Exploring the Response of the Japanese Sardine (Sardinops melanostictus) Stock-Recruitment Relationship to Environmental Changes under Different Structural Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.1.1. Fishery Data
2.1.2. Environmental Factors
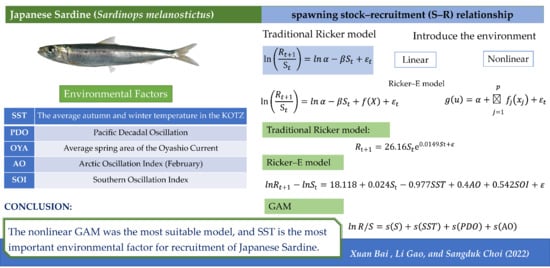
2.2. Model Building Process
2.2.1. Ricker and Ricker-E Model
2.2.2. GAM Model
2.3. Selection Criteria and Validation of Models
3. Results
3.1. Screening of Environmental Factors
3.2. Traditional Ricker Model
3.3. Results of the Ricker Environment Extension Model
3.4. Analysis Results of GAM
3.4.1. Single Factor Analysis of the GAM
3.4.2. Multifactor Analysis
3.5. Fitting Verification of the Optimal Model
4. Discussion
4.1. S-R Model
4.2. The Effect of the Environment on Recruitment
4.2.1. Kuroshio Oyashio Regional
4.2.2. Large-Scale Climatic Patterns
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brander, K. Impacts of climate change on fisheries. J. Mar. Syst. 2010, 79, 389–402. [Google Scholar] [CrossRef]
- Alheit, J. Consequences of regime shifts for marine food webs. Int. J. Earth Sci. 2009, 98, 261–268. [Google Scholar] [CrossRef]
- Kawasaki, T. Recovery and collapse of the Far Eastern sardine. Fish. Oceanogr. 1993, 2, 244–253. [Google Scholar] [CrossRef]
- Alheit, J.; Pohlmann, T.; Casini, M.; Greve, W.; Hinrichs, R.; Mathis, M.; O’Driscoll, K.; Vorberg, R.; Wagner, C. Climate variability drives anchovies and sardines into the North and Baltic Seas. Prog. Oceanogr. 2012, 96, 128–139. [Google Scholar] [CrossRef]
- Barange, M.; Bernal, M.; Cercole, M.C.; Cubillos, L.A.; Daskalov, G.M.; Cunningham, C.L.; de Oliveira, J.A.; Dickey-Collas, M.; Gaughan, D.J.; Hill, K. Current trends in the assessment and management of stocks. In Climate Change and Small Pelagic Fish; Cambridge University Press: Cambridge, UK, 2009; pp. 191–255. [Google Scholar] [CrossRef]
- Allain, G.; Petitgas, P.; Lazure, P. The influence of mesoscale ocean processes on anchovy (Engraulis encrasicolus) recruitment in the Bay of Biscay estimated with a three-dimensional hydrodynamic mode. Fish. Oceanogr. 2001, 10, 151–163. [Google Scholar] [CrossRef]
- Zhao, X.; Hamre, J.; Li, F.; Jin, X.; Tang, Q. Recruitment, sustainable yield and possible ecological consequences of the sharp decline of the anchovy (Engraulis japonicus) stock in the Yellow Sea in the 1990s. Fish. Oceanogr. 2003, 12, 495–501. [Google Scholar] [CrossRef]
- Takasuka, A.; Oozeki, Y.; Kimura, R.; Kubota, H.; Aoki, I. Growth-selective predation hypothesis revisited for larval anchovy in offshore waters: Cannibalism by juveniles versus predation by skipjack tunas. Mar. Ecol. Prog. Ser. 2004, 278, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Checkley, D.M., Jr.; Bakun, A.; Barange, M.A.; Castro, L.R.; Fréon, P.; Guevara-Carrasco, R.; Herrick, S.F., Jr.; MacCall, A.D.; Ommer, R.; Oozeki, Y. Climate Change and Small Pelagic Fish. Cambridge University Press: Cambridge, UK, 2009; pp. 999–1000. [Google Scholar] [CrossRef]
- Funamoto, T.; Aoki, I. Reproductive ecology of Japanese anchovy off the Pacific coast of eastern Honshu, Japan. J. Fish Biol. 2002, 60, 154–169. [Google Scholar] [CrossRef]
- Silva, A. Morphometric variation among sardine (Sardina pilchardus) populations from the northeastern Atlantic and the western Mediterranean. ICES J. Mar. Sci. 2003, 60, 1352–1360. [Google Scholar] [CrossRef]
- Ganias, K.; Somarakis, S.; Machias, A.; Theodorou, A. Evaluation of spawning frequency in a Mediterranean sardine population (Sardina pilchardus sardina). Mar. Biol. 2003, 142, 1169–1179. [Google Scholar] [CrossRef]
- Miranda, A.; Cal, R.M.; Iglesias, J. Effect of temperature on the development of eggs and larvae of sardine Sardina pilchardus Walbaum in captivity. J. Exp. Mar. Biol. Ecol. 1990, 140, 69–77. [Google Scholar] [CrossRef]
- Santos, M.B.; González-Quirós, R.; Riveiro, I.; Cabanas, J.M.; Porteiro, C.; Pierce, G.J. Cycles, trends, and residual variation in the Iberian sardine (Sardina pilchardus) recruitment series and their relationship with the environment. ICES J. Mar. Sci. 2012, 69, 739–750. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, H. Age and growth of Japanese sardine Sardinops melanostictus in Tosa Bay, southwestern Japan during a period of declining stock size. Fish. Sci. 2003, 69, 745–754. [Google Scholar] [CrossRef] [Green Version]
- Ricker, W.E. Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Board Can. 1975, 191, 1–382. [Google Scholar] [CrossRef]
- Lee, H.-H.; Maunder, M.N.; Piner, K.R.; Methot, R.D. Can steepness of the stock-recruitment relationship be estimated in fishery stock assessment models? Fish. Res. 2012, 125, 254–261. [Google Scholar] [CrossRef]
- Nishikawa, H. Relationship between recruitment of Japanese sardine (Sardinops melanostictus) and environment of larval habitat in the low-stock period (1995–2010). Fish. Oceanogr. 2019, 28, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Wada, T.; Jacobson, L.D. Regimes and stock-recruitment relationships in Japanese sardine (Sardinops melanostictus), 1951–1995. Can. J. Fish. Aquat. Sci. 1998, 55, 2455–2463. [Google Scholar] [CrossRef]
- Ganias, K. Linking sardine spawning dynamics to environmental variability. Estuar. Coast. Shelf Sci. 2009, 84, 402–408. [Google Scholar] [CrossRef]
- Takasuka, A.; Oozeki, Y.; Aoki, I. Optimal growth temperature hypothesis: Why do anchovy flourish and sardine collapse or vice versa under the same ocean regime? Can. J. Fish. Aquat. Sci. 2007, 64, 768–776. [Google Scholar] [CrossRef]
- Grip, K.; Blomqvist, S. Marine nature conservation and conflicts with fisheries. Ambio 2020, 49, 1328–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salinger, M. A brief introduction to the issue of climate and marine fisheries. Clim. Chang. 2013, 119, 23–35. [Google Scholar] [CrossRef]
- Sakuramoto, K.; Shimoyama, S.; Suzuki, N. Relationships between environmental conditions and fluctuations in the recruitment of Japanese sardine, Sardinops melanostictus, in the northwestern Pacific. Bull. Jpn. Soc. Fish. Oceanogr. 2010, 74, 88–97. [Google Scholar]
- McClatchie, S.; Goericke, R.; Auad, G.; Hill, K. Re-assessment of the stock-recruit and temperature-recruit relationships for Pacific sardine (Sardinops sagax). Can. J. Fish. Aquat. Sci. 2010, 67, 1782–1790. [Google Scholar] [CrossRef]
- Lindegren, M.; Checkley, D.M., Jr. Temperature dependence of Pacific sardine (Sardinops sagax) recruitment in the California Current Ecosystem revisited and revised. Can. J. Fish. Aquat. Sci. 2013, 70, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Daskalov, G.; Boyer, D.; Roux, J. Relating sardine Sardinops sagax abundance to environmental indices in northern Benguela. Prog. Oceanogr. 2003, 59, 257–274. [Google Scholar] [CrossRef]
- Nishikawa, H.; Yasuda, I. Japanese sardine (Sardinops melanostictus) mortality in relation to the winter mixed layer depth in the Kuroshio Extension region. Fish. Oceanogr. 2008, 17, 411–420. [Google Scholar] [CrossRef]
- Nishikawa, H.; Yasuda, I.; Itoh, S. Impact of winter-to-spring environmental variability along the Kuroshio jet on the recruitment of Japanese sardine (Sardinops melanostictus). Fish. Oceanogr. 2011, 20, 570–582. [Google Scholar] [CrossRef]
- Nakayama, S.I.; Takasuka, A.; Ichinokawa, M.; Okamura, H. Climate change and interspecific interactions drive species alternations between anchovy and sardine in the western North Pacific: Detection of causality by convergent cross mapping. Fish. Oceanogr. 2018, 27, 312–322. [Google Scholar] [CrossRef]
- Asia-Pacific Data-Research Center of the IPRC. Available online: http://apdrc.soest.hawaii.edu/ (accessed on 12 January 2022).
- Japan Meteorological Agency. Available online: https://www.data.jma.go.jp/gmd/kaiyou/data/shindan/b_2/oyashio_exp/oyashio_exp.html (accessed on 25 October 2021).
- NOAA National Centers for Environmental Information. Available online: https://www.ncdc.noaa.gov (accessed on 25 October 2021).
- Yatsu, A.; Watanabe, T.; Ishida, M.; Sugisaki, H.; Jacobson, L.D. Environmental effects on recruitment and productivity of Japanese sardine Sardinops melanostictus and chub mackerel Scomber japonicus with recommendations for management. Fish. Oceanogr. 2005, 14, 263–278. [Google Scholar] [CrossRef]
- Brander, K.; Mohn, R. Effect of the North Atlantic Oscillation on recruitment of Atlantic cod (Gadus morhua). Can. J. Fish. Aquat. Sci. 2004, 61, 1558–1564. [Google Scholar] [CrossRef]
- Watanabe, Y.; Zenitani, H.; Kimura, R. Population decline off the Japanese sardine Sardinops melanostictus owing to recruitment failures. Can. J. Fish. Aquat. Sci. 1995, 52, 1609–1616. [Google Scholar] [CrossRef]
- Cornic, M.; Rooker, J.R. Influence of oceanographic conditions on the distribution and abundance of blackfin tuna (Thunnus atlanticus) larvae in the Gulf of Mexico. Fish. Res. 2018, 201, 1–10. [Google Scholar] [CrossRef]
- Hilborn, R.; Walters, C.J. Quantitative Fisheries Stock Assessment: Choice, Dynamics and Uncertainty; Springer: New York, NY, USA, 1992; Volume 2, pp. 177–178. [Google Scholar]
- Ricker, W.E. Stock and recruitment. J. Fish. Res. Board Can. 1954, 11, 559–623. [Google Scholar] [CrossRef]
- Beverton, R.J.; Holt, S.J. On the dynamics of exploited fish populations. Rev. Fish Biol. Fish. 1994, 4, 259–260. [Google Scholar] [CrossRef]
- Schweight, J.F.; Noakes, D.J. Forecasting pacific herring (Cuplea arengus pallasi) recruitment from spawner abundance and environmental information. In Proceedings of the International Herring Symposium, Anchorage, AK, USA, 23–25 October 1990; pp. 373–387. [Google Scholar]
- Williams, E.H.; Quinn, T.J., II. Pacific herring, Clupea pallasi, recruitment in the Bering Sea and north-east Pacific Ocean, II: Relationships to environmental variables and implications for forecasting. Fish. Oceanogr. 2000, 9, 300–315. [Google Scholar] [CrossRef]
- Solow, A.R. Fisheries recruitment and the North Atlantic oscillation. Fish. Res. 2002, 54, 295–297. [Google Scholar] [CrossRef]
- Arregui, I.; Arrizabalaga, H.; Kirby, D.S.; Martín-González, J.M. Stock-environment-recruitment models for North Atlantic albacore (Thunnus alalunga). Fish. Oceanogr. 2006, 15, 402–412. [Google Scholar] [CrossRef]
- Sakuramoto, K. Does the Ricker or Beverton and Holt type of stock-recruitment relationship truly exist? Fish. Sci. 2005, 71, 577–592. [Google Scholar] [CrossRef]
- Beck, N.; Jackman, S. Beyond linearity by default: Generalized additive models. Am. J. Political Sci. 1998, 42, 596–627. [Google Scholar] [CrossRef]
- Coupé, C. Modeling linguistic variables with regression models: Addressing non-Gaussian distributions, non-independent observations, and non-linear predictors with random effects and generalized additive models for location, scale, and shape. Front. Psychol. 2018, 9, 513. [Google Scholar] [CrossRef] [Green Version]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Pinho, L.G.B.; Nobre, J.S.; Singer, J.M. Cook’s distance for generalized linear mixed models. Comput. Stat. Data Anal. 2015, 82, 126–136. [Google Scholar] [CrossRef]
- Fogarty, M.J. Recruitment in randomly varying environments. ICES J. Mar. Sci. 1993, 50, 247–260. [Google Scholar] [CrossRef]
- Shimoyama, S.; Sakuramoto, K.; Suzuki, N. Proposal for stock-recruitment relationship for Japanese sardine Sardinops melanostictus in North-western Pacific. Fish. Sci. 2007, 73, 1035–1041. [Google Scholar] [CrossRef]
- Myers, R.A. Stock and recruitment: Generalizations about maximum reproductive rate, density dependence, and variability using meta-analytic approaches. ICES J. Mar. Sci. 2001, 58, 937–951. [Google Scholar] [CrossRef] [Green Version]
- Yamada, H.; Takagi, N.; Nishimura, D. Recruitment abundance index of Pacific bluefin tuna using fisheries data on juveniles. Fish. Sci. 2006, 72, 333–341. [Google Scholar] [CrossRef]
- Solanki, H.; Bhatpuria, D.; Chauhan, P. Applications of generalized additive model (GAM) to satellite-derived variables and fishery data for prediction of fishery resources distributions in the Arabian Sea. Geocarto Int. 2017, 32, 30–43. [Google Scholar] [CrossRef]
- Sparholt, H. Causal correlation between recruitment and spawning stock size of central Baltic cod? ICES J. Mar. Sci. 1996, 53, 771–779. [Google Scholar] [CrossRef] [Green Version]
- Langley, A.; Briand, K.; Kirby, D.S.; Murtugudde, R. Influence of oceanographic variability on recruitment of yellowfin tuna (Thunnus albacares) in the western and central Pacific Ocean. Can. J. Fish. Aquat. Sci. 2009, 66, 1462–1477. [Google Scholar] [CrossRef]
- Chen, D.; Irvine, J. A semiparametric model to examine stock recruitment relationships incorporating environmental data. Can. J. Fish. Aquat. Sci. 2001, 58, 1178–1186. [Google Scholar] [CrossRef]
- Deyle, E.R.; Fogarty, M.; Hsieh, C.-H.; Kaufman, L.; MacCall, A.D.; Munch, S.B.; Perretti, C.T.; Ye, H.; Sugihara, G. Predicting climate effects on Pacific sardine. Proc. Natl. Acad. Sci. USA 2013, 110, 6430–6435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardinale, M.; Arrhenius, F. The influence of stock structure and environmental conditions on the recruitment process of Baltic cod estimated using a generalized additive model. Can. J. Fish. Aquat. Sci. 2000, 57, 2402–2409. [Google Scholar] [CrossRef]
- Megrey, B.A.; Lee, Y.-W.; Macklin, S.A. Comparative analysis of statistical tools to identify recruitment-environment relationships and forecast recruitment strength. ICES J. Mar. Sci. 2005, 62, 1256–1269. [Google Scholar] [CrossRef]
- Shih, C.-L.; Chen, Y.-H.; Chien-Chung, H. Modeling the effect of environmental factors on the ricker stock-recruitment relationship for North Pacific albacore using generalized additive models. TAO Terr. Atmos. Ocean. Sci. 2014, 25, 581. [Google Scholar] [CrossRef] [Green Version]
- Murase, H.; Nagashima, H.; Yonezaki, S.; Matsukura, R.; Kitakado, T. Application of a generalized additive model (GAM) to reveal relationships between environmental factors and distributions of pelagic fish and krill: A case study in Sendai Bay, Japan. ICES J. Mar. Sci. 2009, 66, 1417–1424. [Google Scholar] [CrossRef] [Green Version]
- Báez, J.; Santamaría, M.; García, A.; González, J.; Hernández, E.; Ferri-Yáñez, F. Influence of the arctic oscillations on the sardine off Northwest Africa during the period 1976–1996. Vie Et Milieu. 2019, 69, 71–77. [Google Scholar]
- Zwolinski, J.P.; Demer, D.A. Environmental and parental control of Pacific sardine (Sardinops sagax) recruitment. ICES J. Mar. Sci. 2014, 71, 2198–2207. [Google Scholar] [CrossRef] [Green Version]
- Chavez, F.P.; Ryan, J.; Lluch-Cota, S.E.; Ñiquen, C.M. From anchovies to sardines and back: Multidecadal change in the Pacific Ocean. Science 2003, 299, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Sakuramoto, K. A recruitment forecasting model for the Pacific stock of the Japanese sardine (Sardinops melanostictus) that does not assume density-dependent effects. Agri. Sci. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Jacobson, L.D.; MacCall, A.D. Erratum: Stock-recruitment models for Pacific sardine (Sardinops sagax). Can. J. Fish. Aquat. Sci. 1995, 52, 2062. [Google Scholar] [CrossRef]
- Yasuda, I.; Sugisaki, H.; Watanabe, Y.; MINOBE, S.S.; Oozeki, Y. Interdecadal variations in Japanese sardine and ocean/climate. Fish. Oceanogr. 1999, 8, 18–24. [Google Scholar] [CrossRef]
- Okunishi, T.; Ito, S.-i.; Hashioka, T.; Sakamoto, T.T.; Yoshie, N.; Sumata, H.; Yara, Y.; Okada, N.; Yamanaka, Y. Impacts of climate change on growth, migration and recruitment success of Japanese sardine (Sardinops melanostictus) in the western North Pacific. Clim. Chang. 2012, 115, 485–503. [Google Scholar] [CrossRef]
- Takasuka, A. Biological mechanisms underlying climate impacts on population dynamics of small pelagic fish. In Fish Population Dynamics, Monitoring and Management; Springer Nature: Tokyo, Japan, 2018; pp. 19–50. [Google Scholar] [CrossRef]
- Sunami, Y. Modeling of the variability in stock abundance of Japanese sardine from a viewpoint of its food density. J. Shimonoseki Univ. Fish. 1993, 41, 1–8. [Google Scholar]
- Bakun, A.; Broad, K. Environmental ‘loopholes’ and fish population dynamics: Comparative pattern recognition with focus on El Niño effects in the Pacific. Fish. Oceanogr. 2003, 12, 458–473. [Google Scholar] [CrossRef]
- Noto, M.; Yasuda, I. Population decline of the Japanese sardine, Sardinops melanostictus, in relation to sea surface temperature in the Kuroshio Extension. Can. J. Fish. Aquat. Sci. 1999, 56, 973–983. [Google Scholar] [CrossRef]
- Brett, J. Environmental factors and growth. In Fish Physiology: Bioenergetics and Growth; Academic Press: Cambridge, MA, USA, 1979; Volume 8, pp. 599–677. [Google Scholar] [CrossRef]
- Sakurai, Y. An overview of the Oyashio ecosystem. Deep Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 2526–2542. [Google Scholar] [CrossRef] [Green Version]
- Parrish, R.H. A Monterey sardine story. JB Phillips Hist. Fish. Rep. 2000, 1, 2–4. [Google Scholar]
- Hill, K.T.; Lo, N.C.; Macewicz, B.J.; Crone, P.R.; Felix-Uraga, R. Assessment of the Pacific Sardine Resource in 2009 for US Management in 2010: Executive Summary. 2009. Available online: https://repository.library.noaa.gov/view/noaa/3920 (accessed on 9 June 2022).
- Pitcher, T.; Hart, P. Fisheries Ecology; Croom Helm: London, UK, 1982. [Google Scholar]
- Douville, H. Stratospheric polar vortex influence on Northern Hemisphere winter climate variability. Geophys. Res. Lett. 2009, 36. [Google Scholar] [CrossRef] [Green Version]
- Báez, J.C.; Gimeno, L.; Gómez-Gesteira, M.; Ferri-Yáñez, F.; Real, R. Combined effects of the North Atlantic Oscillation and the Arctic Oscillation on sea surface temperature in the Alborán Sea. PLoS ONE 2013, 8, e62201. [Google Scholar] [CrossRef] [Green Version]
- Ohshimo, S.; Tanaka, H.; Hiyama, Y. Long-term stock assessment and growth changes of the Japanese sardine (Sardinops melanostictus) in the Sea of Japan and East China Sea from 1953 to 2006. Fish. Oceanogr. 2009, 18, 346–358. [Google Scholar] [CrossRef]
- Castro-Gutiérrez, J.; Cabrera-Castro, R.; Czerwinski, I.A.; Báez, J.C. Effect of climatic oscillations on small pelagic fisheries and its economic profit in the Gulf of Cadiz. Int. J. Biometeorol. 2022, 66, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Mata, M.; Montemayor-López, G.; Nevárez-Martínez, M. Modeling deterministic effects of age-structure, density-dependence, environmental forcing and fishing on the population dynamics of Sardinops sagax caerulens in the Gulf of California. Calif. Coop. Ocean. Fish. Investig. Rep. 1996, 37, 201–208. [Google Scholar]
- Lluch-Belda, D.; Magallón, F.J.; Schwartzlose, R.A. Large fluctuations in the sardine fishery in the Gulf of California: Possible causes. CalCOFI Rep. 1986, 27, 136–140. [Google Scholar]
- Sogawa, S.; Hidaka, K.; Kamimura, Y.; Takahashi, M.; Saito, H.; Okazaki, Y.; Shimizu, Y.; Setou, T. Environmental characteristics of spawning and nursery grounds of Japanese sardine and mackerels in the Kuroshio and Kuroshio Extension area. Fish. Oceanogr. 2019, 28, 454–467. [Google Scholar] [CrossRef]
- Patterson, K.; Cook, R.; Darby, C.; Gavaris, S.; Kell, L.; Lewy, P.; Mesnil, B.; Punt, A.; Restrepo, V.; Skagen, D.W. Estimating uncertainty in fish stock assessment and forecasting. Fish Fish. 2001, 2, 125–157. [Google Scholar] [CrossRef]
- Sun, M.; Li, Y.; Ren, Y.; Chen, Y. Rebuilding depleted fisheries towards BMSY under uncertainty: Harvest control rules outperform combined management measures. ICES J. Mar. Sci. 2021, 78, 2218–2232. [Google Scholar] [CrossRef]
- Cao, J.; Chen, Y.; Richards, R.A. Improving assessment of Pandalus stocks using a seasonal, size-structured assessment model with environmental variables. Part I: Model description and application. Can. J. Fish. Aquat. Sci. 2017, 74, 349–362. [Google Scholar] [CrossRef]
- Cao, J.; Chen, Y.; Richards, R.A. Improving assessment of Pandalus stocks using a seasonal, size-structured assessment model with environmental variables. Part II: Model evaluation and simulation. Can. J. Fish. Aquat. Sci. 2017, 74, 363–376. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.R.; Cao, J.; Shank, B.V.; Truesdell, S.B.; Mazur, M.D.; Xu, L.; Chen, Y. A model-based approach to incorporate environmental variability into assessment of a commercial fishery: A case study with the American lobster fishery in the Gulf of Maine and Georges Bank. ICES J. Mar. Sci. 2019, 76, 884–896. [Google Scholar] [CrossRef]
- Siple, M.C.; Koehn, L.E.; Johnson, K.F.; Punt, A.E.; Canales, T.M.; Carpi, P.; de Moor, C.L.; De Oliveira, J.A.; Gao, J.; Jacobsen, N.S. Considerations for management strategy evaluation for small pelagic fishes. Fish Fish. 2021, 22, 1167–1186. [Google Scholar] [CrossRef]









| Abbreviation | Meaning |
|---|---|
| ln(R/S) | Logarithm recruitment/spawning stock biomass |
| KOTZ | Kuroshio-Oyashio Transition Zone |
| SST | The average autumn and winter temperature in the KOTZ |
| PDO | Pacific Decadal Oscillation |
| OYA | Average spring area of the Oyashio Current |
| AO | Arctic Oscillation Index (February) |
| SOI | Southern Oscillation Index |
| S-R | Spawning stock-recruitment |
| Formula | Variable | Estimate | Standard Error | t Value | Pr(>|t|) | VIF | AIC | BIC | |
|---|---|---|---|---|---|---|---|---|---|
| ln(R/S) ~ S + SST | 22.087 | 5.149 | 4.290 | 0.0002 | *** | 90.00 | 96.20 | ||
| S | −0.024 | 0.004 | −5.640 | 0.0000 | *** | 1.51 | |||
| SST | −1.226 | 0.335 | −3.660 | 0.0009 | *** | 1.51 | |||
| ln(R/S) ~ S + SST + AO | Intercept | 17.293 | 5.645 | 3.060 | 0.0045 | ** | 88.50 | 96.30 | |
| S | −0.023 | 0.004 | −5.600 | 0.0000 | *** | 1.53 | |||
| SST | −0.918 | 0.366 | −2.510 | 0.0176 | * | 1.93 | |||
| AO | 0.283 | 0.157 | 1.800 | 0.0816 | . | 1.33 | |||
| ln(R/S) ~ S + SST + AO + SOI | Intercept | 18.118 | 5.287 | 3.430 | 0.0018 | ** | 84.60 | 94.00 | |
| S | −0.024 | 0.004 | −6.206 | 0.0000 | *** | 1.55 | |||
| SST | −0.977 | 0.343 | −2.847 | 0.0079 | ** | 1.94 | |||
| AO | 0.400 | 0.155 | 2.575 | 0.0152 | * | 1.49 | |||
| SOI | 0.542 | 0.231 | 2.346 | 0.0258 | * | 1.18 |
| Nvmax | RMSE | Required | MAE | RMSESD | RsquaredSD | MAESD |
|---|---|---|---|---|---|---|
| 1 | 0.931 | 0.686 | 0.747 | 0.334 | 0.338 | 0.277 |
| 2 | 0.789 | 0.610 | 0.653 | 0.365 | 0.323 | 0.292 |
| 3 | 0.935 | 0.653 | 0.781 | 0.299 | 0.282 | 0.264 |
| 4 | 0.749 | 0.705 | 0.617 | 0.264 | 0.297 | 0.235 |
| 5 | 0.806 | 0.641 | 0.668 | 0.331 | 0.295 | 0.286 |
| 6 | 0.820 | 0.656 | 0.687 | 0.299 | 0.285 | 0.263 |
| B.Smooth Terms | edf | Ref. df | F Value | p Value | GCV | Deviance Explained | Rsq.(adj) | AIC |
|---|---|---|---|---|---|---|---|---|
| s(S) | 4.87 | 4.99 | 18.9 | <2 × 10−16 *** | 0.848 | 76.4% | 0.724 | 94 |
| s(SST) | 5.98 | 6.67 | 6.32 | <2 × 10−16 *** | 1.484 | 61.7% | 0.536 | 114 |
| s(PDO) | 1 | 1 | 11.16 | <2 × 10−16 *** | 2.094 | 25.20% | 0.229 | 127 |
| s(OYA) | 5.95 | 6.62 | 2.16 | <2 × 10−16 *** | 2.487 | 35.8% | 0.221 | 132 |
| s(AO) | 1 | 1 | 9.60 | <2 × 10−16 *** | 2.167 | 22.5% | 0.202 | 128 |
| s(SOI) | 3.2 | 3.83 | 0.88 | <2 × 10−16 *** | 2.705 | 15.80% | 0.070 | 136 |
| Model | Formula | edf | t Value | p | adjR2 | Explained | AIC | BIC |
|---|---|---|---|---|---|---|---|---|
| GAM1 | ln(R/S) ~ s(S) | 32.2 | *** | 0.72 | 76.40% | 94.4 | 105.0 | |
| s(S) | 4.87 | *** | ||||||
| GAM2 | ln(R/S) ~ s(S) + s(SST) | 48.1 | *** | 0.88 | 91.90% | 70.6 | 91.8 | |
| s(S) | 4.91 | *** | ||||||
| s(SST) | 6.75 | *** | ||||||
| GAM3 | ln(R/S) ~ s(S) + s(SST) + s(PDO) | 50.9 | *** | 0.89 | 93.40% | 67.2 | 91.3 | |
| s(S) | 4.9 | *** | ||||||
| s(SST) | 6.72 | *** | ||||||
| s(PDO) | 1.83 | |||||||
| GAM4 | ln(R/S) ~ s(S) + s(SST) + s(PDO) + s(AO) | 58.6 | *** | 0.91 | 95.70% | 57.9 | 86.7 | |
| s(S) | 4.83 | *** | ||||||
| s(SST) | 6.87 | *** | ||||||
| s(PDO) | 2.30 | * | ||||||
| s(AO) | 2.49 | . |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Gao, L.; Choi, S. Exploring the Response of the Japanese Sardine (Sardinops melanostictus) Stock-Recruitment Relationship to Environmental Changes under Different Structural Models. Fishes 2022, 7, 276. https://doi.org/10.3390/fishes7050276
Bai X, Gao L, Choi S. Exploring the Response of the Japanese Sardine (Sardinops melanostictus) Stock-Recruitment Relationship to Environmental Changes under Different Structural Models. Fishes. 2022; 7(5):276. https://doi.org/10.3390/fishes7050276
Chicago/Turabian StyleBai, Xuan, Li Gao, and Sangduk Choi. 2022. "Exploring the Response of the Japanese Sardine (Sardinops melanostictus) Stock-Recruitment Relationship to Environmental Changes under Different Structural Models" Fishes 7, no. 5: 276. https://doi.org/10.3390/fishes7050276