Modeling Environmental Impacts of Intensive Shrimp Aquaculture: A Three-Dimensional Hydrodynamic Ecosystem Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Three-Dimensional Hydrodynamic Ecosystem Model
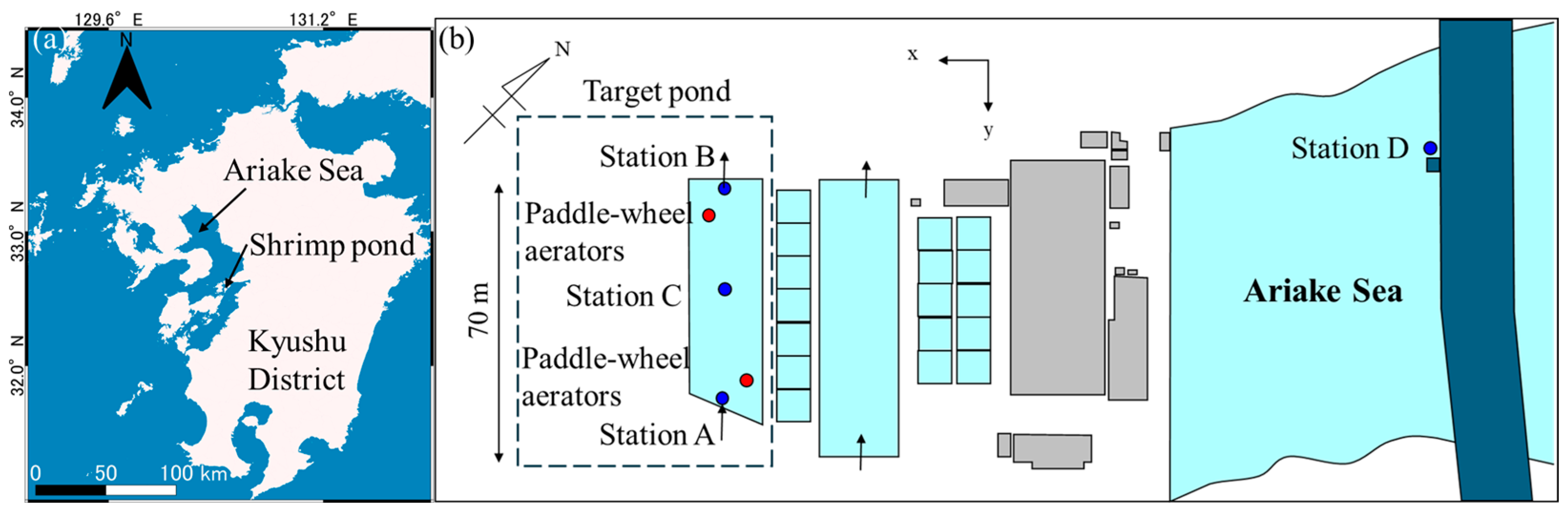
2.2. Case Study
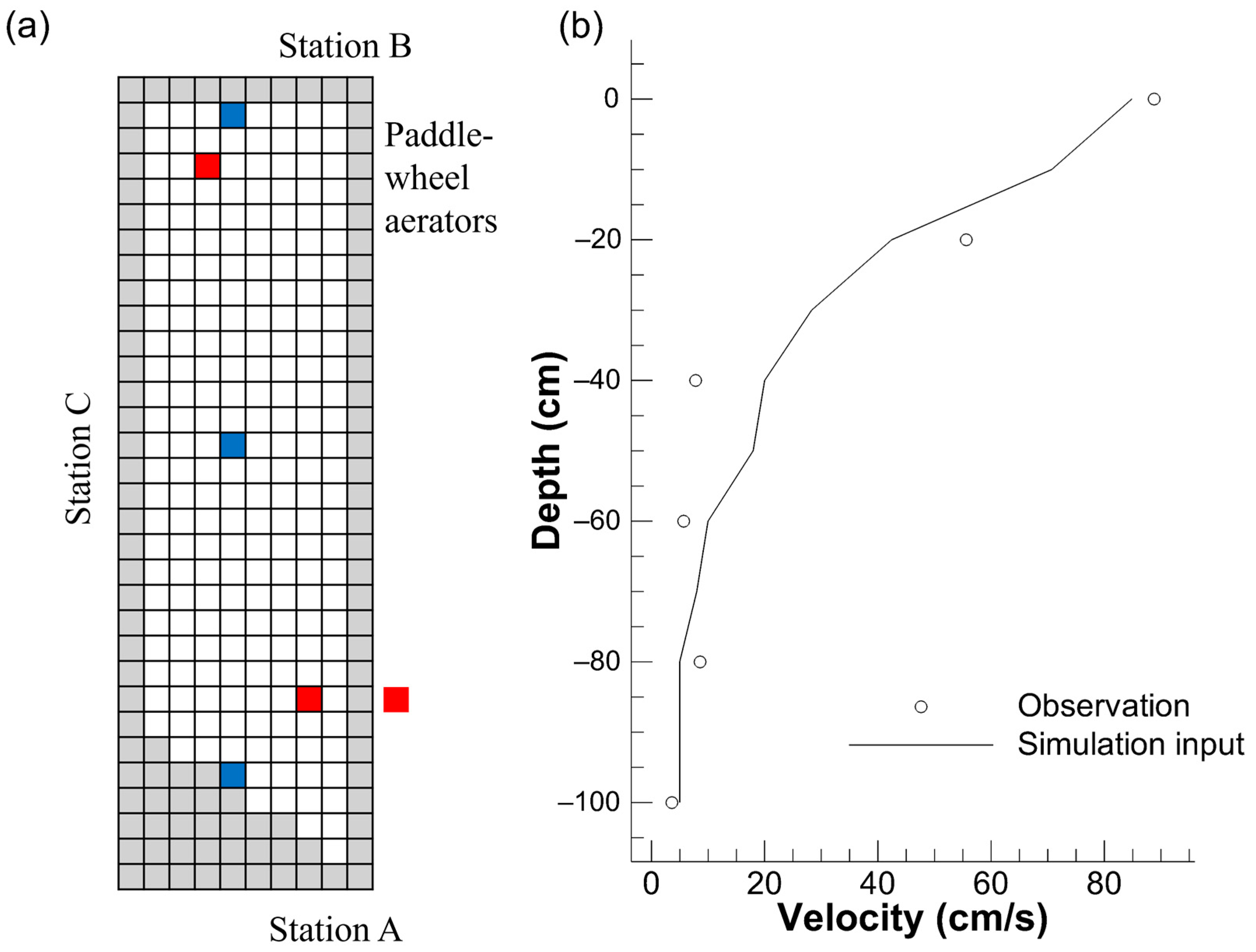
2.3. Computational Conditions
3. Results and Discussion
3.1. Physical Environment
3.2. Water Quality
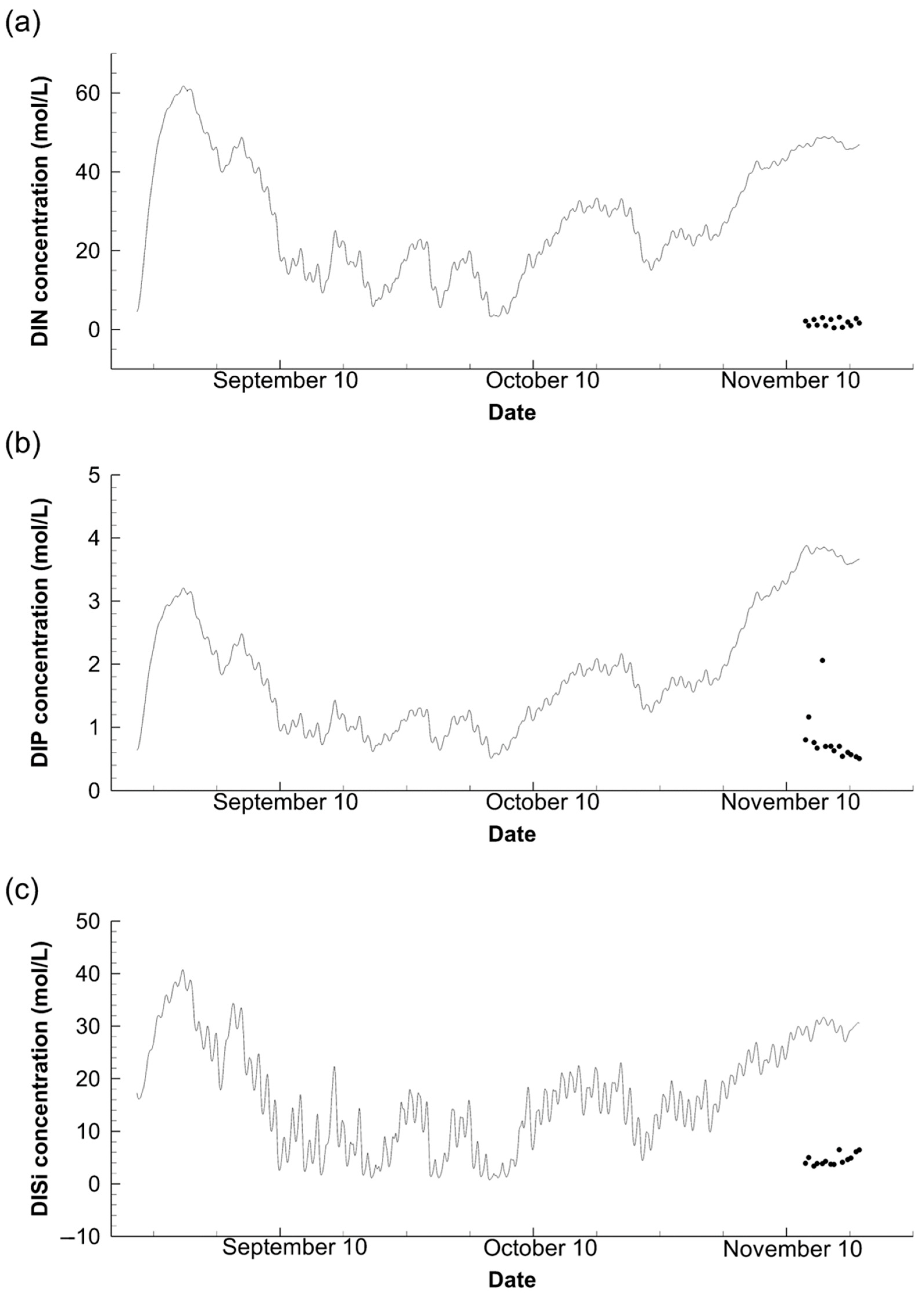
3.3. Nutrient Concentration
3.4. Sludge Accumulation
3.5. Future Research Priorities on Management
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steinmetz, G. This Mind-Bending Drone Footage Shows the Scale of the World’s Largest Shrimp Farm. Available online: https://businessinsider.com/video-shows-scale-of-the-world-largest-shrimp-farm-2017-2 (accessed on 27 January 2024).
- Ottinger, M.; Clauss, K.; Kuenzer, C. Large-Scale assessment of coastal aquaculture ponds with Sentinel-1 time series data. Remote Sens. 2017, 9, 440. [Google Scholar] [CrossRef]
- Shang, Y.C.; Leung, P.; Ling, B.H. Comparative economics of shrimp farming in Asia. Aquaculture 1998, 164, 183–200. [Google Scholar] [CrossRef]
- Páez-Osuna, F. The Environmental impact of shrimp aquaculture: Causes, effects, and mitigating alternatives. Environ. Manag. 2001, 28, 131–140. [Google Scholar] [CrossRef]
- Chevakidagarn, P.; Danteravanich, S. Environmental impact of white shrimp culture during 2012–2013 at Bandon Bay, Surat Thani Province: A case study investigating farm size. Agric. Nat. Resour. 2017, 51, 109–116. [Google Scholar] [CrossRef]
- Wang, J.K. Managing shrimp pond water to reduce discharge problems. Aquac. Eng. 1990, 9, 61–73. [Google Scholar] [CrossRef]
- Tacon, A.G.J. Nutritional studies in crustaceans and the problems of applying research findings to practical farming systems. Aquac. Nutr. 1996, 2, 165–174. [Google Scholar] [CrossRef]
- Burford, M.A.; Jackson, C.J.; Preston, N.P. Reducing nitrogen waste from shrimp farming: An integrated approach. In The New Wave: Proceedings of the Special Session on Sustainable Shrimp Culture; Browdy, C.L., Jory, D.E., Eds.; World Aquaculture Society: Baton Rouge, LA, USA, 2001; pp. 35–43. ISBN 978-1888807059. [Google Scholar]
- Jory, D.E.; Cabrera, T.R.; Dugger, D.M.; Fegan, D.; Lee, P.G.; Lawrence, L.; Jackson, C.J.; Mcintosh, R.P.; Castañeda, J.; International, B.; et al. A global review of shrimp feed management: Status and perspectives. Aquaculture 2001, 2001, 104–152. [Google Scholar]
- Hilborn, R.; Mangel, M. The Ecological Detective: Confronting Models with Data; Princeton University Press: Princeton, NJ, USA, 1997. [Google Scholar]
- Lorenzen, K.; Struve, J.; Cowan, V.J. Impact of farming intensity and water management on nitrogen dynamics in intensive pond culture: A mathematical model applied to Thai commercial shrimp farms. Aquac. Res. 1997, 28, 493–507. [Google Scholar] [CrossRef]
- Burford, M.A.; Costanzo, S.D.; Dennison, W.C.; Jackson, C.J.; Jones, A.B.; McKinnon, A.D.; Preston, N.P.; Trott, L.A. A synthesis of dominant ecological processes in intensive shrimp ponds and adjacent coastal environments in NE Australia. Mar. Pollut. Bull. 2003, 46, 1456–1469. [Google Scholar] [CrossRef]
- Burford, M.A.; Lorenzen, K. Modeling nitrogen dynamics in intensive shrimp ponds: The role of sediment remineralization. Aquaculture 2004, 229, 129–145. [Google Scholar] [CrossRef]
- Kitazawa, D.; Hakuta, K.; Yamayoshi, N.; Tabeta, S. Field measurement and modelling of the material cycle in the cultivation pond of penaeid shrimp Penaeus japonicus. In Proceedings of the ASME 2007 26th International Conference on Offshore Mechanics and Arctic Engineering (OMAE), San Diego, CA, USA, 10–15 June 2007; pp. 53–60. [Google Scholar] [CrossRef]
- Peterson, E.L.; Walker, M.B. Effect of speed on Taiwanese paddlewheel aeration. Aquac. Eng. 2002, 26, 129–147. [Google Scholar] [CrossRef]
- Kang, Y.H.; Lee, M.O.; Choi, S.D.; Sin, Y.S. 2-D Hydrodynamic model simulating paddlewheel-driven circulation in rectangular shrimp culture ponds. Aquaculture 2004, 231, 163–179. [Google Scholar] [CrossRef]
- Kittiwanich, J.; Songsangjinda, P.; Yamamoto, T.; Fukami, K.; Muanagyao, P. Modeling the effect of nitrogen input from feed on the nitrogen dynamics in an enclosed intensive culture pond of black tiger shrimp (Penaeus monodon). Coas. Mar. Sci. 2012, 35, 39–51. [Google Scholar]
- Poersch, L.H.; Milach, Â.M.; Cavalli, R.O.; Wasielesky, W.J.R.; Möller, O.; Castello, J.P. Use of a mathematical model to estimate the impact of shrimp pen culture at Patos Lagoon Estuary, Brazil. An. Acad. Bras. Ciênc. 2014, 86, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, F.S.; Ghorbani, R.; Hosseini, S.A.; Haghighi, F.P.; Saravi, H.N. Associations between shrimp farming and nitrogen dynamic: A model in the Caspian Sea. Aquaculture 2019, 510, 323–328. [Google Scholar] [CrossRef]
- Soriano, F.A.C.; Junquera, V.I.; Minakata, P.E.; Cano, O.M.; Paz, J.d.J.O.; Ramirez, J.C.A.; Willerer, A.O.M. Nitrogen dynamics model in zero water exchange, low salinity intensive ponds of white shrimp, Litopenaeus vannamei, at Colima, Mexico. Lat. Am. J. Aquat. Res. 2013, 41, 68–79. [Google Scholar] [CrossRef]
- Silfiana; Widowati; Putro, S.P.; Udjiani, T. Modeling of nitrogen transformation in an integrated multi-trophic aquaculture (IMTA). J. Phys. Conf. Ser. 2018, 983, 12122. [Google Scholar] [CrossRef]
- Widowati; Putro, S.P.; Silfiana. Stability analysis of the phytoplankton effect model on changes in nitrogen concentration on integrated multi-trophic aquaculture systems. J. Phys. Conf. Ser. 2018, 1025, 12088. [Google Scholar] [CrossRef]
- Araneda, M.E.; Hernández, J.M.; Gasca-Leyva, E.; Vela, M.A. Growth modelling including size heterogeneity: Application to the intensive culture of white shrimp (P. vannamei) in freshwater. Aquac. Eng. 2013, 56, 1–12. [Google Scholar] [CrossRef]
- de Melo Filho, M.E.S.; Owatari, M.S.; Mouriño, J.L.P.; Carciofi, B.A.M.; Soares, H.M. Empirical modeling of feed conversion in pacific white shrimp (Litopenaeus vannamei) growth. Ecol. Model. 2020, 437, 109291. [Google Scholar] [CrossRef]
- Dong, S.; Liu, D.; Zhu, B.; Yu, L.; Shan, H.; Wang, F. A dynamic energy budget model for kuruma shrimp Penaeus japonicus: Parameterization and application in integrated marine pond aquaculture. Animals 2022, 12, 1828. [Google Scholar] [CrossRef] [PubMed]
- Zarzar, C.A.; Fernandes, T.J.; Cardoso de Oliveira, I.R. Modeling the growth of pacific white shrimp (Litopenaeus vannamei) using the new Bayesian hierarchical approach based on correcting bias caused by incomplete or limited data. Ecol. Inform. 2023, 77, 102271. [Google Scholar] [CrossRef]
- Lin, Q.; Yang, W.; Zheng, C.; Lu, K.; Zheng, Z.; Wang, J.; Zhu, J. Deep-learning based approach for forecast of water quality in intensive shrimp culture ponds. Indian J. Fish. 2018, 65, 75–80. [Google Scholar] [CrossRef]
- Ma, Z.; Song, X.; Wan, R.; Gao, L.; Jiang, D. Artificial neural network modeling of the water quality in intensive Litopenaeus vannamei shrimp tanks. Aquaculture 2014, 433, 307–312. [Google Scholar] [CrossRef]
- Ahmad, T.; Boyd, C.E. Design and peroormance of paddle wheel aerators. Aquac. Eng. 1988, 7, 39–62. [Google Scholar] [CrossRef]
- Chatvijitkul, S.; Boyd, C.E.; Davis, D.A. Nitrogen, phosphorus, and carbon concentrations in some common aquaculture feeds. J. World Aquac. Soc. 2017, 49, 477–483. [Google Scholar] [CrossRef]
- Ginot, V.; Hervé, J.-C. Estimating the parameters of dissolved oxygen dynamics in shallow ponds. Ecol. Modell. 1994, 73, 169–187. [Google Scholar] [CrossRef]
- Marques, G.M.; Augustine, S.; Lika, K.; Pecquerie, L.; Domingos, T.; Kooijman, S.A.L.M. The AmP project: Comparing species on the basis of dynamic energy budget parameters. PLoS Comput. Biol. 2018, 14, e1006100. [Google Scholar] [CrossRef] [PubMed]
- Junda, M. Development of intensive shrimp farming, Litopenaeus vannamei in land-based ponds: Production and management. J. Phys. Conf. Ser. 2018, 1028, 012020. [Google Scholar] [CrossRef]
- Alfiansah, Y.R.; Hassenrück, C.; Kunzmann, A.; Taslihan, A.; Harder, J.; Gärdes, A. Bacterial abundance and community composition in pond water from shrimp aquaculture systems with different stocking densities. Front. Microbiol. 2018, 9, 2457. [Google Scholar] [CrossRef]
- Huang, Q.; Olenin, S.; Li, L.; Sun, S.; De Troch, M. Meiobenthos as food for farmed shrimps in the earthen ponds: Implications for sustainable feeding. Aquaculture 2020, 521, 735094. [Google Scholar] [CrossRef]
- Yuvanatemiya, V.; Boyd, C.E.; Thavipoke, P. Pond bottom management at commercial shrimp farms in Chantaburi Province, Thailand. J. World Aquac. Soc. 2011, 42, 618–632. [Google Scholar] [CrossRef]
- Shinji, J.; Nohara, S.; Yagi, N.; Wilder, M. Bio-economic analysis of super-intensive closed shrimp farming and improvement of management plans: A case study in Japan. Fish. Sci. 2019, 85, 1055–1065. [Google Scholar] [CrossRef]
- Wardhany, V.A.; Yuliandoko, H.; Subono; Harun, A.M.U.; Astawa, I.G.P. Smart system and monitoring of Vanammei shrimp ponds. Int. J. Adv. Sci. Eng. Inf. Technol. 2021, 11, 1366. [Google Scholar] [CrossRef]

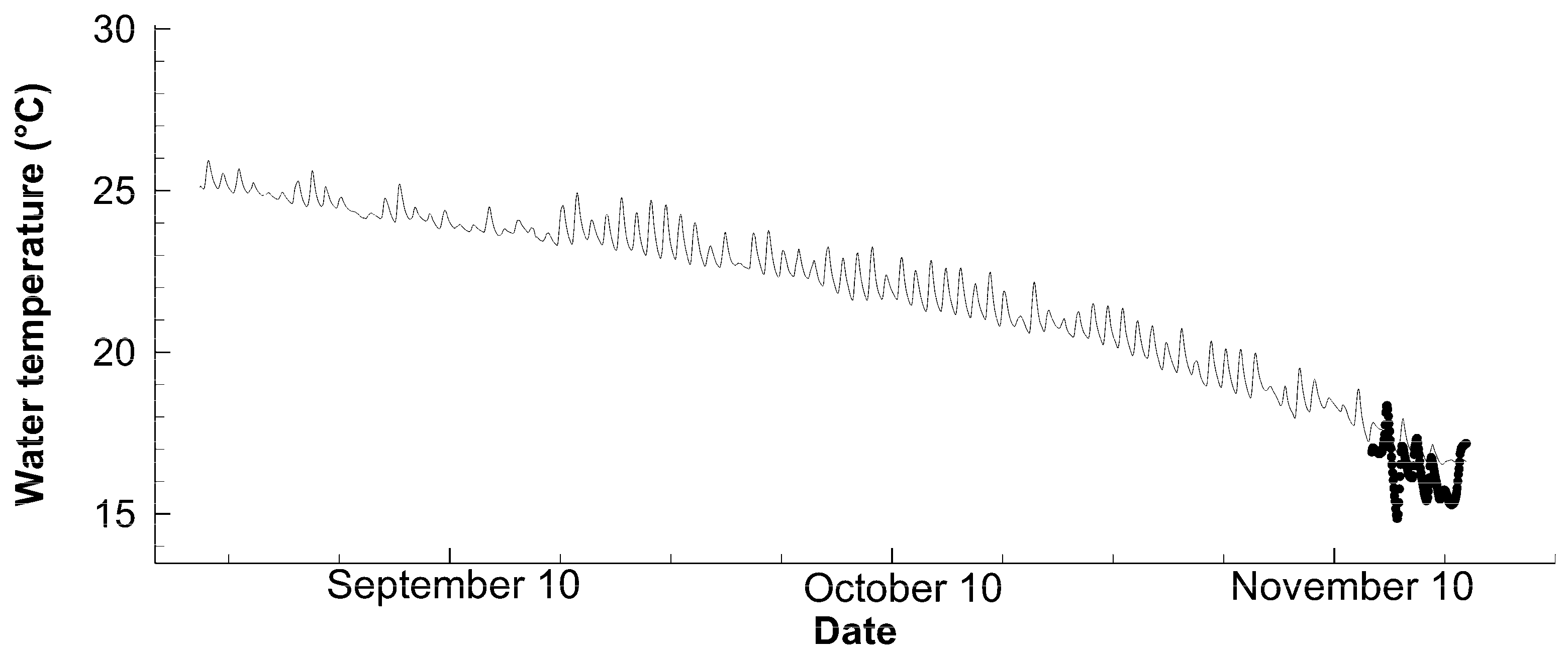

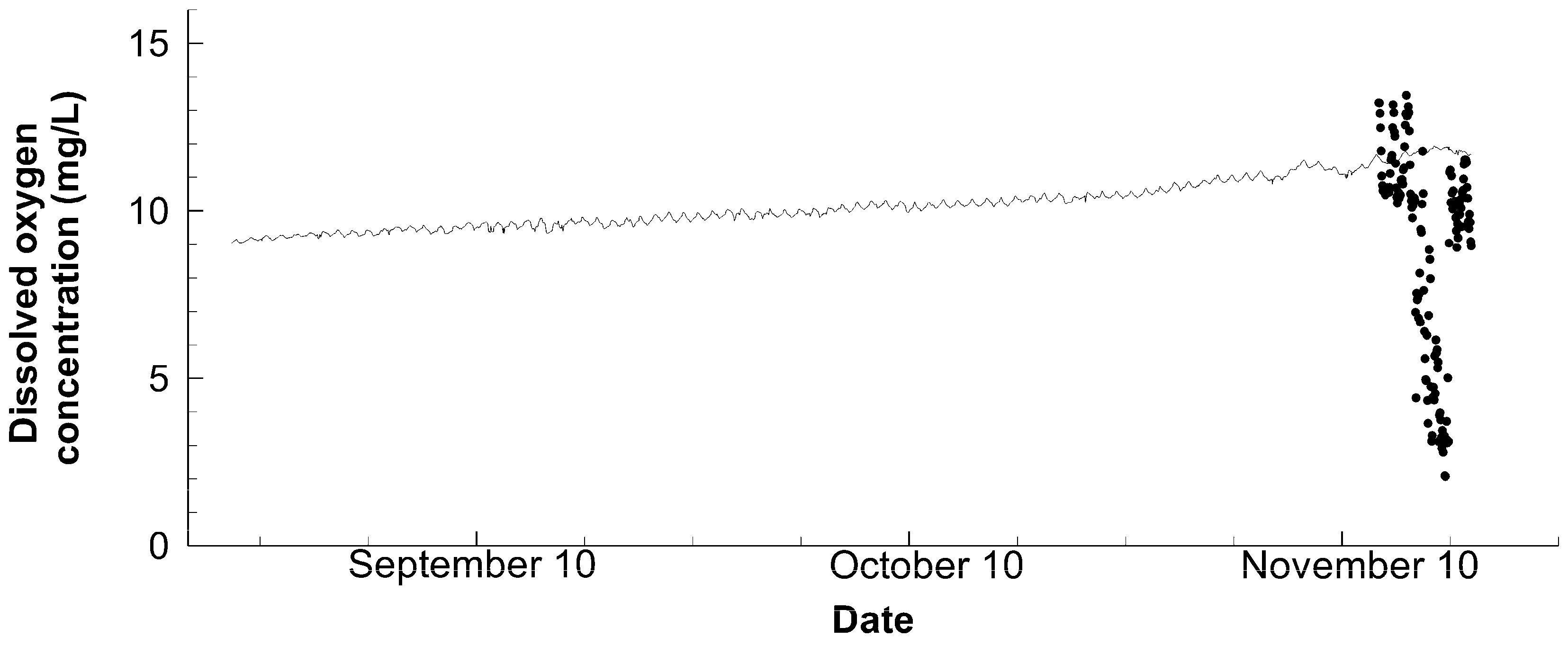

| Items | Instrument | Setup |
|---|---|---|
| Water temperature | Compact-CT | Every 30 min values at 40 cm below the water surface of Station C |
| Chlorophyll a | Compact-CLW | Every 10 min values at 40 cm below the water surface of Station C |
| Dissolved oxygen | Compact-DOW | Every 10 min values at 80 cm below the water surface of Station C |
| Nutrient | Water samples | 50 mL each at 6:00 and 15:00 every day from the drawing pipe at Station A and from the surface water at Station B |
| Water treatment and analysis | immediately frozen in the refrigerator before the laboratory; filtered using a Whatman GF/F glass filter paper and analyzed by the Auto Analyzer in the laboratory |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Tu, T.; Wang, H.; Kitazawa, D. Modeling Environmental Impacts of Intensive Shrimp Aquaculture: A Three-Dimensional Hydrodynamic Ecosystem Approach. Fishes 2024, 9, 126. https://doi.org/10.3390/fishes9040126
Zhou J, Tu T, Wang H, Kitazawa D. Modeling Environmental Impacts of Intensive Shrimp Aquaculture: A Three-Dimensional Hydrodynamic Ecosystem Approach. Fishes. 2024; 9(4):126. https://doi.org/10.3390/fishes9040126
Chicago/Turabian StyleZhou, Jinxin, Teng Tu, Huajin Wang, and Daisuke Kitazawa. 2024. "Modeling Environmental Impacts of Intensive Shrimp Aquaculture: A Three-Dimensional Hydrodynamic Ecosystem Approach" Fishes 9, no. 4: 126. https://doi.org/10.3390/fishes9040126





