Preliminary Results on Light Conditions Manipulation in Octopus vulgaris (Cuvier, 1797) Paralarval Rearing
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Ethical Statement
3.2. Octopus vulgaris Broodstock
3.3. Experiment 1—Effect of Polarized Light on Prey Capture
3.4. Experiment 2—Effect of Blue Filter or the Elimination of Light Wall Reflection on the Rearing of O. vulgaris Paralarvae
3.5. Growth and Survival
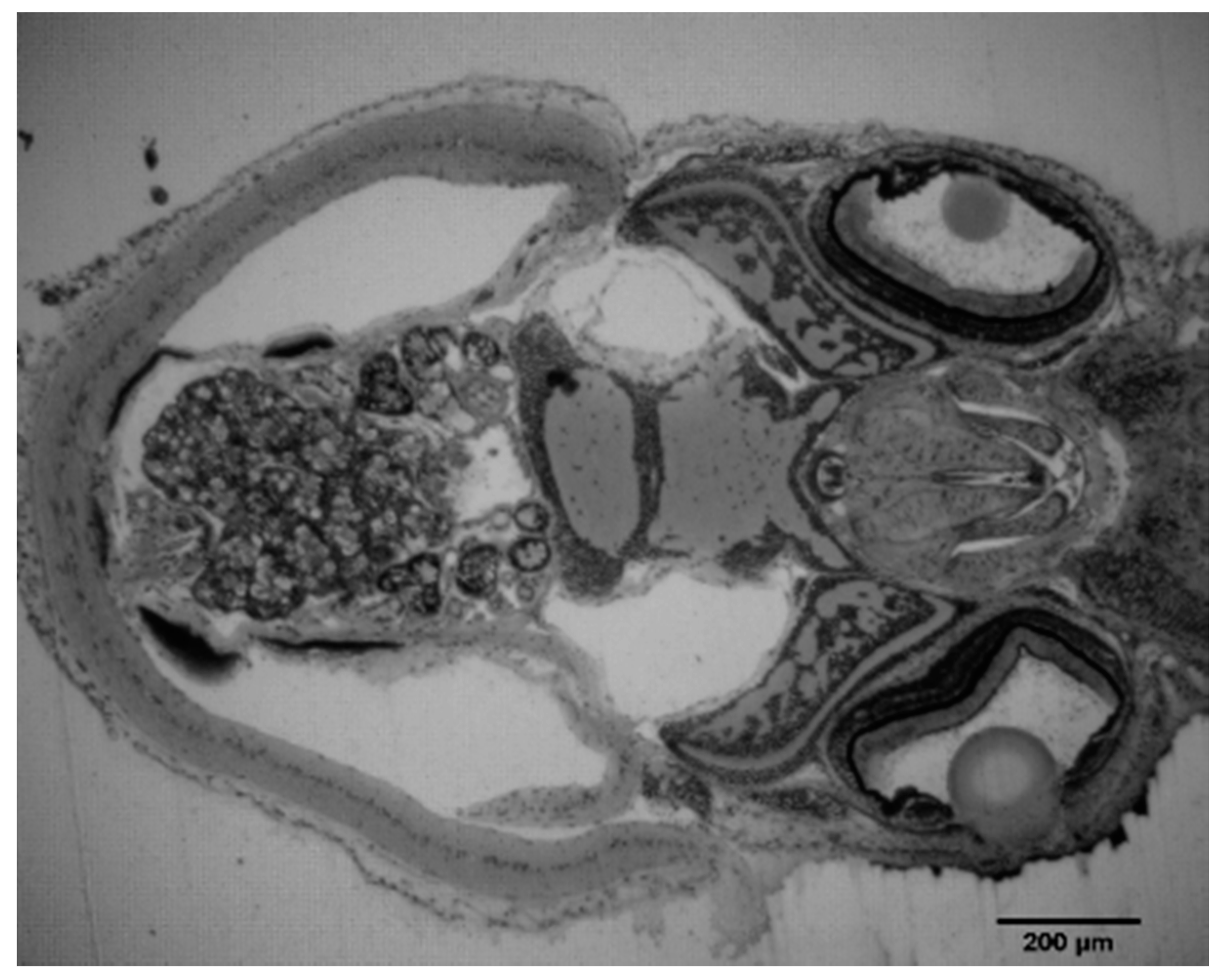
3.6. Histology
3.7. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vaz-Pires, P.; Seixas, P.; Barbosa, A. Aquaculture potential of the common octopus (Octopus vulgaris Cuvier 1797): A review. Aquaculture 2004, 238, 221–238. [Google Scholar] [CrossRef]
- Iglesias, J.; Fuentes, L. Octopus vulgaris. Paralarval Culture. In Cephalopod Culture; Iglesias, J., Fuentes, L., Villanueva, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 427–450. [Google Scholar]
- Okumura, S.; Kurihara, A.; Iwamoto, A.; Takeuchi, T. Improved survival and growth in Octopus vulgaris paralarvae by feeding large type Artemia and Pacific sandeel, Ammodytes personatus: Improved survival and growth of common octopus paralarvae. Aquaculture 2005, 244, 147–157. [Google Scholar] [CrossRef]
- Márquez, L.; Quintana, D.; Almansa, E.; Navas, J.I. Effects of visual conditions and prey density on feeding kinetics of paralarvae of Octopus vulgaris from a laboratory spawning. J. Molluscan Stud. 2007, 73, 117–121. [Google Scholar] [CrossRef]
- Sykes, A.V.; Domingues, P.M.; Márquez, L.; Andrade, J.P. The effects of tank colours on the growth and survival of cuttlefish (Sepia officinalis, Linnaeus 1758) hatchlings and juveniles. Aquac. Res. 2011, 42, 441–449. [Google Scholar] [CrossRef]
- Fernández-López, A.; Roo, F.J.; Socorro, J.; Hernández-Cruz, M.C.; Férnandez-Palacios, H.; Izquierdo, M.S. Crecimiento y Supervivencia de Paralarvas de Octopus vulgaris Cultivadas bajo Diferentes Intensidades de Luz. In Proceedings of the X Congreso Nacional de Acuicultura, Valencia, Spain, 17–21 October 2005; Sociedad Española de Acuicultura: Valencia, Spain, 2005; pp. 374–375. [Google Scholar]
- Shashar, N.; Sabbah, S.; Cronin, T.W. Transmission of linearly polarized light in seawater: Implications for polarization signaling. J. Exp. Biol. 2004, 207, 3619–3628. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, B.; Dera, J. Introduction: Absorption of Sunlight in the Ocean. In Light Absorption in Sea Water; Laurence, A.M., Kevin, H., Eds.; Springer: New York, NY, USA, 2007; Volume 33, pp. 1–10. [Google Scholar]
- Marshall, J.; Cronin, T. Polarization visión. Curr. Biol. 2011, 21, R101–R105. [Google Scholar] [CrossRef] [PubMed]
- Cartron, L.; Josef, N.; Lerner, A.; Mc Cusker, S.D.; Darmaillacq, A.-S.; Dickel, L.; Shashar, N. Polarization vision can improve object detection in turbid waters by cuttlefish. J. Exp. Mar. Biol. Ecol. 2013, 447, 80–85. [Google Scholar] [CrossRef]
- Shashar, N.; Hanlon, R.T.; Petz, A.D.M. Polarization vision helps detect transparent prey. Nature 1998, 393, 222–223. [Google Scholar] [CrossRef]
- Moody, M.F.; Parriss, J.R. The discrimination of polarized light by Octopus: A behavioural and morphological study. Zeitschrift Für Vergleichende Physiologie 1961, 44, 268–291. [Google Scholar] [CrossRef]
- Shashar, N.; Cronin, T.W. Polarization contrast vision in Octopus. J. Exp. Biol. 1996, 199, 999–1004. [Google Scholar] [PubMed]
- Villanueva, R.; Norman, M.D. Biology of the planktonic stages of benthic octopuses. Oceanogr. Mar. Biol. Annu. Rev. 2008, 46, 105–202. [Google Scholar]
- Villanueva, R.; Nozais, C.; von Boletzky, S. Swimming behaviour and food searching in planktonic Octopus vulgaris Cuvier from hatching to settlement. J. Exp. Mar. Biol. Ecol. 1997, 208, 169–184. [Google Scholar] [CrossRef]
- Iglesias, J.; Sánchez, F.J.; Bersano, J.G.F.; Carrasco, J.F.; Dhont, J.; Fuentes, L.; Linares, F.; Muñoz, J.L.; Okumura, S.; Roo, J.; et al. Rearing of Octopus vulgaris paralarvae: Present status, bottlenecks and trends. Aquaculture 2007, 266, 1–15. [Google Scholar] [CrossRef]
- Lavens, P.; Sorgeloos, P. Manual on the Production and Use of Live Food for Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 1996; Volume 361, p. 295. [Google Scholar]
- Olmos-Pérez, L.; Roura, Á.; Pierce, G.J.; Boyer, S.; González, Á.F. Diet composition and variability of wild Octopus vulgaris and Alloteuthis media (Cephalopoda) paralarvae: A metagenomic approach. Front. Physiol. 2017, 8, 321. [Google Scholar] [CrossRef] [PubMed]
- Roura, Á.; González, Á.F.; Pascual, S.; Guerra, Á. A molecular approach to identifying the prey of cephalopod paralarvae. ICES J. Mar. Sci. 2010, 67, 1408–1412. [Google Scholar] [CrossRef]
- Roura, Á. Ecología de Paralarvas Planctónicas de Cefalópodos en Áreas de Afloramiento Costero. Ecology of Planktonic Cephalopod Paralarvae in Coastal Upwelling Systems. Ph.D. Thesis, Universidad de Vigo, Vigo, Spain, 2013; p. 219. [Google Scholar]
- Lourenço, S.; Roura, Á.; Fernández-Reiriz, M.S.; Narciso, L.; González, A. Feeding relationship between Octopus vulgaris (Cuvier, 1797) early life-cycle stages and their prey in the western Iberian upwelling system: Correlation of reciprocal lipid and fatty acid contents. Front. Physiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Boletzky, S.V.; Hanlon, R.T. A review of the laboratory maintenance, rearing and culture of cephalopod molluscs. Mem. Natl. Mus. Vic. 1983, 44, 147–187. [Google Scholar] [CrossRef]
- Roura, Á.; González, Á.; Redd, K.; Guerra, Á. Molecular prey identification in wild Octopus vulgaris paralarvae. Mar. Biol. 2012, 159, 1335–1345. [Google Scholar] [CrossRef] [Green Version]
- Mangold, K. Octopus vulgaris. In Cephalopod Life Cycles Vol. I: Species Acounts; Boyle, P.R., Ed.; Academic Press: London, UK, 1983; p. 475. [Google Scholar]
- Otero, J.; Álvarez-Salgado, X.A.; González, A.F.; Gilcoto, M.; Guerra, A. High-frequency coastal upwelling events influence Octopus vulgaris larval dynamics on the NW Iberian shelf. Mar. Ecol.-Prog. Ser. 2009, 386, 123–132. [Google Scholar] [CrossRef]
- Roura, Á.; Antón Álvarez-Salgado, X.; González, Á.F.; Gregori, M.; Rosón, G.; Otero, J.; Guerra, Á. Life strategies of cephalopod paralarvae in a coastal upwelling system (NW Iberian Peninsula): Insights from zooplankton community and spatio-temporal analyses. Fish. Oceanogr. 2016, 25, 241–258. [Google Scholar] [CrossRef]
- Kiefer, D.; Strickland, J.D.H. A comparative study of photosynthesis in seawater samples incubated under two types of light attenuator. Limnol. Oceanogr. 1970, 15, 408–412. [Google Scholar] [CrossRef]
- Almansa, E.; (Spanish Institute of Oceanography, Santa Cruz de Tenerife, Spain). Personal communication, 2012.
- Vidal, E.A.G.; Dimarco, F.P.; Wormuth, J.H.; Lee, P.G. Optimizing rearing conditions of hatchling loliginid squid. Mar. Biol. 2002, 140, 117–127. [Google Scholar]
- Hulet, W.H.; Villoch, M.R.; Hixon, R.F.; Hanlon, R.T. Fin damage in capture and reared squids. Lab. Anim. Sci. 1979, 29, 528–533. [Google Scholar] [PubMed]
- Sykes, A.V.; Quintana, D.; Andrade, J.P. The effects of light intensity on growth and survival of cuttlefish (Sepia officinalis, Linnaeus 1758) hatchlings and juveniles. Aquac. Res. 2014, 45, 2032–2040. [Google Scholar] [CrossRef]
- Sykes, A.V.; Gonçalves, R.A.; Andrade, J.P. Early weaning of cuttlefish (Sepia officinalis, L.) with frozen grass shrimp (Palaemonetes varians) from the first day after hatching. Aquac. Res. 2013, 44, 1815–1823. [Google Scholar] [CrossRef]
- Shashar, N.; Hagan, R.; Boal, J.G.; Hanlon, R.T. Cuttlefish use polarization sensitivity in predation on silvery fish. Vis. Res. 2000, 40, 71–75. [Google Scholar] [CrossRef]
- Nanton, D.A.; Castell, J.D. The effects of dietary fatty acids on the fatty acid composition of the harpacticoid copepod, Tisbe sp., for use as a live food for marine fish larvae. Aquaculture 1998, 163, 251–261. [Google Scholar] [CrossRef]
- Støttrup, J.G. Production and Nutritional Value of Copepods. In Live Feeds in Marine Aquaculture; Støttrup, J.G., McEvoy, L., Eds.; Blackwell Science Ltd.: Bodmin, Cornwall, UK, 2007; pp. 145–205. [Google Scholar]
- Repolho, T.; Baptista, M.; Pimentel, M.S.; Dionisio, G.; Trubenbach, K.; Lopes, V.M.; Lopes, A.R.; Calado, R.; Diniz, M.; Rosa, R. Developmental and physiological challenges of octopus (Octopus vulgaris) early life stages under ocean warming. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2014, 184, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Fuentes, L.; Sánchez, J.; Otero, J.J.; Moxica, C.; Lago, M.J. First feeding of Octopus vulgaris Cuvier, 1797 paralarvae using Artemia: Effect of prey size, prey density and feeding frequency. Aquaculture 2006, 261, 817–822. [Google Scholar] [CrossRef]
- Uriarte, I.; Hernández, J.; Dörner, J.; Paschke, K.; Farías, A.; Crovetto, E.; Rosas, C. Rearing and growth of the octopus Robsonella fontaniana (Cephalopoda: Octopodidae) from planktonic hatchlings to benthic juveniles. Biol. Bull. 2010, 218, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Franco-Santos, R.M.; Perales-Raya, C.; Almansa, E.; De Troch, M.; Garrido, D. Beak microstructure analysis as a tool to identify potential rearing stress for Octopus vulgaris paralarvae. Aquac. Res. 2016, 47, 3001–3015. [Google Scholar] [CrossRef]
- Oh, Y.K.; Seol, E.; Kim, M.S.; Park, S. Photoproduction of hydrogen from acetate by a chemoheterotrophic bacterium Rhodopseudomonas palustris P4. Int. J. Hydrog. Energy 2004, 29, 1115–1121. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice-Hall Inc.: Upper Saddle River, NT, USA, 1999. [Google Scholar]
- Fowler, J.; Cohen, L.; Jarvis, P. Practical Statistics for Field Biology; Wiley: New York, NY, USA, 1998; p. 256. [Google Scholar]
| Artemia sp. | Tisbe sp. | (Two-Way ANOVA) | F Values and Degrees Freedom | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Light | P | UP | P | UP | Light | Prey | Interaction | Light | Prey | Interaction |
| Trial 1 | 35.3 ± 2.3 | 32.0 ± 2.0 | 20.7 ± 4.2 | 19.0 ± 3.0 | - | * | - | F: 2.1; d.f. 1 | F: 64.4; d.f. 1 | F: 0.2; d.f. 1 |
| Trial 2 | 30.0 ± 2.0 | 27.3 ± 8.1 | 10.7 ± 2.3 | 11.3 ± 2.3 | - | * | - | F: 0.6; d.f. 1 | F: 43.6; d.f. 1 | F: 0.3; d.f. 1 |
| Control | Blue Filter | Focused Light | |
|---|---|---|---|
| VML (mm) | 1.63 ± 0.11 | 1.58 ± 0.07 | 1.70 ± 0.07 |
| SGR VML (%/day) | 0.74 ± 0.49 | 0.50 ± 0.33 | 1.01 ± 0.28 |
| DML (mm) | 2.17 ± 0.13 | 2.10 ± 0.12 | 2.23 ± 0.12 |
| SGR DML (%/day) | 0.89 ± 0.45 | 0.68 ± 0.41 | 1.09 ± 0.38 |
| Dry weight (mg) | 0.45 ± 0.08 | 0.41 ± 0.10 | 0.56 ± 0.15 |
| SGR weight (%/day) | 4.92 ± 1.42 | 4.29 ± 1.74 | 6.44 ± 1.84 |
| Survival rate (%) | 66.6 ± 11.7 | 60.4 ± 9.0 | 56.1 ± 11.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido, D.; Reis, D.B.; Orol, D.; Gonçalves, R.A.; Martín, M.V.; Sykes, A.V.; Rodríguez, C.; Felipe, B.C.; Zheng, X.; Lagos, L.; et al. Preliminary Results on Light Conditions Manipulation in Octopus vulgaris (Cuvier, 1797) Paralarval Rearing. Fishes 2017, 2, 21. https://doi.org/10.3390/fishes2040021
Garrido D, Reis DB, Orol D, Gonçalves RA, Martín MV, Sykes AV, Rodríguez C, Felipe BC, Zheng X, Lagos L, et al. Preliminary Results on Light Conditions Manipulation in Octopus vulgaris (Cuvier, 1797) Paralarval Rearing. Fishes. 2017; 2(4):21. https://doi.org/10.3390/fishes2040021
Chicago/Turabian StyleGarrido, Diego, Diana B. Reis, Diego Orol, Rui A. Gonçalves, M. Virginia Martín, António V. Sykes, Covadonga Rodríguez, Beatriz C. Felipe, Xiaodong Zheng, Luis Lagos, and et al. 2017. "Preliminary Results on Light Conditions Manipulation in Octopus vulgaris (Cuvier, 1797) Paralarval Rearing" Fishes 2, no. 4: 21. https://doi.org/10.3390/fishes2040021






