- and prisms-related shell matrix proteins in the pen shell, Atrina pectinata. Comp. Biochem. Physiol. D—Genom. Proteom. 2022, 44, 101025. [Google Scholar] [CrossRef] [PubMed]
- Magnabosco, G.; Giuri, D.; Di Bisceglie, A.P.; Scarpino, F.; Fermani, S.; Tomasini, C.; Falini, G. New Material perspective for waste seashells by covalent functionalization. ACS Sustain. Chem. Eng. 2021, 9, 6203–6208. [Google Scholar] [CrossRef]
- Marin, F.; Marie, B.; Benhamada, S.; Silva, P.; Le Roy, N.; Guichard, N.; Wolf, S.; Montagnani, C.; Joubert, C.; Piquemal, D.; et al. ‘Shellome’: Proteins involved in mollusk shell biomineralization-diversity, functions. In Recent Advances in Pearl Research: Proceedings of the International Symposium on Pearl Research 2011; Watabe, S., Maeyama, K., Nagasawa, H., Eds.; Terrapub: Tokyo, Japan, 2013; pp. 149–166. [Google Scholar]
- Mouriès, L.P.; Almeida, M.-J.; Milet, C.; Berland, S.; Lopez, E. Bioactivity of nacre water-soluble organic matrix from the bivalve mollusk Pinctada maxima in three mammalian cell types: Fibroblasts, bone marrow stromal cells and osteoblasts. Comp. Biochem. Physiol. B—Biochem. Mol. Biol. 2002, 132, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, M.; Bouzalguet, H.; Biagianti, J.; Duplat, D.; Milet, C.; Lopez, E.; Bédouet, L. Low molecular weight molecules of oyster nacre induce mineralization of the MC3T3-E1 cells. J. Biomed. Mater. Res. Part A 2008, 85A, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Bédouet, L.; Rusconi, F.; Rousseau, M.; Duplat, D.; Marie, A.; Dubost, L.; Le Ny, K.; Berland, S.; Péduzzi, J.; Lopez, E. Identification of low molecular weight molecules as new components of the nacre organic matrix. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 144, 532–543. [Google Scholar] [CrossRef]
- Sud, D.; Doumenc, D.; Lopez, E.; Milet, C. Role of water-soluble matrix fraction, extracted from the nacre of Pinctada maxima, in the regulation of cell activity in abalone mantle cell culture (Haliotis tuberculata). Tissue Cell 2001, 33, 154–160. [Google Scholar] [CrossRef]
- Milet, C.; Berland, S.; Lamghari, M.; Mouriès, L.; Jolly, C.; Borzeix, S.; Doumenc, D.; Lopez, E. Conservation of signal molecules involved in biomineralization control in calcifying matrices of bone and shell. Comptes-Rendus Palevol 2004, 3, 493–501. [Google Scholar] [CrossRef]
- Frémy, M.E. Recherches chimiques sur les os. Ann. Chim. Phys. 1855, 43, 47–107. [Google Scholar]
- Grégoire, C. Structure of the molluscan shell. In Chemical Zoology, Vol. VII: Mollusca; Florkin, M., Scheer, B.T., Eds.; Academic Press: New York, NY, USA, 1972; pp. 45–102. [Google Scholar]
- Wilbur, K.M.; Saleuddin, A.S.M. Shell formation. In The Mollusca, Vol.4 Physiology, Part 1; Saleuddin, A.S.M., Wilbur, K.M., Eds.; Academic Press: New York, NY, USA, 1983; pp. 235–287. [Google Scholar]
- Almeida, M.; Machado, J.; Coelho, M.V.; da Silva, P.S.; Coimbra, J. L-3,4-dihydroxyphenylalanine (L-DOPA) secreted by oyster (Crassostrea gigas) mantle cells: Functional aspects. Comp. Biochem. Physiol B Biochem Mol. Biol. 1998, 120, 709–713. [Google Scholar] [CrossRef]
- Levi-Kalisman, Y.; Falini, G.; Addadi, L.; Weiner, S. Structure of the nacreous organic matrix of a bivalve mollusk shell examined in the hydrated state using Cryo-TEM. J. Struct. Biol. 2001, 135, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Traub, W.; Lowenstam, H.A. Organic matrix in calcified exoskeletons. In Biomineralization and Biological Metal Accumulation—Biological and Geological Perspectives; Westbroek, P., de Jong, E.W., Eds.; D. Reidel Publishing Company: Dordrecht, Holland, 1983; pp. 205–224. [Google Scholar]
- Carter, J.G. (Ed.) Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends; Van Nostrand Reinhold: New York, NY, USA, 1990; Volume 1, 832p. [Google Scholar]
- Hare, P.E.; Abelson, P.H. Amino acid composition of some calcified proteins. Carnegie Inst. Wash. Yearb. 1965, 64, 223–232. [Google Scholar]
- Marie, B.; Marin, F.; Marie, A.; Bédouet, L.; Dubost, L.; Alcaraz, G.; Milet, C.; Luquet, G. Evolution of nacre: Biochemistry and ‘shellomics’ of the shell organic matrix of the cephalopod Nautilus macromphalus. ChemBioChem 2009, 10, 1495–1510. [Google Scholar] [CrossRef]

| Mollusc Species | Mineralogy and Shell Microstructure * | Matrix Fraction | Mean Matrix Quantity (mg) per Gram of Shell Powder | % Matrix in Shell Powder | % AIM/Total Matrix | |
|---|---|---|---|---|---|---|
| Magallana gigas Pacific oyster |  | CALCITIC OL: prismatic IL: foliated + chalky lenses | AIM | 4.35 | 0.57% | 76.74% |
| ASM > 10 kDa | 1.14 | |||||
| 1 < ASM < 10 kDa | 0.18 | |||||
| Cerastoderma edule Common cockle |  | ARAGONITIC OL: crossed-lamellar IL: complex crossed-lamellar | AIM | 0.51 | 0.08% | 65.25% |
| ASM > 10 kDa | 0.10 | |||||
| 1 < ASM < 10 kDa | 0.18 | |||||
| Venerupis philippinarum Manila clam |  | ARAGONITIC OL: composite prismatic IL: crossed-lamellar becoming homogeneous | AIM | 1.18 | 0.15% | 77.96% |
| ASM > 10 kDa | 0.22 | |||||
| 3 < ASM < 10 kDa | 0.09 | |||||
| 1 < ASM < 3 kDa | 0.03 | |||||
| Venus verrucosa Warty venus |  | ARAGONITIC OL: composite prismatic ML: crossed-lamellar IL: homogeneous | AIM | 1.11 | 0.20% | 55.23% |
| ASM > 10 kDa | 0.72 | |||||
| 1 < ASM < 10 kDa | 0.18 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lutet-Toti, C.; Da Silva Feliciano, M.; Debrosse, N.; Thomas, J.; Plasseraud, L.; Marin, F. Diverting the Use of Hand-Operated Tablet Press Machines to Bioassays: A Novel Protocol to Test ‘Waste’ Insoluble Shell Matrices. Methods Protoc. 2024, 7, 30. https://doi.org/10.3390/mps7020030
Lutet-Toti C, Da Silva Feliciano M, Debrosse N, Thomas J, Plasseraud L, Marin F. Diverting the Use of Hand-Operated Tablet Press Machines to Bioassays: A Novel Protocol to Test ‘Waste’ Insoluble Shell Matrices. Methods and Protocols. 2024; 7(2):30. https://doi.org/10.3390/mps7020030
Chicago/Turabian StyleLutet-Toti, Camille, Marie Da Silva Feliciano, Nelly Debrosse, Jérôme Thomas, Laurent Plasseraud, and Frédéric Marin. 2024. "Diverting the Use of Hand-Operated Tablet Press Machines to Bioassays: A Novel Protocol to Test ‘Waste’ Insoluble Shell Matrices" Methods and Protocols 7, no. 2: 30. https://doi.org/10.3390/mps7020030





