Sol–Gel Synthesis of Dy Co-Doped ZnO:V Nanoparticles for Optoelectronic Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation Details
2.2. Characterization Techniques
3. Results and Discussion
3.1. Structural and Morphological Analysis
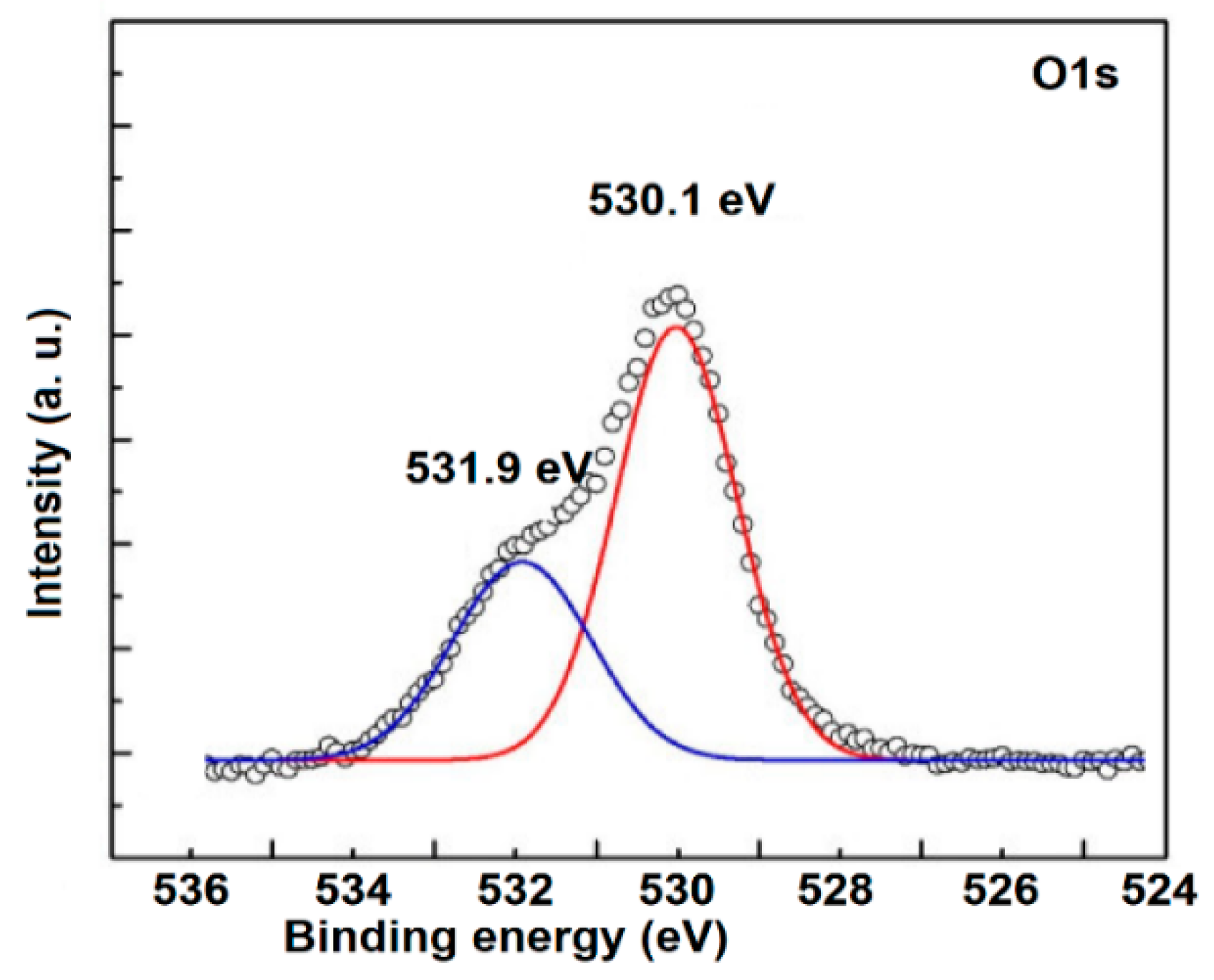
3.2. Elemental Analysis
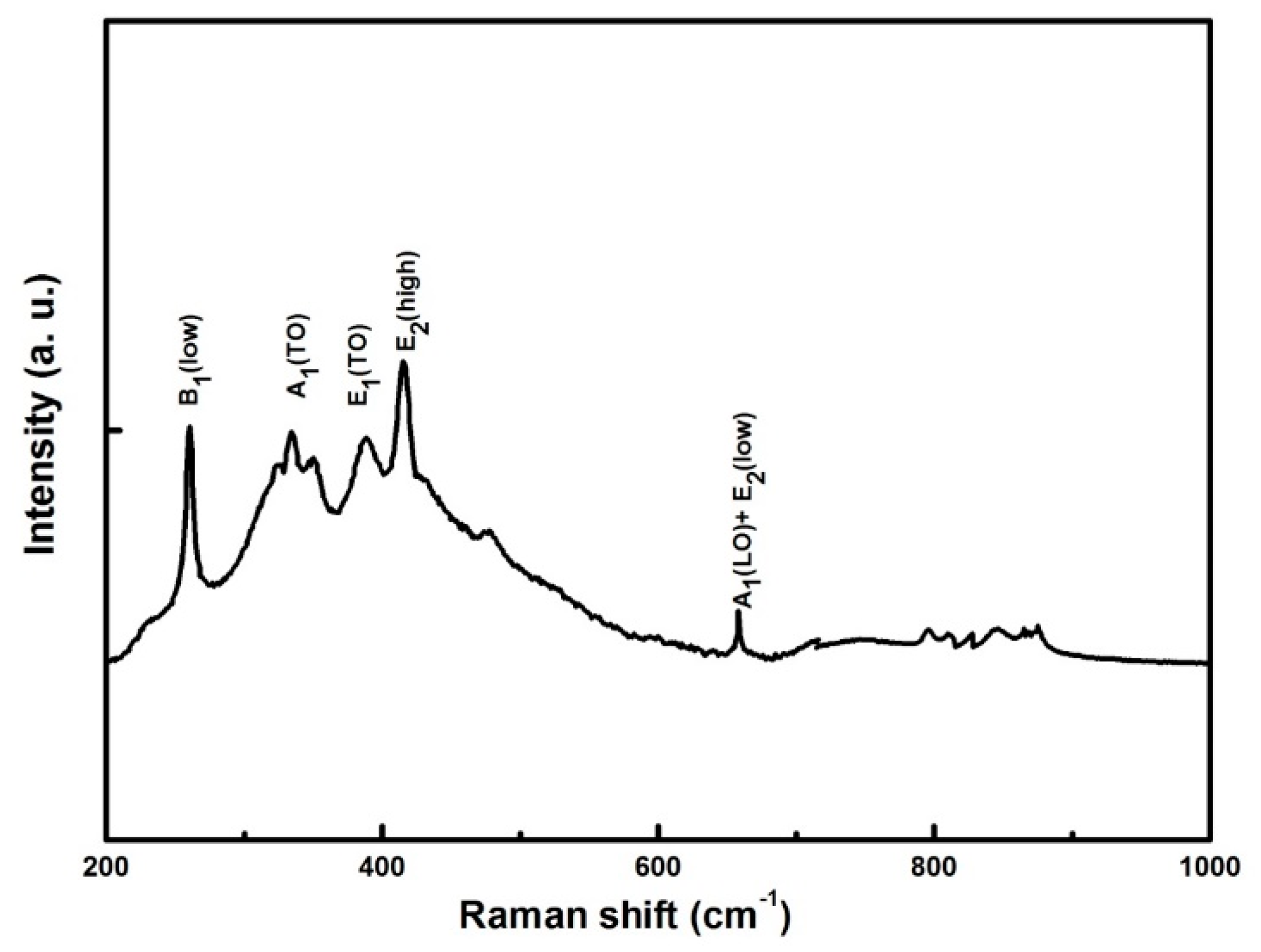
3.3. Raman Analysis
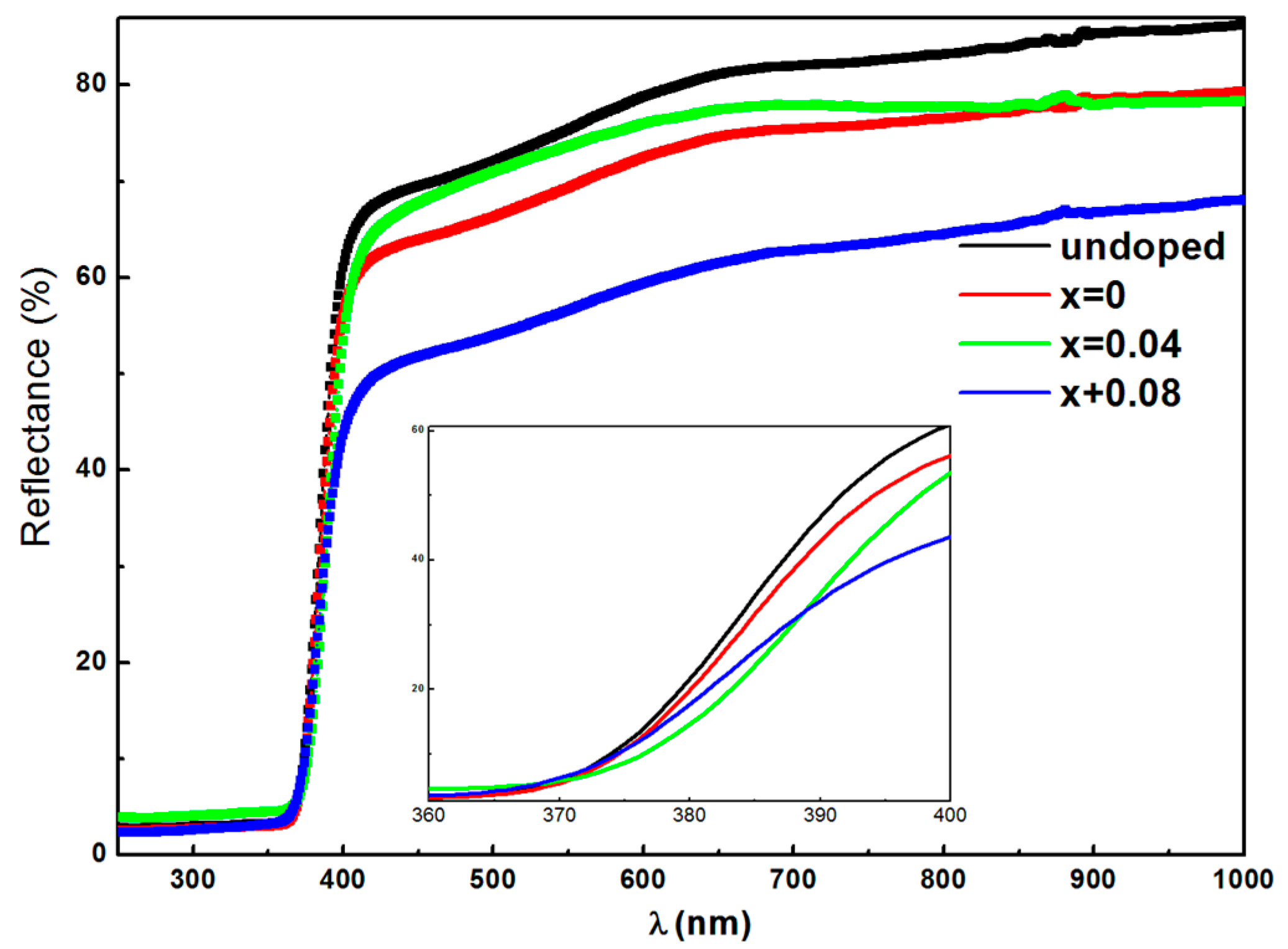
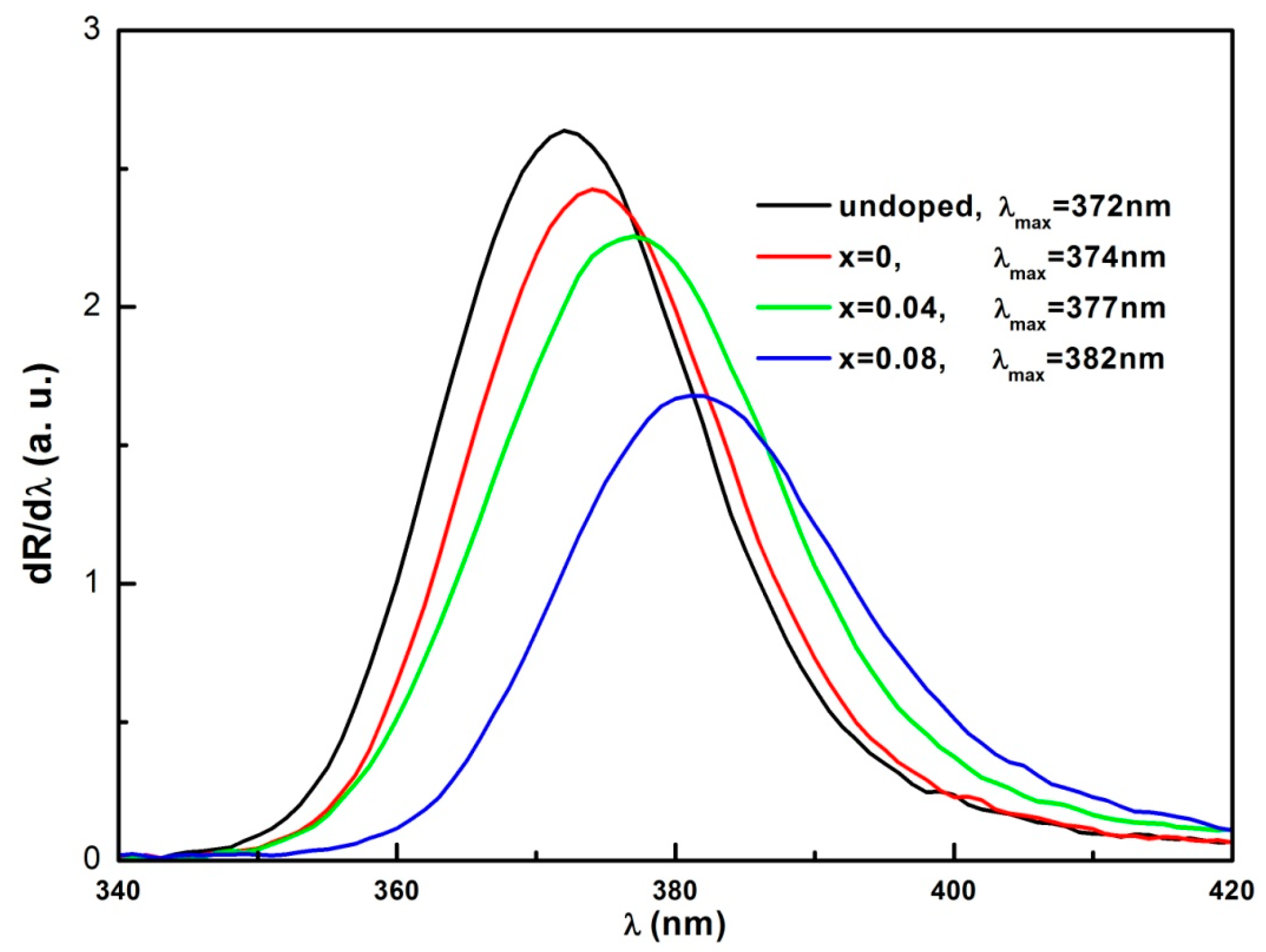
3.4. Optical Properties
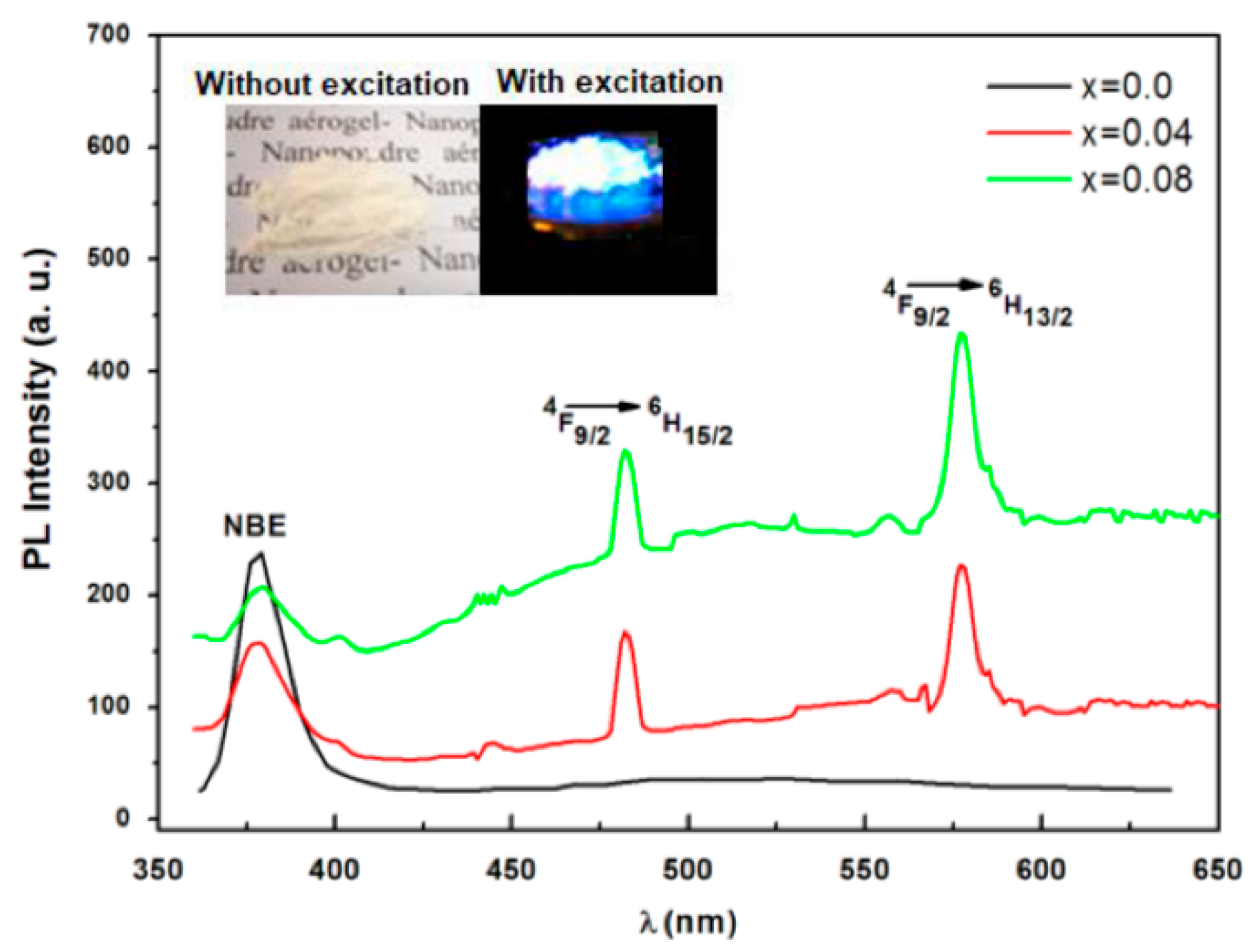
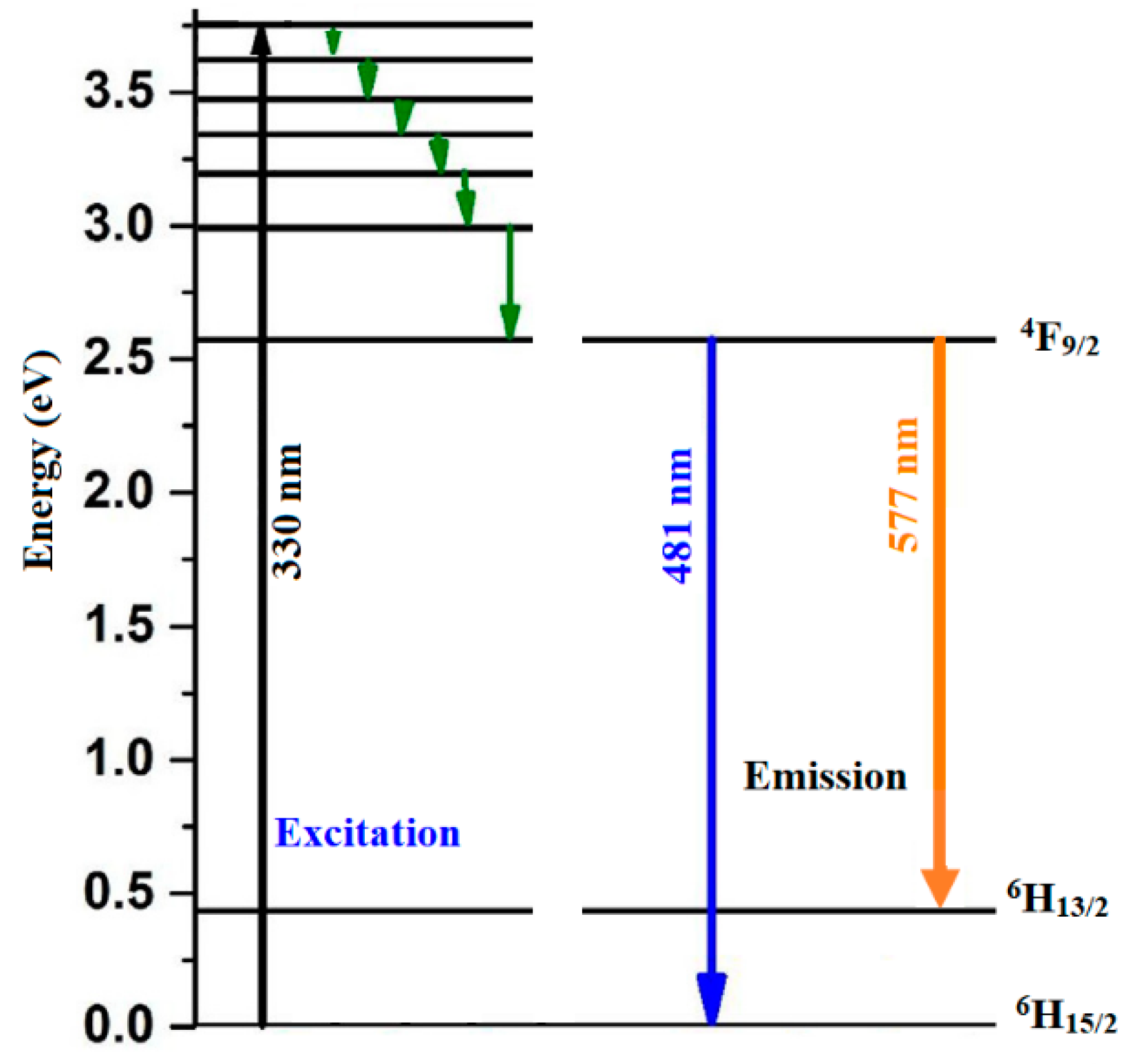
3.5. Photoluminescence (PL)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellmer, K.; Klein, A. ZnO and Its Applications; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2008; Volume 104, p. 1. [Google Scholar]
- Calnan, S.; Tiwari, A.N. High mobility transparent conducting oxides for thin film solar cells. Thin Solid Film. 2010, 518, 1839. [Google Scholar] [CrossRef]
- El Ghoul, J. Synthesis of vanadium doped ZnO nanoparticles by sol–gel method and its characterization. J. Mater. Sci.-Mater. Electron. 2016, 27, 2159. [Google Scholar] [CrossRef]
- Janotti, A.; van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef] [Green Version]
- El Ghoul, J.; Bouguila, N.; Gómez-Lopera, S.A.; El Mir, L. Structural and optical properties of nanoparticles (V, Al) co-doped ZnO synthesized by sol–gel processes. Superlattices Microstruct. 2013, 64, 451. [Google Scholar] [CrossRef]
- Mehrabian, M.; Azimirad, R.; Mirabbaszadeh, K.; Afarideh, H.; Davoudian, M. UV detecting properties of hydrothermal synthesized ZnO nanorods. Phys. E 2011, 43, 1141. [Google Scholar] [CrossRef]
- Willander, M.; Nur, O.; Sadaf, J.R.; Qadir, M.I.; Zaman, S.; Zainelabdin, A.; Bano, N.; Hussain, I. Luminescence from Zinc Oxide Nanostructures and Polymers and Their Hybrid Devices. Materials 2010, 3, 2643. [Google Scholar] [CrossRef]
- Willander, M.; Nur, O.; Zhao, Q.X.; Yang, L.L.; Lorenz, M.; Cao, B.Q.; Perez, J.Z.; Zekalla, C.; Zimmermann, G.; Grundmann, M.; et al. Zinc oxide nanorod based photonic devices: Recent progress in growth, light emitting diodes and lasers. Nanotechnology 2009, 20, 332001. [Google Scholar] [CrossRef]
- Ishizumi, A.; Kanemitsu, Y. Structural and luminescence properties of Eu-doped ZnO nanorods fabricated by a microemulsion method. Appl. Phys. Lett. 2005, 86, 253106. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Luo, W.; Li, R.; Chen, X. Spectroscopic evidence of the multiple-site structure of Eu3+ ions incorporated in ZnO nanocrystals. Opt. Lett. 2007, 32, 566. [Google Scholar] [CrossRef]
- Armelao, L.; Bottaro, G.; Pascolini, M.; Sessolo, M.; Tondello, A. Structure−Luminescence correlations in europium-doped sol−gel ZnO nanopowders. J. Phys. Chem. C 2008, 112, 4049. [Google Scholar] [CrossRef]
- Taguchi, S.; Ishizumi, A.; Tayagaki, T.; Kanemitsu, Y. Mn–Mn couplings in Mn-doped CdS nanocrystals studied by magnetic circular dichroism spectroscopy. Appl. Phys. Lett. 2009, 94, 173101. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, R.; Luo, W.; Zhu, H.; Chen, X. Optical spectroscopy of Sm3+ and Dy3+ doped ZnO nanocrystals. Spectrosc. Lett. 2010, 43, 343. [Google Scholar] [CrossRef]
- Wu, G.S.; Zhuang, Y.L.; Lin, Z.Q.; Yuan, X.Y.; Xie, T.; Zhang, L.D. Synthesis and photoluminescence of Dy-doped ZnO nanowires. Phys. E 2006, 31, 5. [Google Scholar] [CrossRef]
- Liu, E.Z.; Liu, Y.; He, J.Z. Ferromagnetism induced by defect complex in Co-doped ZnO. Appl. Phys. Lett. 2008, 93, 132506. [Google Scholar] [CrossRef]
- Kim, D.; Yang, J.; Hong, J. Ferromagnetism induced by Zn vacancy defect and lattice distortion in ZnO. J. Appl. Phys. 2009, 106, 013908. [Google Scholar] [CrossRef]
- Chen, B.; Yu, Q.X.; Gao, Q.Q.; Liao, Y.; Wang, G.Z. Structural reconstruction and defects transition in mediating room temperature ferromagnetism in Co-doped ZnO film. Appl. Phys. Lett. 2013, 102, 132405. [Google Scholar] [CrossRef]
- Kaushik, A.; Dalela, B.; Rathore, R.; Vats, V.S.; Choudhary, B.L.; Alvi, P.A.; Kumar, S.; Dalela, S. Influence of Co doping on the structural, optical and magnetic properties of ZnO nanocrystals. J. Alloys Compd. 2013, 578, 328. [Google Scholar] [CrossRef]
- Wibowo, J.A.; Djaja, N.F.; Saleh, R. Cu-and Ni-doping effect on structure and magnetic properties of Fe-doped ZnO nanoparticles. Adv. Mater. Phys. Chem. 2013, 3, 48. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Chen, C.L.; Dong, C.L.; Ho, Y.K.; Lee, J.F.; Chan, T.S.; Thangavel, R.; Chen, T.K.; Mok, B.H.; Rao, S.M.; et al. Structural, optical, and magnetic characterization of Co and N co-doped ZnO nanopowders. J. Mater. Sci. 2013, 48, 2618. [Google Scholar] [CrossRef]
- Kaur, P.; Pandey, S.K.; Kumar, S.; Negi, N.S.; Chen, C.L.; Rao, S.M.; Wu, M.K. Tuning ferromagnetism in zinc oxide nanoparticles by chromium doping. Appl. Nano 2015, 5, 975. [Google Scholar] [CrossRef] [Green Version]
- Murmu, P.P.; Kennedy, J.; Ruck, B.J.; Williams, G.V.M.; Markwit, A.; Rubanov, S.; Suvorova, A.A. Effect of annealing on the structural, electrical and magnetic properties of Gd-implanted ZnO thin films. J. Mater. Sci. 2012, 47, 1119. [Google Scholar] [CrossRef]
- Kumar, S.; Kaur, P.; Chen, C.L.; Thangavel, R.; Dong, C.L.; Ho, Y.K.; Lee, J.F.; Chan, T.S.; Chen, T.K.; Mok, B.H.; et al. optical and magnetic characterization of Ru doped ZnO nanorods. J. Alloys Compd. 2014, 588, 705. [Google Scholar] [CrossRef]
- Kumar, S.; Thangavel, R. Gd doping effect on structural, electrical and magnetic properties of ZnO thin films synthesized by sol-gel spin coating technique. Elecon. Mater. Lett. 2017, 13, 129. [Google Scholar] [CrossRef]
- Kaur, P.; Kumar, S.; Chen, C.L.; Hsu, Y.Y.; Chan, T.S.; Dong, C.L.; Srivastava, C.; Singh, A.; Rao, S.M. Investigations on structural, magnetic and electronic structure of Gd-doped ZnO nanostructures synthesized using sol–gel technique. Appl. Phys. A 2016, 122, 1. [Google Scholar] [CrossRef]
- Wu, Z.F.; Cheng, K.; Zhang, F.; Guan, R.F.; Wuc, X.M.; Zhuge, L.J. Effect of Al co-doping on the electrical and magnetic properties of Cu-doped ZnO nanorods. J. Alloys Compd. 2014, 615, 521. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Nath, T.K.; Behan, A.J.; Neal, J.R.; Score, D.; Feng, Q.; Fox, A.M.; Gehring, G.A. Enhancement of room temperature ferromagnetism of Fe-doped ZnO epitaxial thin films with Al co-doping. J. Magn. Magn. Mater. 2011, 323, 1033. [Google Scholar] [CrossRef]
- Liu, L.; Yu, P.Y.; Ma, Z.; Mao, S.S. Enhancement of room temperature ferromagnetism of Fe-doped ZnO epitaxial thin films with Al co-doping. Phys. Rev. Lett. 2008, 100, 127203. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Schmidt, H.; Hochmuth, H.; Lorenz, M.; Setzer, A.; Esquinazi, P.; Meinecke, C.; Grundmann, M. Room temperature ferromagnetism in Nd-and Mn-codoped ZnO films. J. Phys. D Appl. Phys. 2008, 41, 105012. [Google Scholar] [CrossRef]
- El Ghoul, J.; Al-Harbi, F.F. Synthesis, Structural and Optical Properties of Er and V Codoping ZnO Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2021, 31, 272. [Google Scholar] [CrossRef]
- Assadi, M.H.N.; Zhang, Y.B.; Photongkam, P.; Li, S. Intrinsic ambient ferromagnetism in ZnO: Co induced by Eu codoping. J. Appl. Phys. 2011, 109, 013909. [Google Scholar] [CrossRef]
- Huang, H.; Ou, Y.; Xu, S.; Fang, G.; Li, M.; Zhao, X.Z. Properties of Dy-doped ZnO nanocrystalline thin films prepared by pulsed laser deposition. Appl. Surf. Sci. 2008, 254, 2013. [Google Scholar] [CrossRef]
- Omri, K.; el Ghoul, J.; Lemine, O.M.; Bououdina, M.; Zhang, B.; El Mir, L. Magnetic and optical properties of manganese doped ZnO nanoparticles synthesized by sol-gel technique. Superlattices Microstruct. 2013, 60, 139. [Google Scholar] [CrossRef]
- El Ghoul, J.; Al-Harbi, F.F. Synthesis, structural, optical and magnetic properties of Gd co-doped ZnO: V nanoparticles. Solid State Commun. 2020, 314, 113916. [Google Scholar] [CrossRef]
- Mourad, S.; El Ghoul, J.; Omri, K.; Khirouni, K. Indium doping effect on properties of ZnO nanoparticles synthesized by sol–gel method. Chin. Phys. B 2019, 28, 047701. [Google Scholar] [CrossRef]
- Powder Diffraction File. Joint Committee for Powder Diffraction Studies (JCPDS) File No. 36-1451.
- Thangeeswari, T.; Murugasen, P.; Velmuruga, J. Influence of Co and Dy doping on the optical and magnetic properties of ZnO nanoparticles for DMS application. J. Supercond. Nov. Magn. 2015, 28, 2505. [Google Scholar] [CrossRef]
- Zhan, F.; Yang, Y.; Li, W.; Li, J.; Liu, W.; Li, Y.; Chen, Q. Preparation of DyVO4/WO3 heterojunction plate array films with enhanced photoelectrochemical activity. RSC Adv. 2016, 6, 10393. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, D.R.; Jiao, X.L. Monoclinic structured BiVO4 nanosheets: Hydrothermal preparation, formation mechanism, and coloristic and photocatalytic properties. J. Phys. Chem. B 2006, 110, 2668. [Google Scholar] [CrossRef] [PubMed]
- Jayachandraiah, C.; Kumar, K.S.; Krishnaiah, G.; Rao, N.M. Influence of Dy dopant on structural and photoluminescence of Dy-doped ZnO nanoparticles. J. Alloys Compd. 2015, 623, 248. [Google Scholar] [CrossRef]
- Russo, V.; Ghidelli, M.; Gondoni, P.; Casari, C.S.; Bassi, A.L. Multi-wavelength Raman scattering of nanostructured Al-doped zinc oxide. J. Appl. Phys. 2014, 115, 073508. [Google Scholar] [CrossRef] [Green Version]
- Lung, C.; Toma, M.; Pop, M.; Marconi, D.; Pop, A. Characterization of the structural and optical properties of ZnO thin films doped with Ga, Al and (Al+ Ga). J. Alloys Compd. 2017, 725, 1238. [Google Scholar] [CrossRef]
- Batista, C.; Teixeira, V.; Carneiro, J.O. Structural and morphological characterization of magnetron sputtered nanocrystalline vanadium oxide films for thermochromic smart surfaces. J. Nano Res. 2008, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Thaslin, S.; Fathima, N.; Anandhan, A.; Ganesan, A.R.K.P.; Karthikeyan, M.; Marimuthu, T. Surface texture and luminous analysis of Sol-Gel spin coated Dy-doped ZnO thin films. IRJET 2017, 4, 89. [Google Scholar]
- Kumar, J.S.; Pavani, K.; Babu, A.M.; Giri, N.K.; Rai, S.B.; Moorthy, L.R. Fluorescence characteristics of Dy3+ ions in calcium fluoroborate glasses. J. Lumin. 2010, 130, 1916. [Google Scholar] [CrossRef]
- Gruber, J.B.; Zandi, B.; Valiev, U.V.; Rakhimov, S.A. Energy levels of Dy3+(4f9) in orthoaluminate crystals. J. Appl. Phys. 2003, 94, 1030. [Google Scholar] [CrossRef]
- Van Dijken, A.; Makkinje, J.; Meijerink, A. The influence of particle size on the luminescence quantum efficiency of nanocrystalline ZnO particles. J. Lumin. 2001, 92, 323. [Google Scholar] [CrossRef]
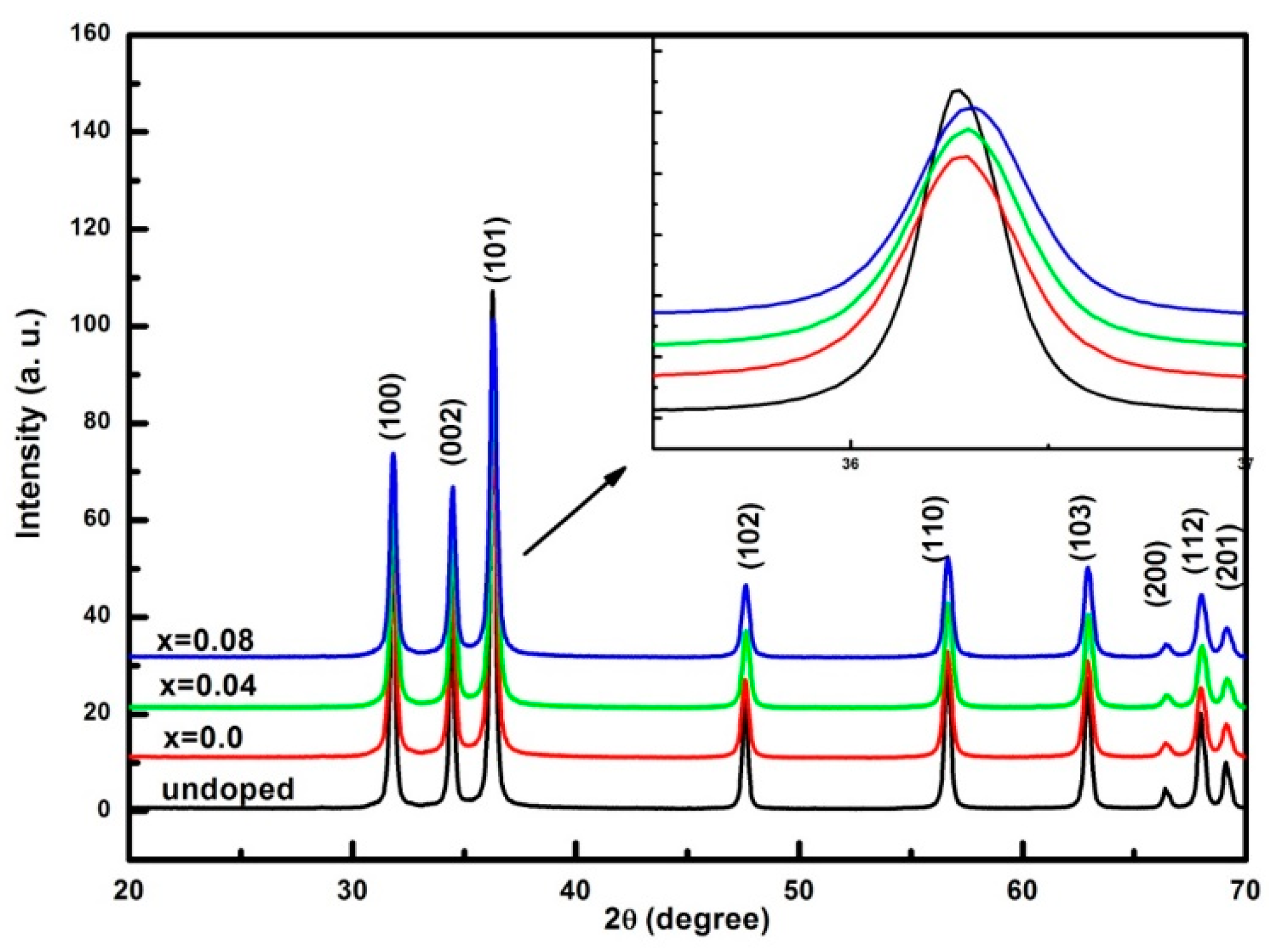
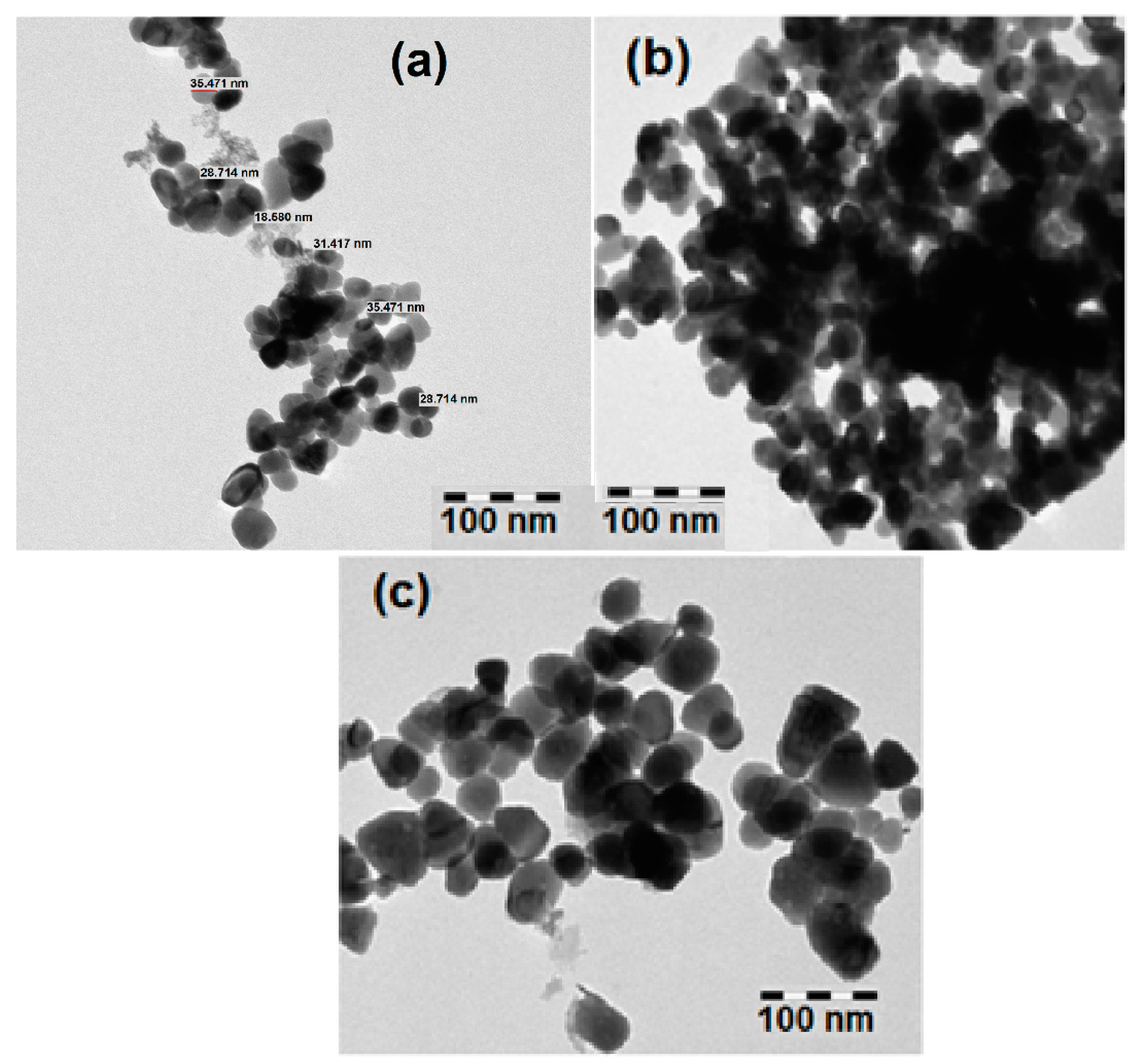
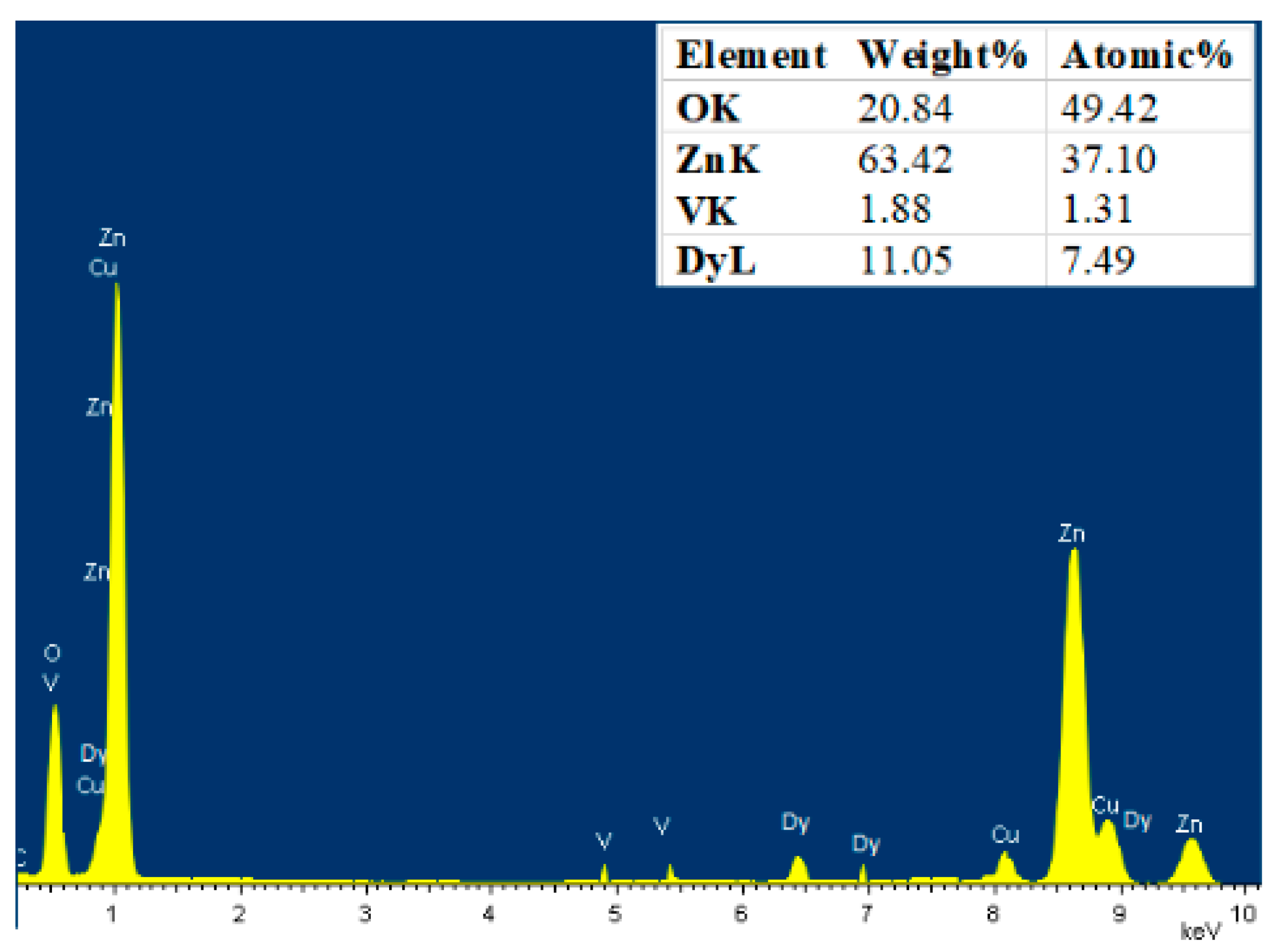
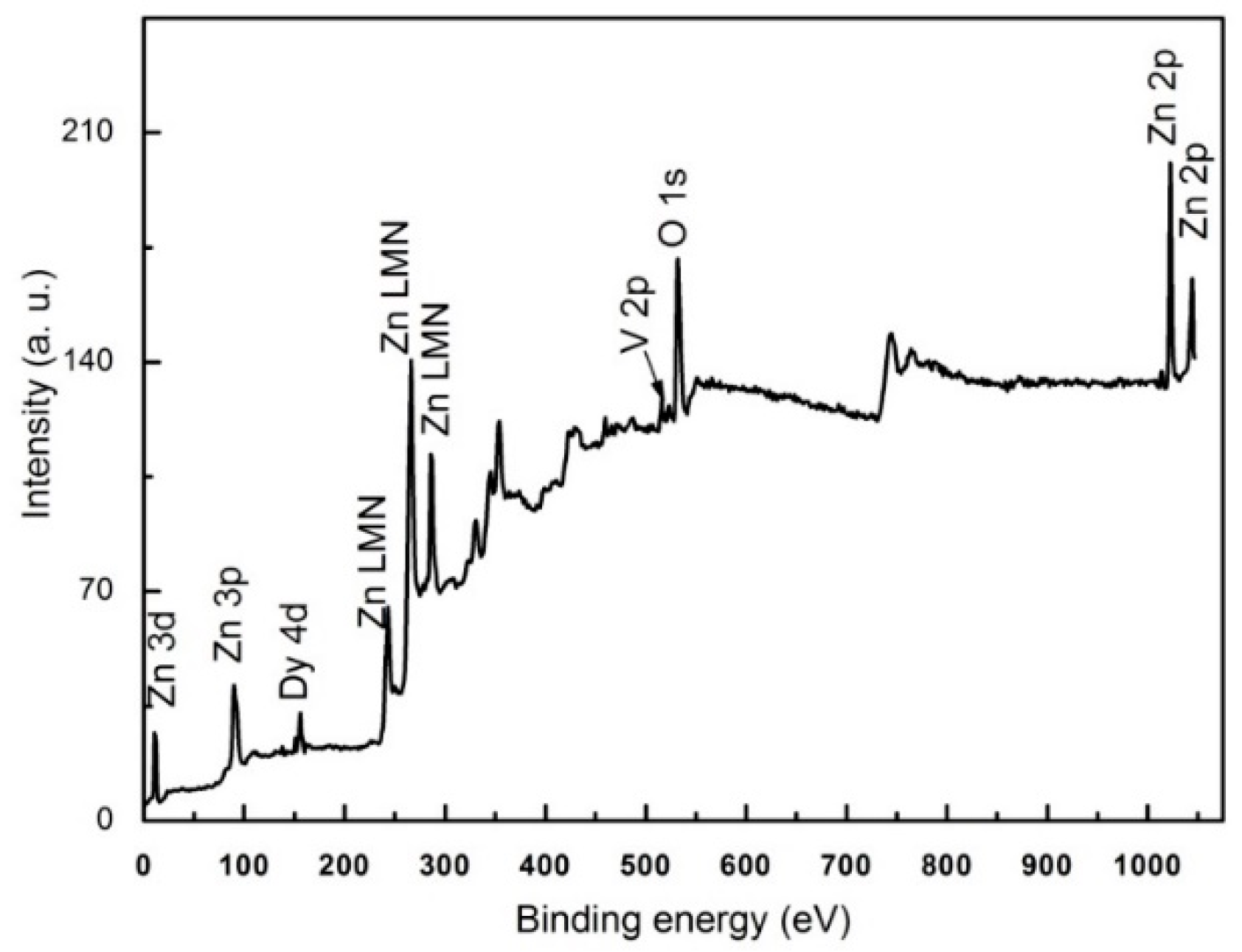
| Sample | a (Å) | C (Å) | Crystallite Size (nm) | Strain (ε) × 10−4 | Bandgap (eV) |
|---|---|---|---|---|---|
| Undoped ZnO | 3.2498 | 5.2063 | 34.71 | 1.59 | 3.333 |
| x = 0.0 | 3.2497 | 5.2063 | 32.84 | 1.48 | 3.315 |
| x = 0.04 | 3.2494 | 5.2064 | 29.73 | 1.39 | 3.287 |
| x = 0.08 | 3.2495 | 5.2065 | 27.38 | 1.31 | 3.246 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Harbi, F.F.; El Ghoul, J.M. Sol–Gel Synthesis of Dy Co-Doped ZnO:V Nanoparticles for Optoelectronic Applications. Condens. Matter 2021, 6, 35. https://doi.org/10.3390/condmat6030035
Al-Harbi FF, El Ghoul JM. Sol–Gel Synthesis of Dy Co-Doped ZnO:V Nanoparticles for Optoelectronic Applications. Condensed Matter. 2021; 6(3):35. https://doi.org/10.3390/condmat6030035
Chicago/Turabian StyleAl-Harbi, Fatemah F., and Jaber Mohamed El Ghoul. 2021. "Sol–Gel Synthesis of Dy Co-Doped ZnO:V Nanoparticles for Optoelectronic Applications" Condensed Matter 6, no. 3: 35. https://doi.org/10.3390/condmat6030035





