Magnetically Recoverable and Reusable Antimicrobial Nanocomposite Based on Activated Carbon, Magnetite Nanoparticles, and Silver Nanoparticles for Water Disinfection
Abstract
:1. Introduction
- The superior antimicrobial properties of AgNPs for water disinfection;
- The magnetic properties of MNPs for easy and fast removal of AgNPs from treated water and for making antimicrobial agent recoverable and reusable;
- The superior adsorption ability of AC for organic and inorganic species;
- And the immobilization of well dispersed nanoparticles on the AC and/or the MAC surfaces that prevents the aggregation of magnetite and silver nanoparticles in water, helping retain the superior properties of these nanoparticles.
2. Materials and Methods
2.1. Materials
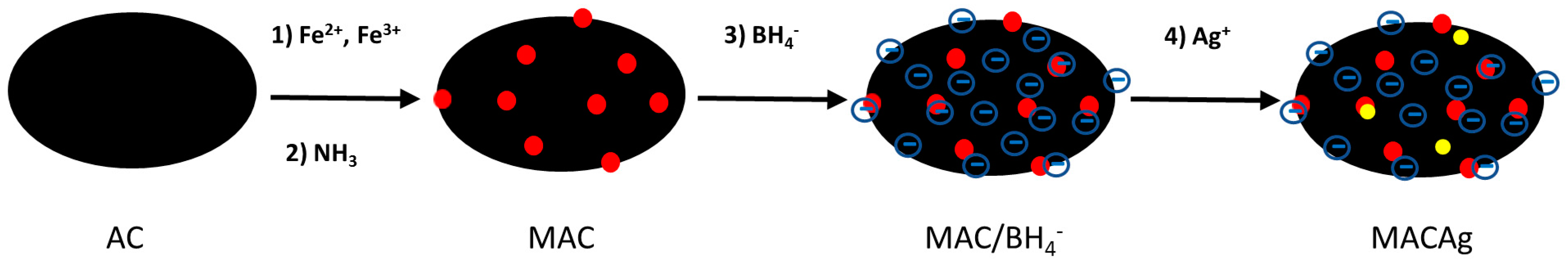
2.2. Synthesis of Magnetite-Activiated Carbon-Silver (MACAg) Nanocomposite
2.3. Characterization
2.4. Antimicrobial Activity of MACAg Nanocomposite
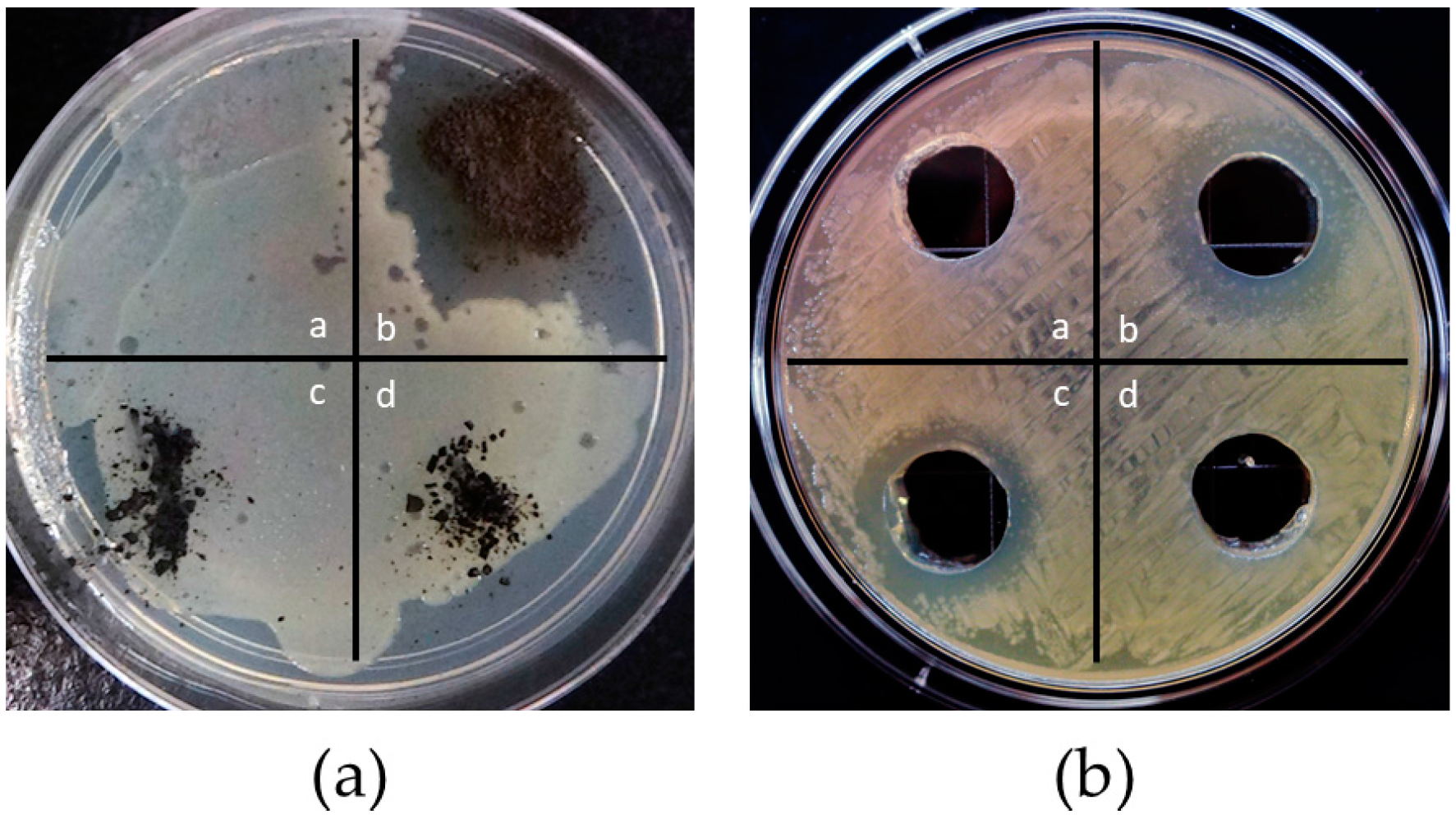
2.4.1. Zone of Inhibition
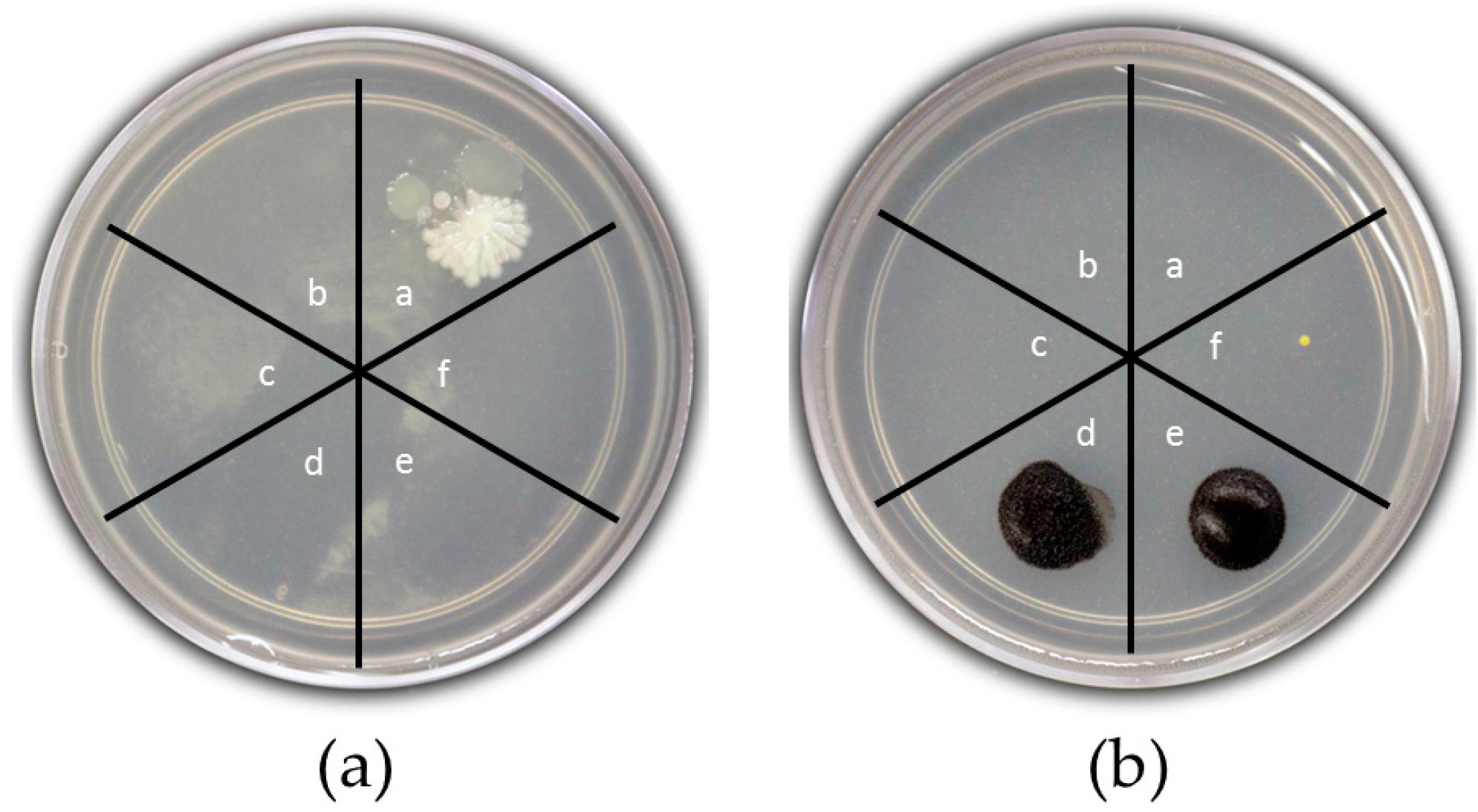
2.4.2. Shaking Tests
2.4.3. Repeated Use of MACAg Nanocomposite
2.4.4. Disinfection of Long Island Sound (LIS) Surface Water
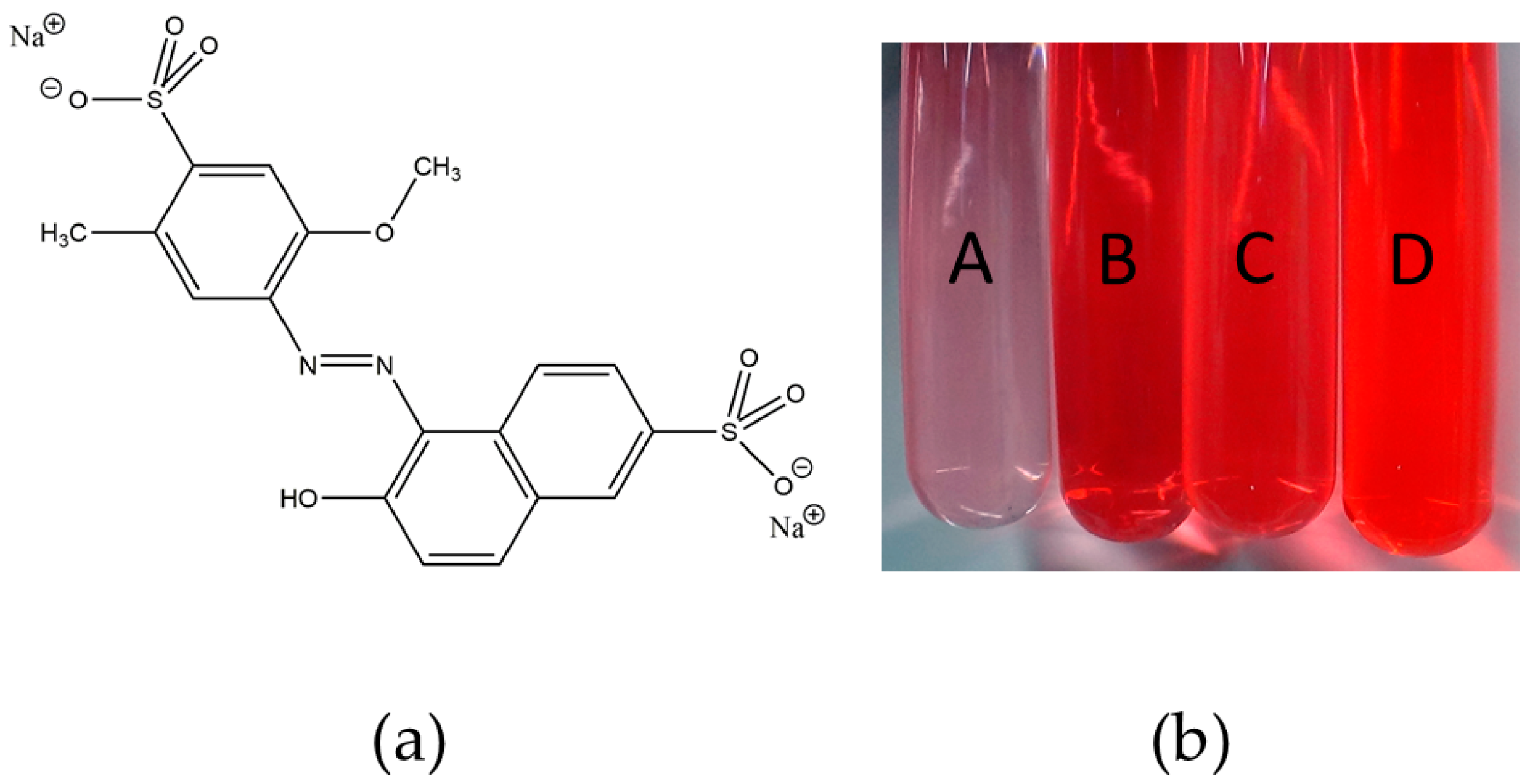
2.5. Release of Silver
3. Results and Discussion
3.1. Synthesis and Characterization of MACAg
3.2. Assessment of Antimicrobial Activity of MACAg
3.2.1. Zone of Inhibition
3.2.2. Shaking Tests
3.2.3. Repeated Use of MACAg Nanocomposite
3.2.4. Disinfection of Long Island Sound (LIS) Surface Water
3.3. Release of Silver
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization (WHO). “Water, Sanitation and Hygiene Links to Health Facts and Figures”. 2004. Available online: http://www.who.int/water_sanitation_health/en/factsfigures04.pdf (accessed on 31 May 2017).
- Seiden, J.M.; Way, C.; Rivkin, R.B. Microbial hitchhikers: Dynamics of bacterial populations in a trans-Pacific voyage of a bulk carrier. Aquat. Invasions 2010, 5, 13–22. [Google Scholar] [CrossRef]
- First, M.R.; Drake, L.A. Approaches for determining the effects of UV radiation on microorganisms in ballast water. Manag. Biol. Invasions 2013, 4, 87–99. [Google Scholar] [CrossRef]
- Krasner, S.W.; Weinberg, H.S.; Richardson, S.D.; Pastor, S.J.; Chinn, R.; Sclimenti, M.J.; Onstad, G.D.; Thruston, A.D., Jr. Occurrence of a new generation of disinfection byproducts. Environ. Sci. Technol. 2006, 40, 7175–7185. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. “The Safe Water System—Disinfection by-Products”. Available online: www.cdc.gov/safewater/chlorination-byproducts.html (accessed on 31 May 2017).
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, G.J.; Marinas, B.J.; Mayers, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Tan, S.; Zou, W.; Jiang, F.; Tan, S.; Liu, Z.; Yuan, D. Facile fabrication of copper-supported ordered mesoporous carbon for antibacterial behavior. Mater. Lett. 2010, 64, 2163–2166. [Google Scholar] [CrossRef]
- Damm, C.; Munstedt, H.; Rosch, A.J. Long-term antimicrobial polyamide 6/silver nanocomposites. Mater. Sci. 2007, 42, 6067–6073. [Google Scholar] [CrossRef]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Viga, H.; Kakinen, A.; Titma, T.; Heinlaan, M.; et al. Size-dependent toxicity of silver nanoparticles to bacteria, yeast, algae, crustaceans and mammalian cells in vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef] [PubMed]
- Panyala, N.R.; Pena-Mendez, E.M.; Havel, J. Silver or silver nanoparticels: A hazardous threat to the environment and human health? J. Appl. Biomed. 2008, 6, 47–59. [Google Scholar]
- Okafor, F.; Janen, A.; Kukhtareva, T.; Edwards, V.; Curley, M. Green synthesis of silver nanoparticles, their characterization, application and antibacterial activity. Int. J. Environ. Res. Public Health 2010, 10, 5221–5238. [Google Scholar] [CrossRef] [PubMed]
- Alt, V.; Bechert, T.; Steinrucke, P.; Wagener, M.; Seidel, P.; Dingeldein, E.; Domann, E.; Schnettler, R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials 2004, 25, 4383–4391. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Xue, J.; He, J. Controlled in-situ synthesis of silver nanoparticles in natural cellulose fibers toward highly efficient antimicrobial materials. J. Nanosci. Nanotechnol. 2009, 9, 3067–3074. [Google Scholar] [CrossRef] [PubMed]
- Furlan, P.Y.; Melcer, M.E. Removal of aromatic pollutant surrogate from water by recyclable magnetite-activated nanocomposite: An experiment for general chemistry. J. Chem. Educ. 2014, 91, 1966–1970. [Google Scholar] [CrossRef]
- Furlan, P.Y.; Ackerman, B.M.; Melcer, M.E.; Perez, S.E. Reusable magnetic nanocomposite sponges for removing oil from water discharges. J. Ship Prod. Des. 2016, 32, 1–10. [Google Scholar]
- Dankovich, T.A.; Gray, D.G. Bactericidal paper impregnated with silver nanoparticles for point-of-use water treatment. Environ. Sci. Technol. 2011, 5, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Roque, E.B.; Fonseca, F.V.D.; Borges, C.P. High flux microfiltration membranes with silver nanoparticles for water disinfection. Desalin. Water Treat. 2015, 56, 3590–3598. [Google Scholar] [CrossRef]
- Ren, D.; Smith, J.A. Retention and transport of silver nanoparticles in a ceramic porous medium used for point-of use water treatment. Environ. Sci. Technol. 2013, 47, 3825–3832. [Google Scholar] [CrossRef] [PubMed]
- Quang, D.V.; Sarawade, P.B.; Jeon, S.J.; Kim, S.H.; Km, J.; Chai, Y.G.; Kim, H.T. Effective water disinfection using silver nanoparticle containing silica beads. Appl. Surf. Sci. 2013, 266, 280–287. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, Y.; Shi, Y.; Pan, G.; Yan, H.; Xu, J.; Guo, M.; Qin, L.; Liu, Y. Facile modification of thin-film composite nanofiltration membrane with silver nanoparticles for anti-biofouling. J. Polym. Res. 2016, 23, 105. [Google Scholar] [CrossRef]
- Karthik, C.; Radha, K.V. Silver nanoparticle loaded activated carbon: An escalated nanocomposite with antimicrobial property. Orient. J. Chem. 2016, 32, 735–741. [Google Scholar] [CrossRef]
- Eltugral, N.; Simsir, H.; Karagoz, S. Preparation of nano-silver-supported activated carbon using different ligands. Res. Chem. Intermed. 2016, 42, 1663. [Google Scholar] [CrossRef]
- Azmier, M.; Alrozi, R. Removal of malachite green dye from aqueous solution using rambutan peel-based activated carbon: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2011, 171, 510–516. [Google Scholar]
- Cook, D.; Newcombe, G.; Sztajnbok, P. The application of powdered activated carbon for MIB and geosmin removal: Predicting PAC doses in four waters. Water Res. 2001, 35, 9. [Google Scholar] [CrossRef]
- Crittenden, J.; Vaitheeswaran, K.; Hand, D.; Howe, E.; Aieta, E. Removal of dissolved organic carbon using granular activated carbon. Water Res. 1993, 27, 7. [Google Scholar] [CrossRef]
- Removal of Inorganic Contaminants by Activated Carbon Adsorption. Available online: http://www.webapps.cee.vt.edu/ewr/environmental/teach/gwprimer/group23/inorganics.html (accessed on 31 May 2017).
- Rao, M.M.; Ramana, D.K.; Seshaiah, K.; Wang, M.C.; Chien, S.W.C. Removal of some metal ions by activated carbon prepared from Phaseolus aureus hulls. J. Hazard. Mater. 2009, 166, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Furlan, P.Y.; Fisher, A.J.; Melcer, M.E.; Furlan, A.Y.; Warren, J.B. Preparing and testing a magnetic antimicrobial nanocomposite for water disinfection to gain experience at nanochemistry-microbiology interface. J. Chem. Educ. 2017, 94, 488–493. [Google Scholar] [CrossRef]
- Berger, P.; Adelman, N.B.; Beckman, K.J.; Campbell, D.J.; Eillis, A.B.; Lisensky, G.C. Preparation and properties of an aqueous ferrofluid. J. Chem. Educ. 1999, 76, 943–948. [Google Scholar] [CrossRef]
- Solomon, S.D.; Bahadory, M.; Jeyarajasingam, A.V.; Rutkowsky, S.A.; Boritz, C. Synthesis and study of silver nanoparticles. J. Chem. Educ. 2007, 84, 322–325. [Google Scholar]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Le Quay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Liu, J.Y.; Hurt, R.H. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Shuval, H. Stabilized formulation of hydrogen peroxide with silver: A secondary disinfectant. In Providing Safe Drinking Water in Small Systems: Technology, Operations, and Economics; Cotruvo, J.A., Carun, G.F., Hearne, N., Eds.; Lewis Publishers: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 1999; p. 184. [Google Scholar]
- Water Technology: Silver, Coper Have Antibacterial Effects. Available online: http://www.watertechonline.com/silver-copper-have-anti-bacterial-effects/ (accessed on 31 May 2017).



| No. Cycles 1 | MACAg (0.22% Ag) | MACAg (0.15% Ag) | MACAg (0.06% Ag) |
|---|---|---|---|
| 1 | 0.03 ppm | 0.01 ppm | 0.02 ppm |
| 3 | 0.05 ppm | 0.01 ppm | 0.01 ppm |
| 5 | 0.09 ppm | − | − |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furlan, P.Y.; Fisher, A.J.; Furlan, A.Y.; Melcer, M.E.; Shinn, D.W.; Warren, J.B. Magnetically Recoverable and Reusable Antimicrobial Nanocomposite Based on Activated Carbon, Magnetite Nanoparticles, and Silver Nanoparticles for Water Disinfection. Inventions 2017, 2, 10. https://doi.org/10.3390/inventions2020010
Furlan PY, Fisher AJ, Furlan AY, Melcer ME, Shinn DW, Warren JB. Magnetically Recoverable and Reusable Antimicrobial Nanocomposite Based on Activated Carbon, Magnetite Nanoparticles, and Silver Nanoparticles for Water Disinfection. Inventions. 2017; 2(2):10. https://doi.org/10.3390/inventions2020010
Chicago/Turabian StyleFurlan, Ping Y., Adam J. Fisher, Alexander Y. Furlan, Michael E. Melcer, David W. Shinn, and John B. Warren. 2017. "Magnetically Recoverable and Reusable Antimicrobial Nanocomposite Based on Activated Carbon, Magnetite Nanoparticles, and Silver Nanoparticles for Water Disinfection" Inventions 2, no. 2: 10. https://doi.org/10.3390/inventions2020010





