Optimization of the Amplification of Equine Muscle-Derived Mesenchymal Stromal Cells in a Hollow-Fiber Bioreactor
Abstract
:1. Introduction
2. Material and Methods
2.1. Cell Cultures
2.1.1. Equine Muscle Microbiopsy and Mesenchymal Stromal Cell Culture
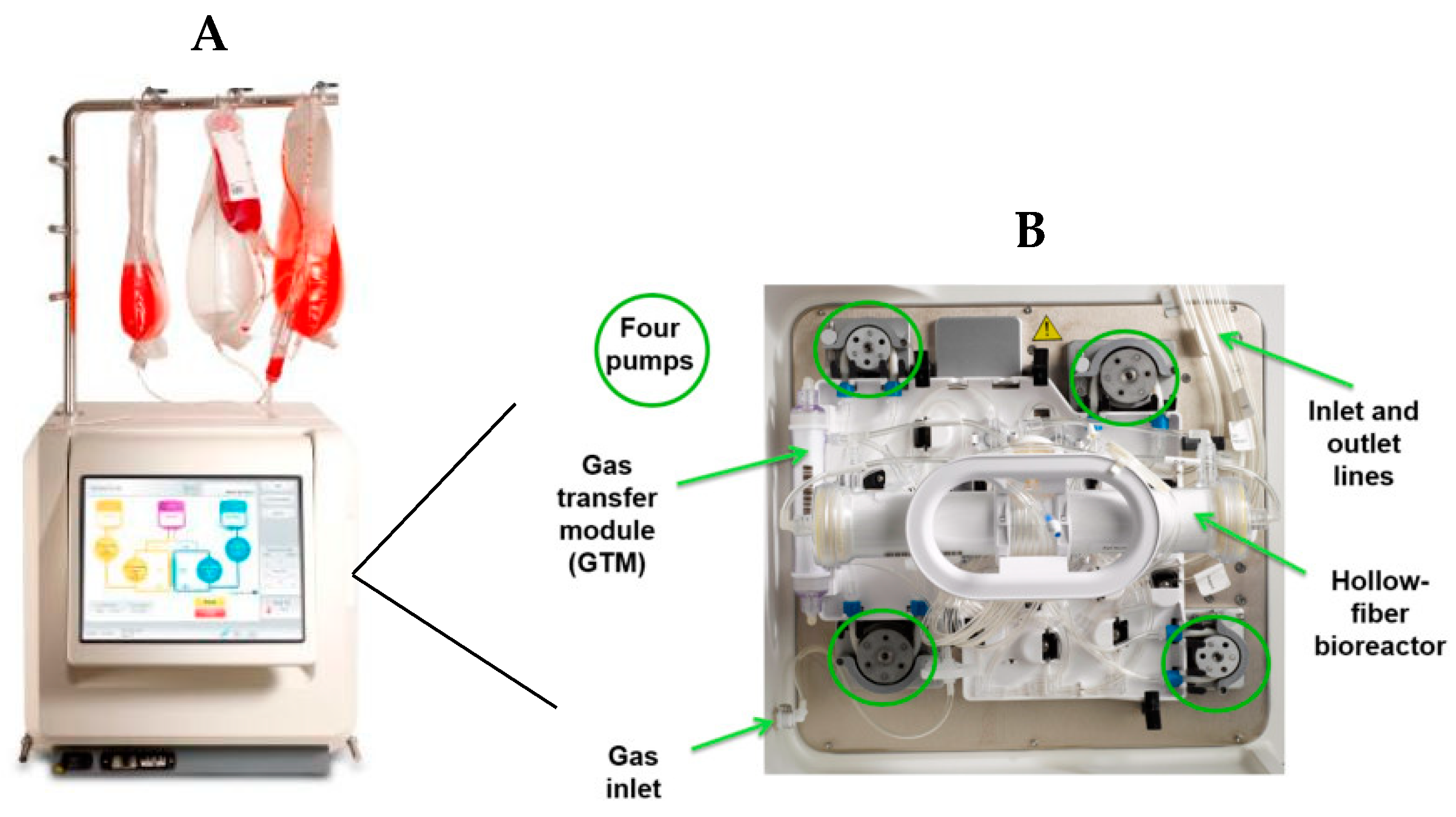
2.1.2. Quantum™ Preparation and Amplification of the Equine Muscle-Derived Mesenchymal Stromal Cells
Priming and Coating
Loading and Feeding
Cell Harvest
2.2. Flow Cytometry Analysis of the Immunophenotype of the Generated Cells
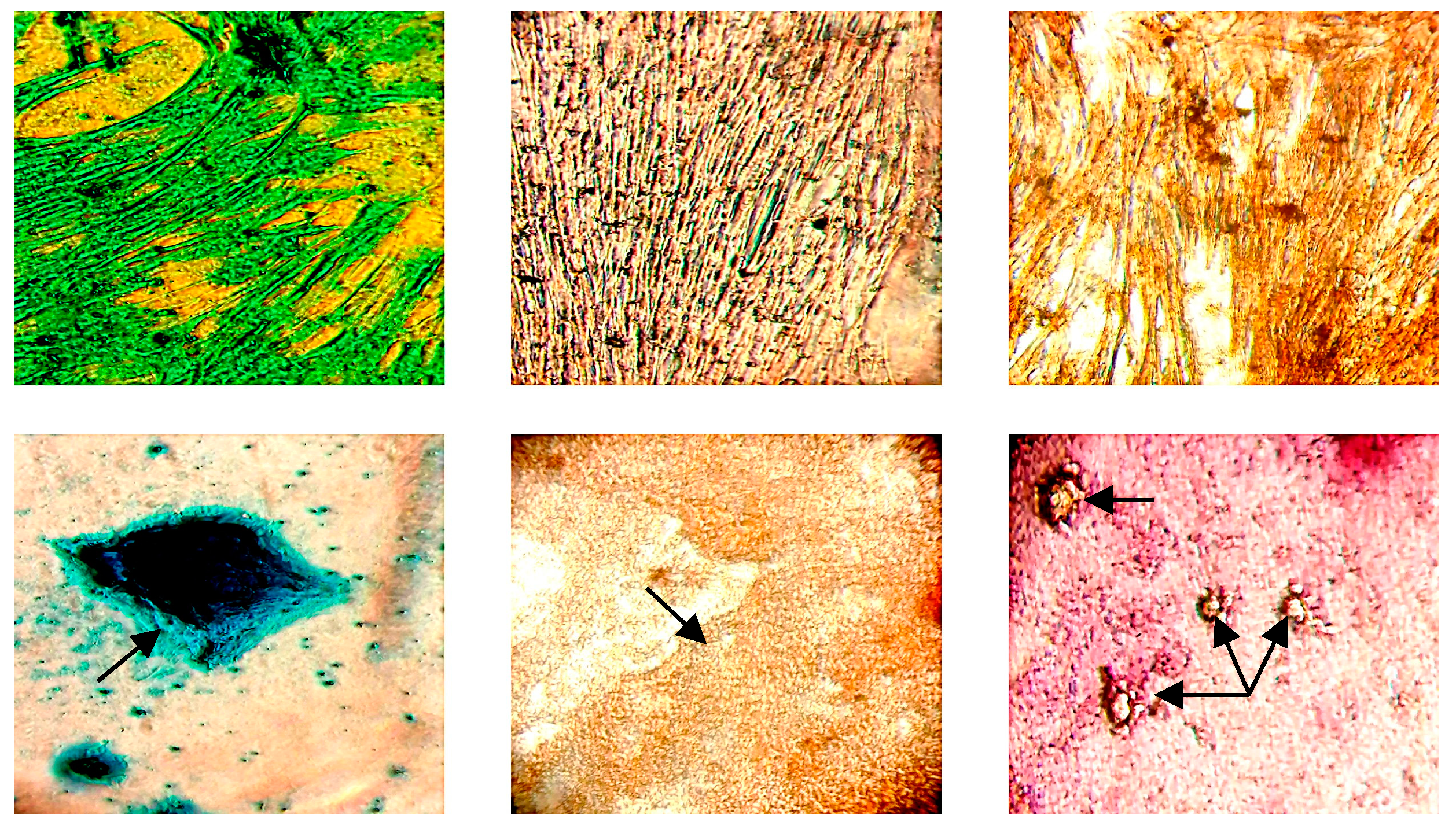
2.3. Trilineage Differentiation of Generated Equine Mesenchymal Stromal Cells
3. Results
Muscle-Derived Mesenchymal Stromal Cell Culture, Amplification and Characterization
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tamzali, Y.; Marguet, C.; Priymenko, N. Gastrointestinal disorders and colic in the horse: Review of recent developments and future perspectives. Vet. J. 2008, 177, 42–49. [Google Scholar]
- Mair, T.; Divers, T. (Eds.) Equine Gastrointestinal Surgery; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Navarro, M.A.; Arroyo, L.G.; Uzal, F.A. Special section on diseases of the equine gastrointestinal tract. J. Vet. Diagn. Investig. 2022, 34, 353. [Google Scholar] [CrossRef] [PubMed]
- Cook, V.; Hassel, D. Evaluation of the colic in horses: Decision for referral. Vet. Clin. N. Am. Equine Pract. 2014, 30, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Bowden, A.; England, G.C.W.; Brennan, M.L.; Mair, T.S.; Furness, W.A.; Freeman, S.L.; Burford, J.H. Indicators of ‘critical’ outcomes in 941 horses seen ‘out-of-hours’ for colic. Vet. Rec. 2020, 187, 105881. [Google Scholar] [CrossRef] [PubMed]
- Blangy-Letheule, A.; Vergnaud, A.; Dupas, T.; Rozec, B.; Lauzier, B.; Leroux, A.A. Spontaneous Sepsis in Adult Horses: From Veterinary to Human Medicine Perspectives. Cells 2023, 12, 1052. [Google Scholar] [CrossRef] [PubMed]
- Sousa, B.R.; Parreira, R.C.; Fonseca, E.A.; Amaya, M.J.; Tonelli, F.M.; Lacerda, S.M.; Lalwani, P.; Santos, A.K.; Gomes, K.N.; Ulrich, H.; et al. Human adult stem cells from diverse origins: An overview from multiparametric immunophenotyping to clinical applications. Cytom. Part A 2014, 85, 43–77. [Google Scholar] [CrossRef] [PubMed]
- Shammaa, R.; El-Kadiry, A.E.; Abusarah, J.; Rafei, M. Mesenchymal stromal cells Beyond Regenerative Medicine. Front. Cell Dev. Biol. 2020, 18, 8–72. [Google Scholar]
- Al Naem, M.; Bourebaba, L.; Kucharczyk, K.; Röcken, M.; Marycz, K. Therapeutic mesenchymal stromal stem cells: Isolation, characterization and role in equine regenerative medicine and metabolic disorders. Stem Cell Rev. Rep. 2020, 16, 301–322. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.G.; MacDonald, E.S. Use of Biologics and Stem Cells in the Treatment of Other Inflammatory Diseases in the Horse. Vet. Clin. N. Am. Equine Pract. 2023, 39, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Rojewski, M.T.; Fekete, N.; Baila, S.; Nguyen, K.; Fürst, D.; Antwiler, D.; Dausend, J.; Kreja, L.; Ignatius, A.; Sensebé, L.; et al. GMP-compliant isolation and expansion of bone marrow-derived MSCs in the closed, automated device quantum cell expansion system. Cell Transplant. 2013, 22, 1981–2000. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, T.; Papantoniou, I.; Rice, B.; Schrooten, J.; Luyten, F.P.; Aerts, J.M. Large-scale progenitor cell expansion for multiple donors in a monitored hollow fibre bioreactor. Cytotherapy 2016, 18, 1219–1233. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.; Lefavor, R.; Zubair, A. Characterization and cost-benefit analysis of automated bioreactor-expanded mesenchymal stromal cells for clinical applications. Transfusion 2018, 58, 2374–2382. [Google Scholar] [CrossRef] [PubMed]
- Ceusters, J.; Lejeune, J.P.; Sandersen, C.; Niesten, A.; Lagneaux, L.; Serteyn, D. From skeletal muscle to stem cells: An innovative and minimally-invasive process for multiple species. Sci. Rep. 2017, 7, 696. [Google Scholar] [CrossRef] [PubMed]
- de Schauwer, C.; Meyer, E.; van de Walle, G.R.; van Soom, A. Markers of stemness in equine mesenchymal stromal cells: A plea for uniformity. Theriogenology 2011, 75, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.J.R.; Luquet, E.; Pletenka, J.; Leonard, A.; Warter, E.; Gurchenkov, B.; Carrere, J.; Rieu, C.; Hardouin, J.; Moncaubeig, F.; et al. Engineering 3D micro-compartments for highly efficient and scale-independent expansion of human pluripotent stem cells in bioreactors. Biomaterials 2023, 295, 122033. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Clone | Dilution |
|---|---|---|
| CD-44 | CVS18 | 25 |
| CD-45 | F-10-89-4 | 5 |
| MHCII | CVS20 | 25 |
| CD-90 | DH24A | 50 |
| Run | Horse | Passage | Fresh/ Frozen | Loading | Seeding | Duration (Days) | Harvest | Cell Viability | Final Count (Viable Cells) | Multiplication Factor |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Horse 1 | P5 | Fresh | Classic | 25 × 106 | 7 | 217 × 106 | 91% | 197 × 106 | 7.88 |
| 2 | Horse 1 | P3 | Fresh | Classic | 10 × 106 | 15 | 30 × 106 | 60% | 18 × 106 | 1.80 |
| 3 | Horse 1 | P6 | Frozen | Classic | 16.5 × 106 | 8 | 79 × 106 | 73% | 57.7 × 106 | 3.50 |
| 4 | Horse 2 | P6 | Fresh | Classic | 25 × 106 | 8 | 92 × 106 | 78% | 71.8 × 106 | 2.87 |
| 5 | Horse 3 | P4 | Fresh | Bull’s eye | 25 × 106 | 8 | 216 × 106 | 83% | 179 × 106 | 7.16 |
| 6 | Horse 3 | P4 | Frozen | Bull’s eye | 20 × 106 | 10 | 170 × 106 | 82% | 140 × 106 | 7 |
| 7 | Horse 4 | P5 | Frozen | Bull’s eye | 25 × 106 | 9 | 326 × 106 | 91% | 297 × 106 | 11.88 |
| 8 | Horse 5 | P4 | Frozen | Bull’s eye | 10 × 106 | 9 | 220 × 106 | 85% | 187 × 106 | 18.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duysens, J.; Graide, H.; Niesten, A.; Mouithys-Mickalad, A.; Ceusters, J.; Serteyn, D. Optimization of the Amplification of Equine Muscle-Derived Mesenchymal Stromal Cells in a Hollow-Fiber Bioreactor. Methods Protoc. 2024, 7, 32. https://doi.org/10.3390/mps7020032
Duysens J, Graide H, Niesten A, Mouithys-Mickalad A, Ceusters J, Serteyn D. Optimization of the Amplification of Equine Muscle-Derived Mesenchymal Stromal Cells in a Hollow-Fiber Bioreactor. Methods and Protocols. 2024; 7(2):32. https://doi.org/10.3390/mps7020032
Chicago/Turabian StyleDuysens, Julien, Hélène Graide, Ariane Niesten, Ange Mouithys-Mickalad, Justine Ceusters, and Didier Serteyn. 2024. "Optimization of the Amplification of Equine Muscle-Derived Mesenchymal Stromal Cells in a Hollow-Fiber Bioreactor" Methods and Protocols 7, no. 2: 32. https://doi.org/10.3390/mps7020032





