Quantification of Statins in Pharmaceutical Products Using Screen-Printed Sensors Based of Multi-Walled Carbon Nanotubes and Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Electrochemical Measurements
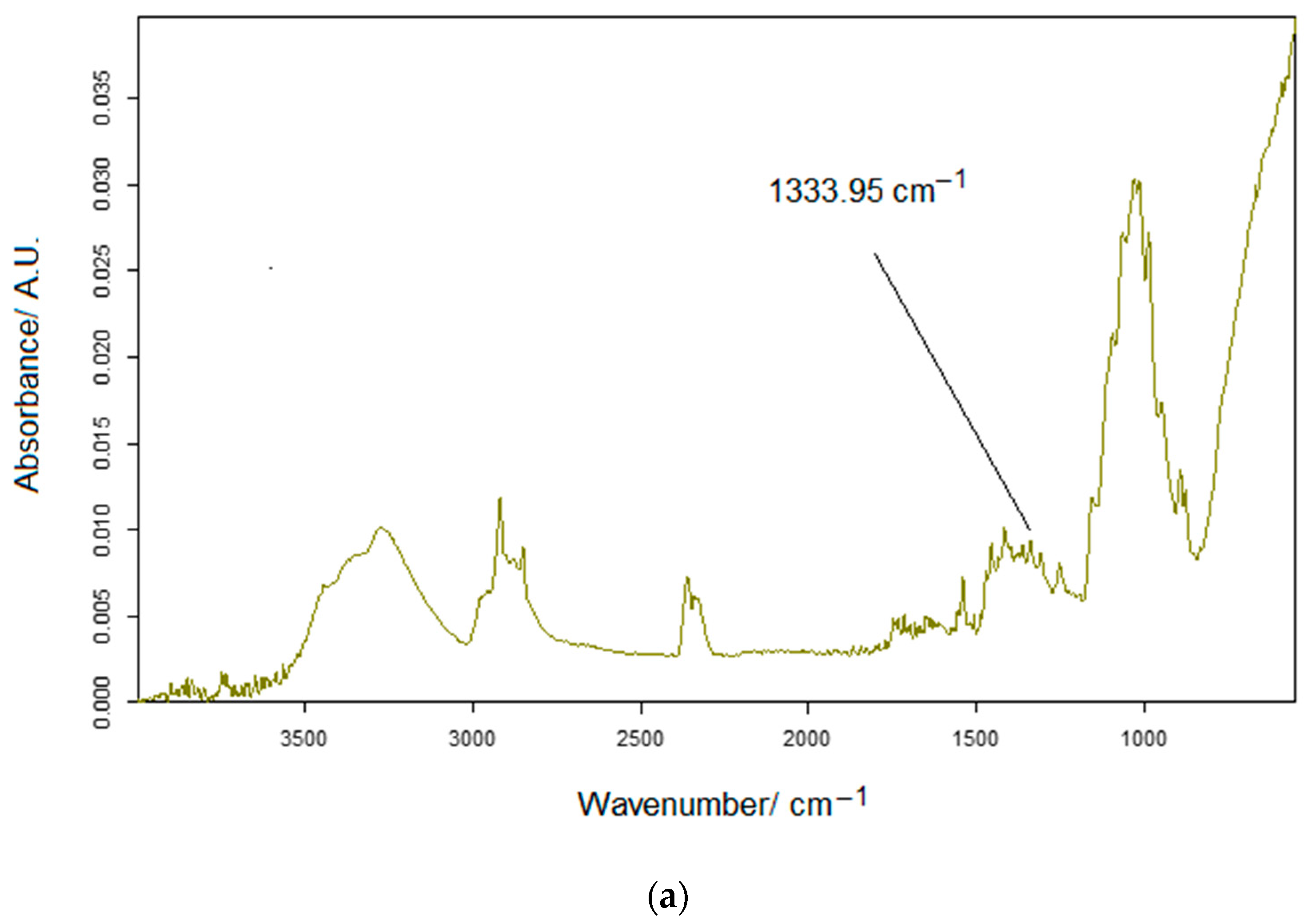
2.3. Spectrometric Measurements
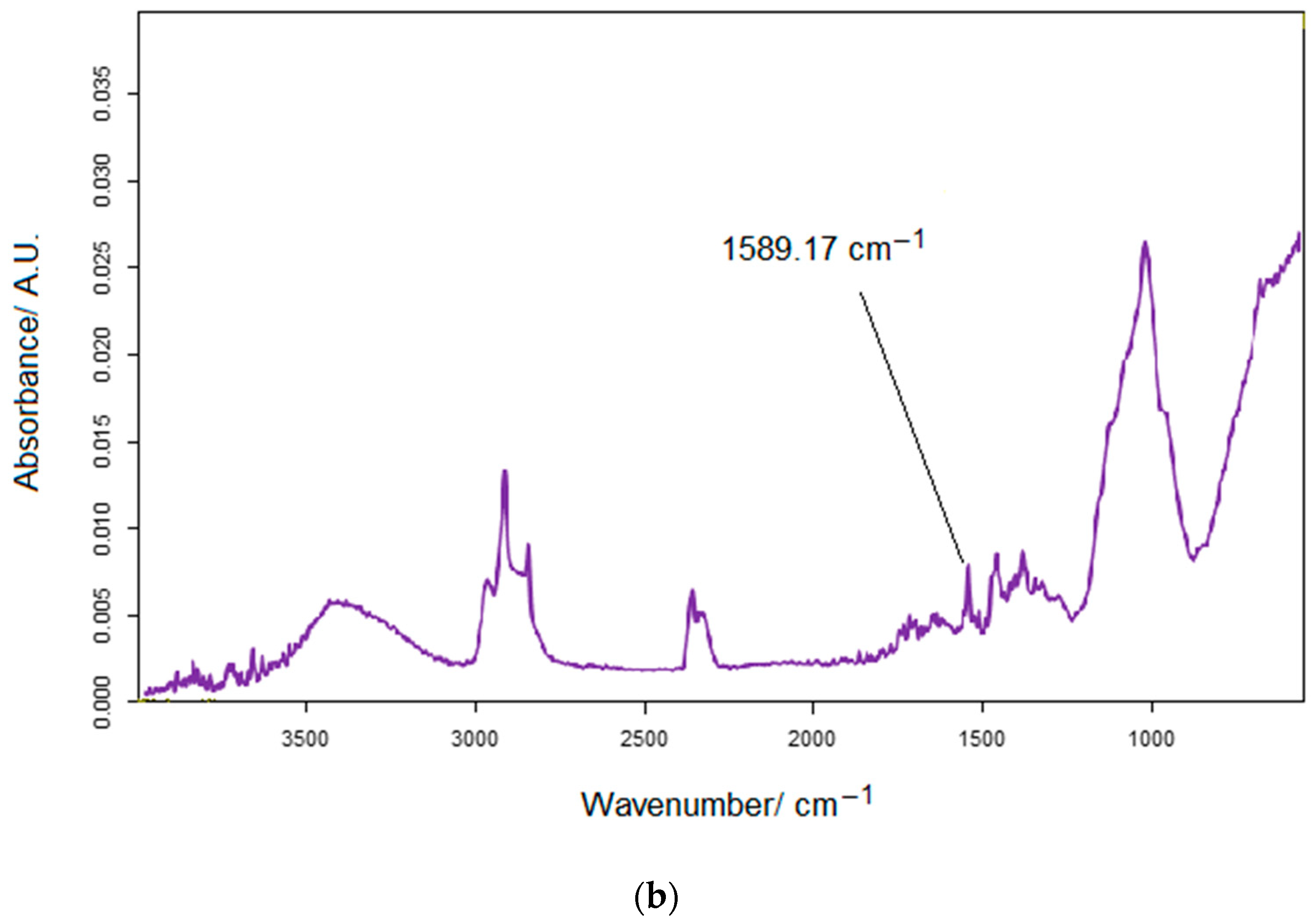
2.4. Pharmaceutical Samples and Preparation of Solutions to Be Analyzed
3. Results and Discussion
3.1. Characterization of the Active Surface of the Sensors
3.2. Characterization of the Electrochemical Behavior of Sensors in Catechol Solution
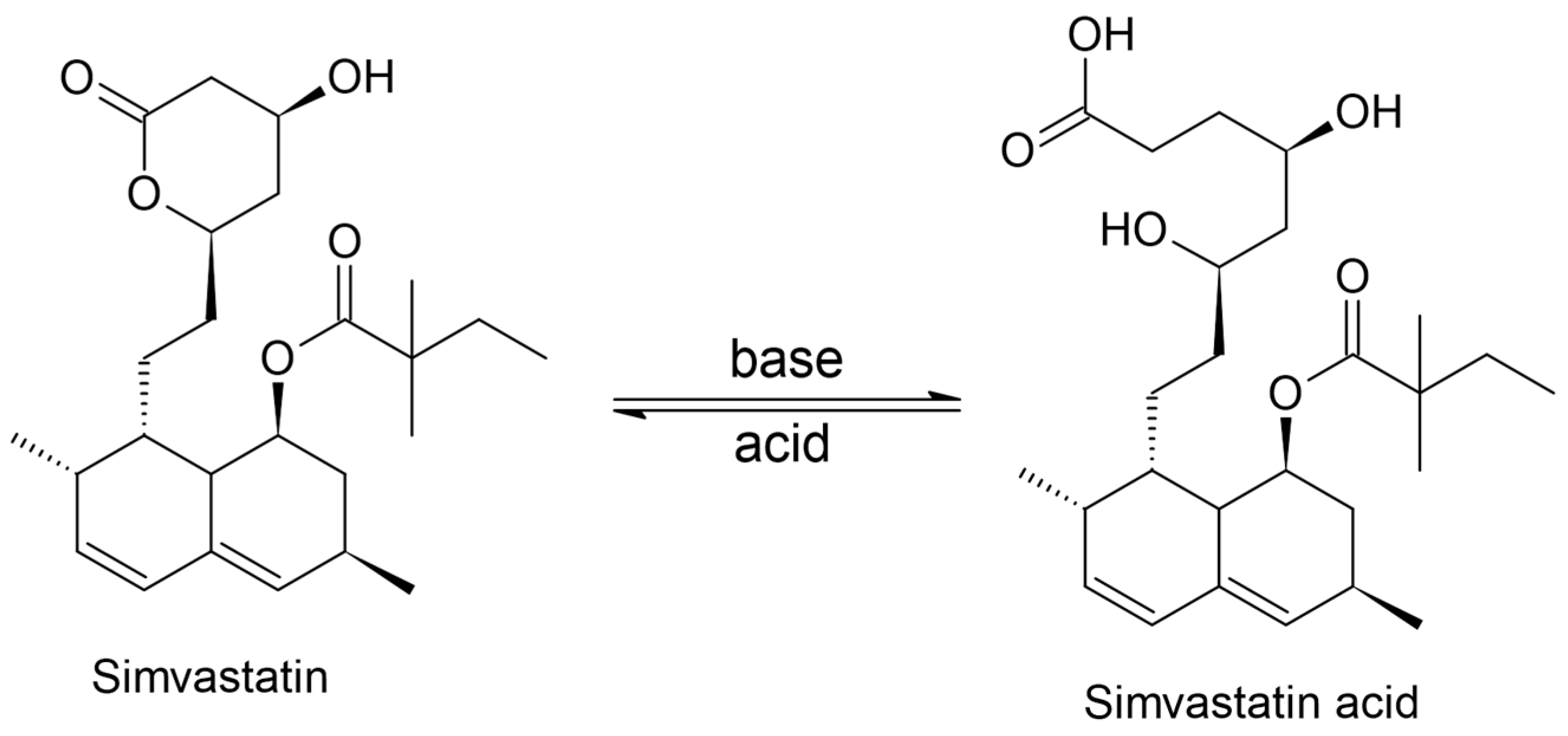
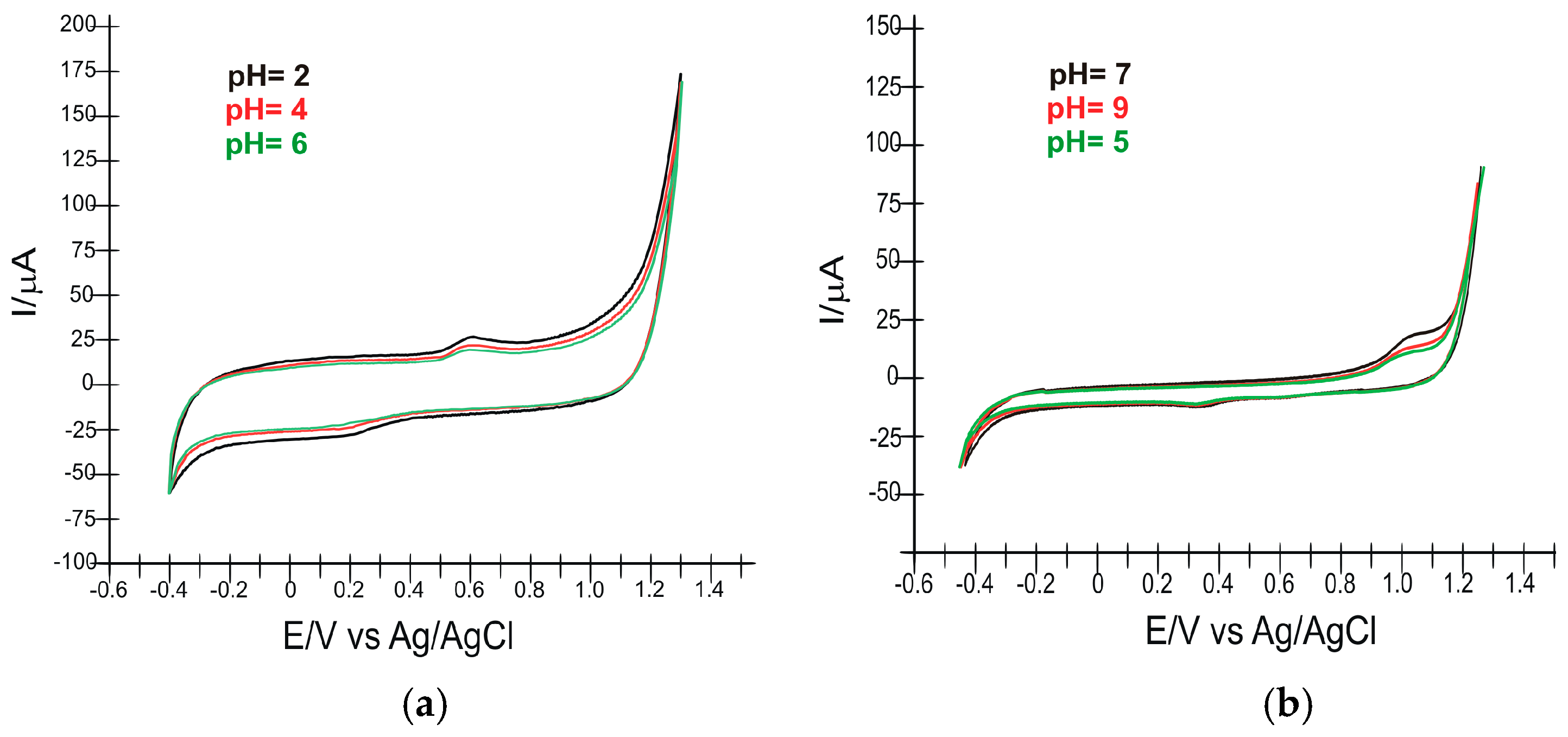
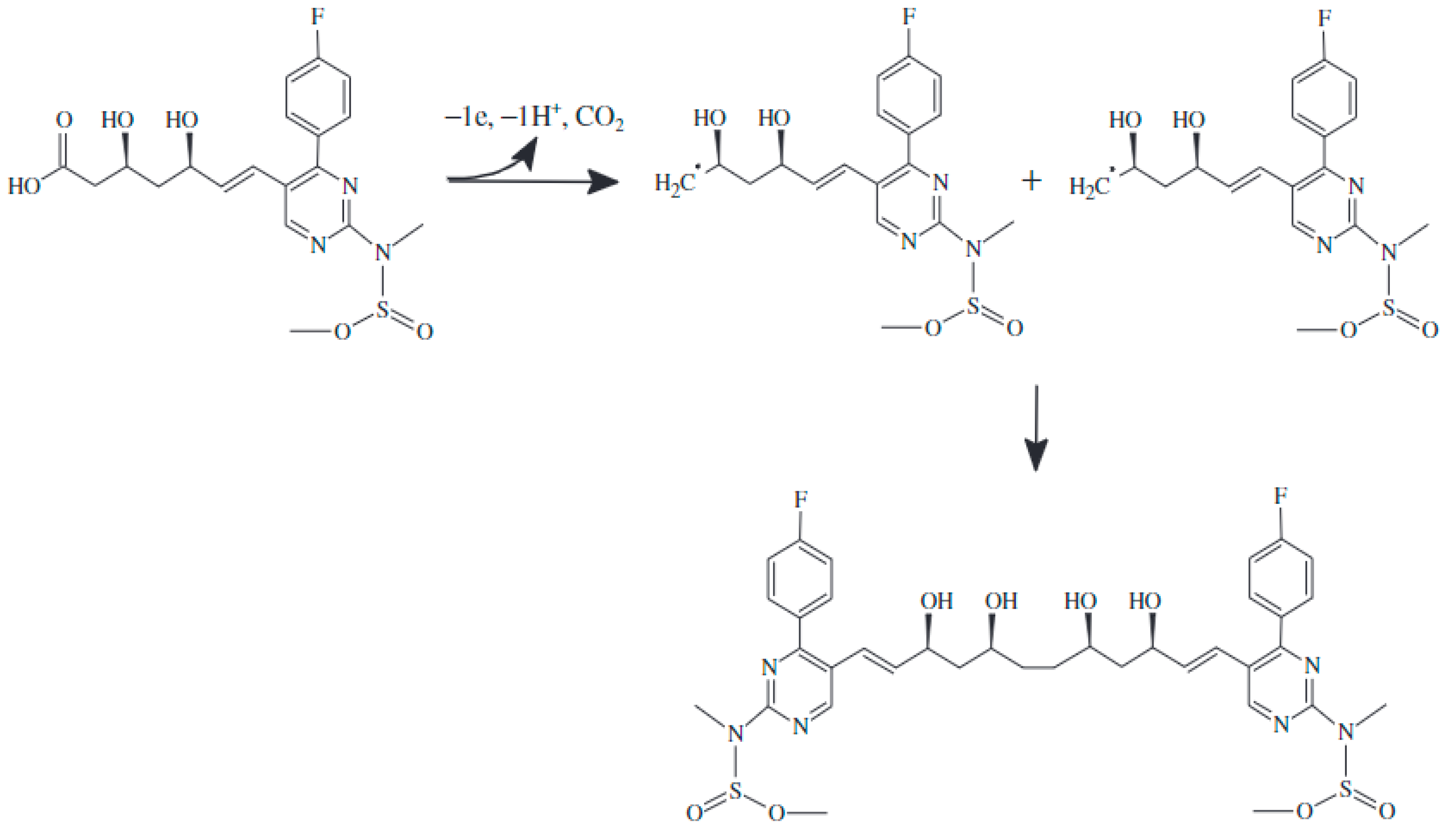
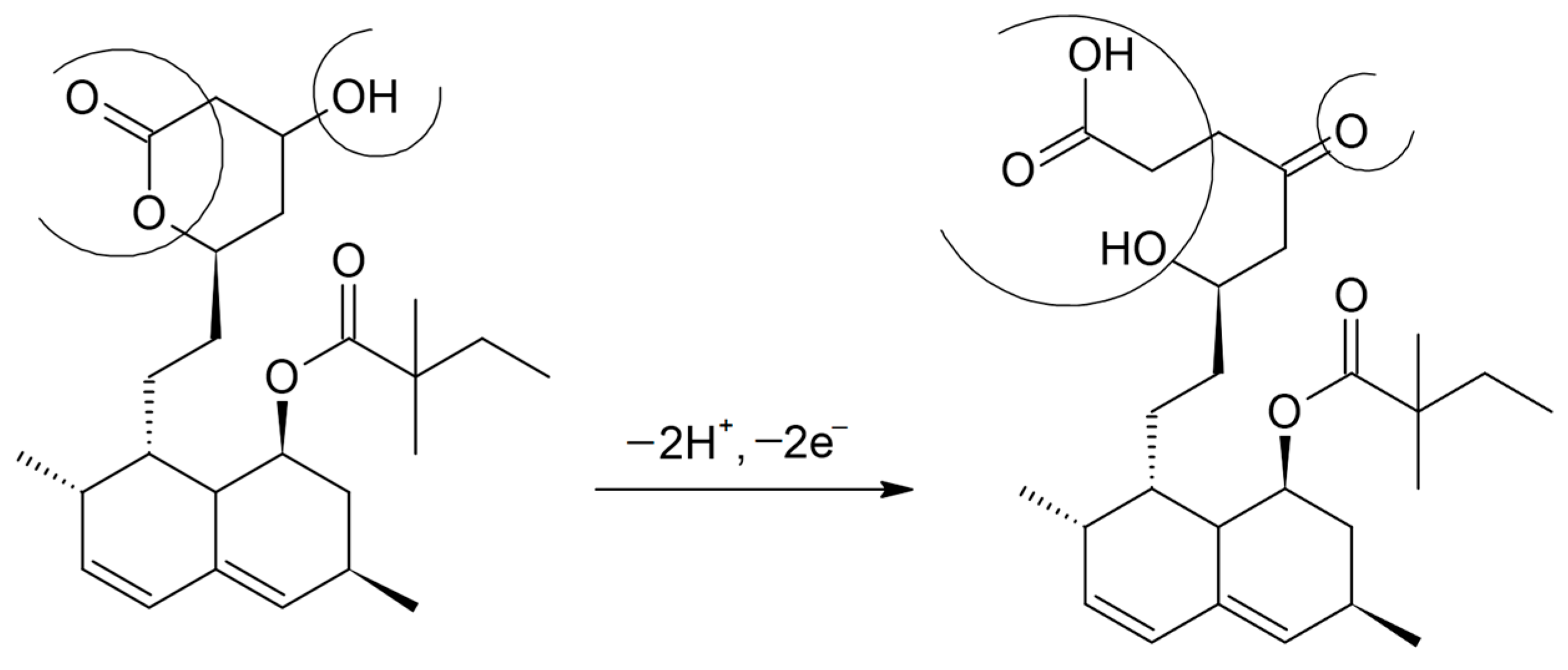
3.3. Study of the Electrochemical Behavior of the Sensors in Rosuvastatin and Simvastatin Solutions
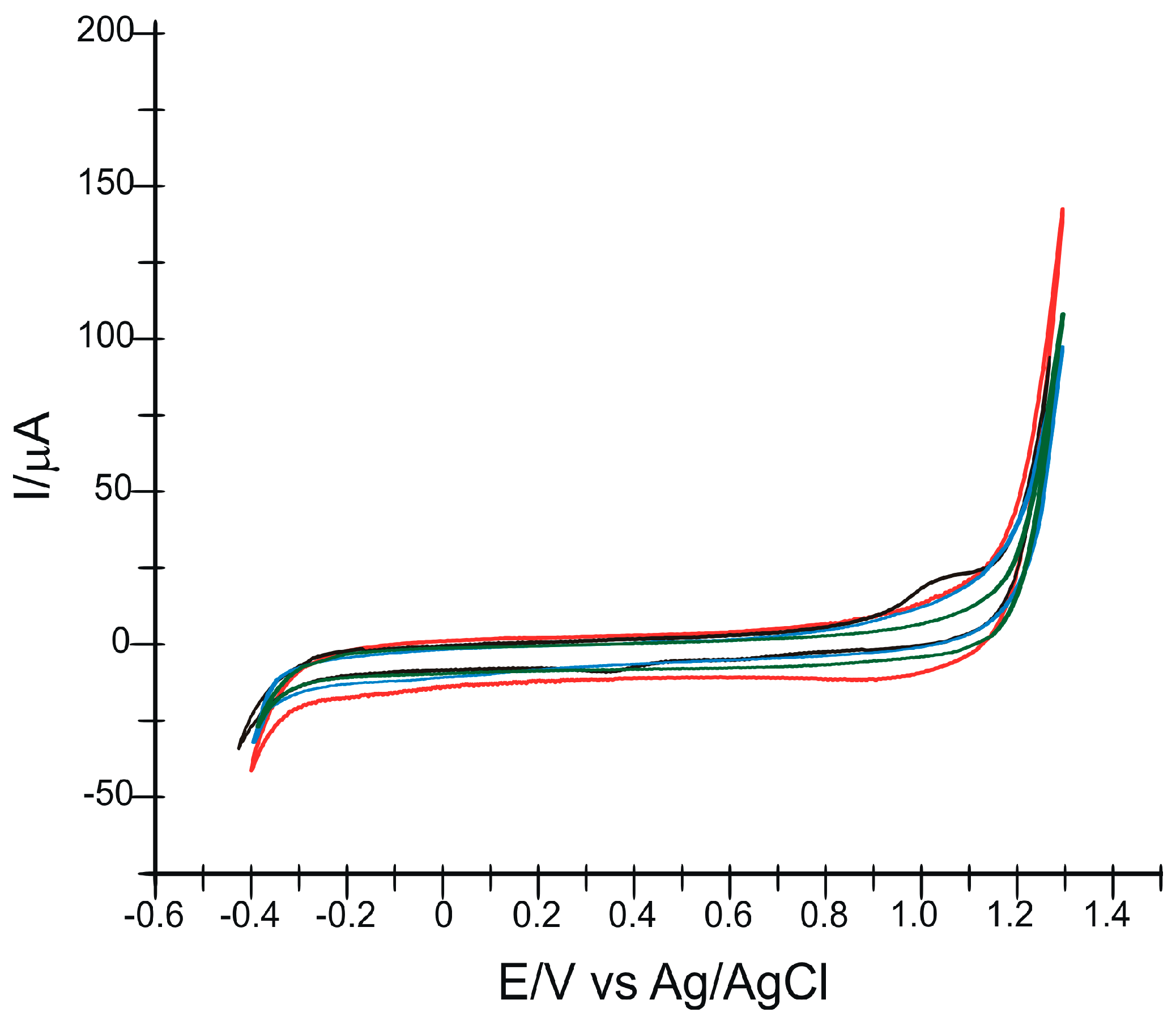
3.4. Influence of Scan Rate on Sensor Responses
- Г—concentration of the active species adsorbed on the electrode surface, mol × cm−2;
- I—current corresponding to the peak, A;
- A—electrode surface, cm2;
- n—number of electrons transferred during redox processes;
- F—Faraday’s constant, 96,485 C × mol−1;
- R—universal gas constant, 8.314 J/mol K;
- T—absolute temperature, 298 K.
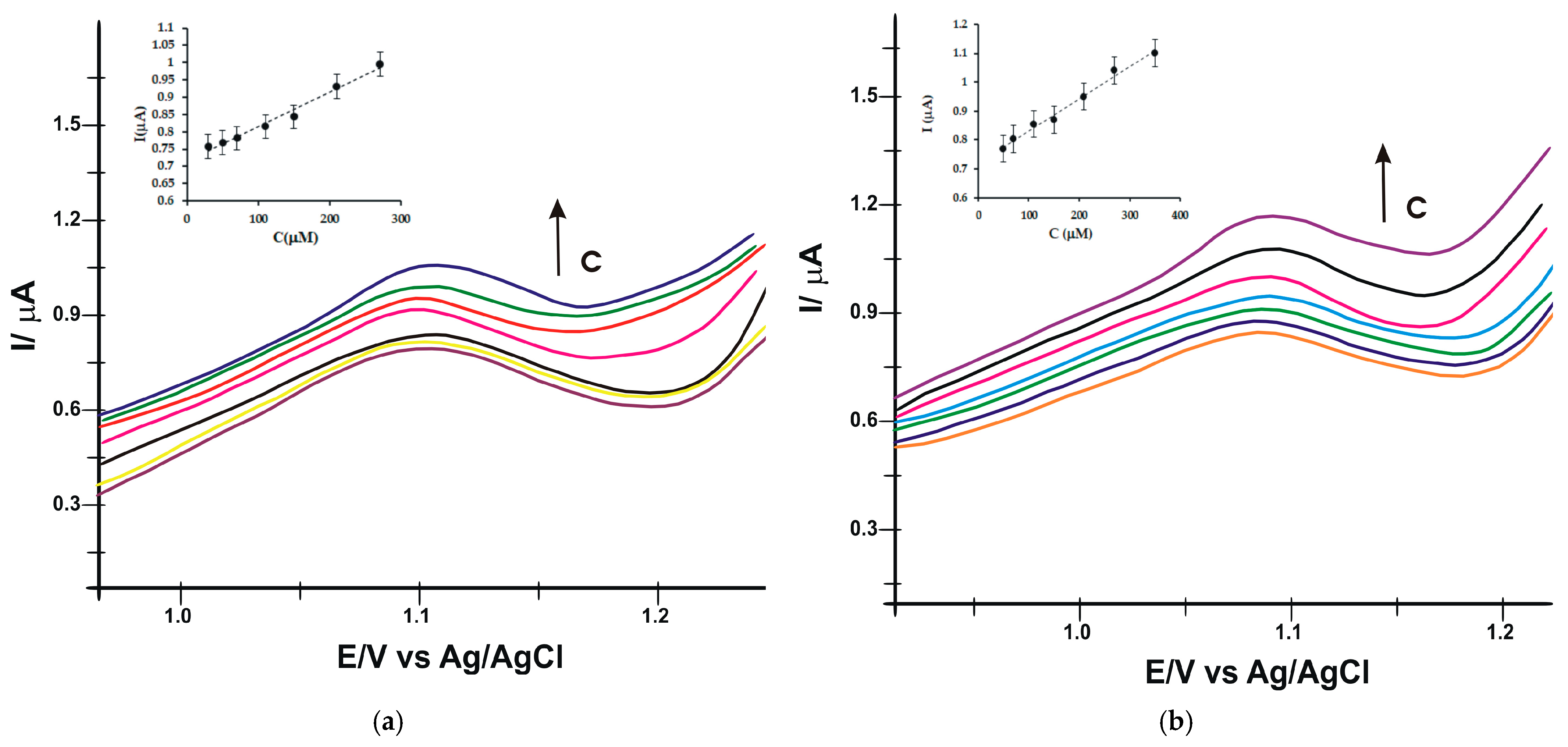
3.5. Determination of LOD and LOQ
3.6. Recovery Studies
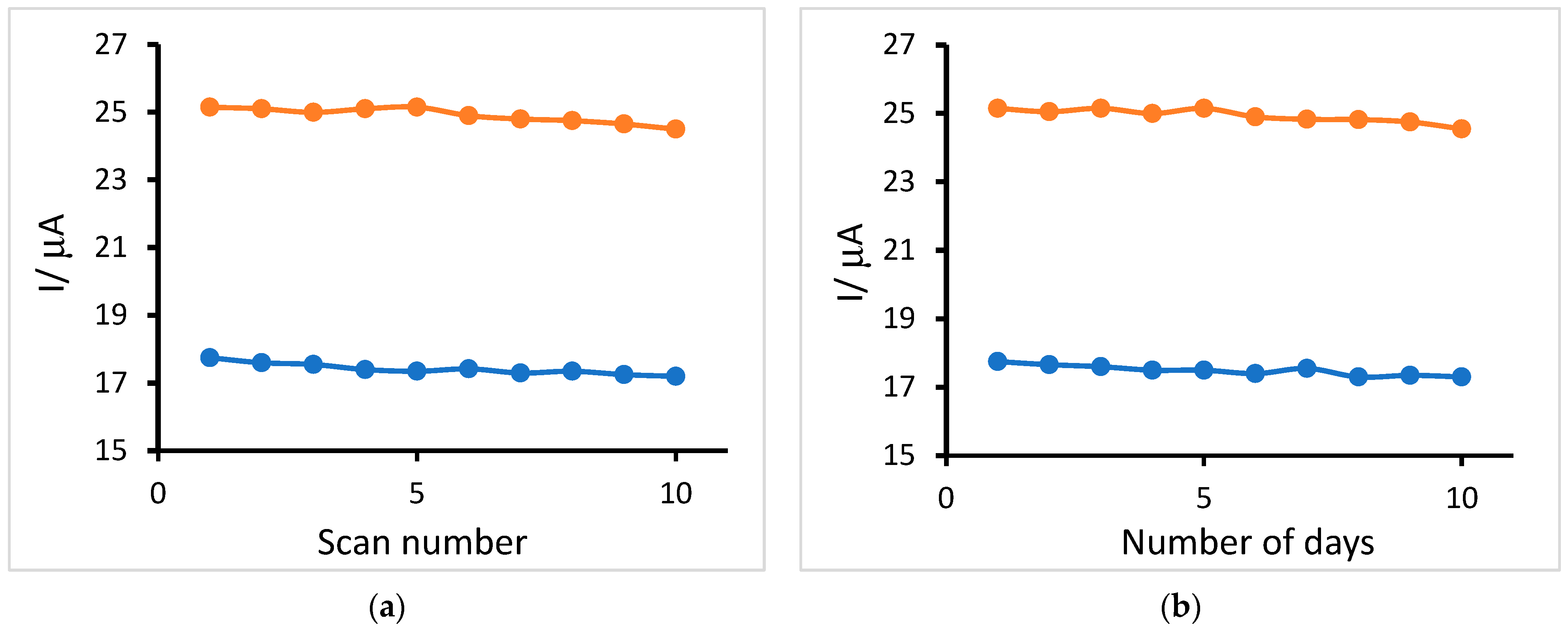
3.7. Stability and Reproducibility Studies
3.8. Interference Studies
3.9. Quantification of Statins in the Analyzed Pharmaceutical Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adhyaru, B.B.; Jacobson, T.A. Safety and Efficacy of Statin Therapy. Nat. Rev. Cardiol. 2018, 15, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Tsoukas, M.A.; Mantzoros, C.S. Lipodystrophy Syndromes. In Endocrinology: Adult and Pediatric; Elsevier: Amsterdam, The Netherlands, 2016; pp. 648–661.e5. ISBN 978-0-323-18907-1. [Google Scholar]
- West of Scotland Coronary Prevention Group. West of Scotland Coronary Prevention Study: Identification of High-Risk Groups and Comparison with Other Cardiovascular Intervention Trials. Lancet 1996, 348, 1339–1342. [Google Scholar] [CrossRef]
- Ochiai, H.; Uchiyama, N.; Imagaki, K.; Hata, S.; Kamei, T. Determination of Simvastatin and Its Active Metabolite in Human Plasma by Column-Switching High-Performance Liquid Chromatography with Fluorescence Detection after Derivatization with 1-Bromoacetylpyrene. J. Chromatogr. B Biomed. Sci. Appl. 1997, 694, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Mol, M.J.T.M.; Leuven, J.A.G.; Erkelens, D.W.; Schouten, T.A.; Stalenhoef, A.F.H. Effects of synvinolin (mk-733) on plasma lipids in familial hypercholesterolaemia. Lancet 1986, 328, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, C.; Wu, S.; Hu, S. Enhanced Oxidation of Simvastatin at a Multi-Walled Carbon Nanotubes-Dihexadecyl Hydrogen Phosphate Composite Modified Glassy Carbon Electrode and the Application in Determining Simvastatin in Pharmaceutical Dosage Forms. Electroanalysis 2005, 17, 749–754. [Google Scholar] [CrossRef]
- Krisnamurti, D.; Farida, S.; Putri, R.; Fachri, W.; Purwaningsih, E. The Effect of Simvastatin–Acalypha Indica Linn. Combination on the Improvement of Fatty Pancreas in Rats Induced with a High Fructose and Cholesterol Diet. J. Adv. Vet. Anim. Res. 2022, 9, 346. [Google Scholar] [CrossRef]
- Dyade, G.K.; Sawant, R.L.; Joshi, H.A.; Shinde, A.D.; Bandal, R.S.; Gadhingleskar, S.V. A Novel Approach: Effect of Polarity Index of Mobile Phase on Retention Time of Antihyperlipidemic Antihypertensive and Angiotensin Inhibiting Drugs in RP-HPLC Method. Res. Jour. Pharm. Technol. 2020, 13, 3065. [Google Scholar] [CrossRef]
- Alonso, R.; Cuevas, A.; Cafferata, A. Diagnosis and Management of Statin Intolerance. J. Atheroscler. Thromb. 2019, 26, 207–215. [Google Scholar] [CrossRef]
- Aydoğmuş, Z.; Asfoor, A. Electroanalytical Methods for Determination of Antiviral Drugs in Pharmaceutical Formulation and Biological Fluids: A Review. Chin. J. Anal. Chem. 2022, 50, 100063. [Google Scholar] [CrossRef]
- Antal, I.; Koneracka, M.; Zavisova, V.; Kubovcikova, M.; Kormosh, Z.; Kopcansky, P. Statins Determination: A Review of Electrochemical Techniques. Crit. Rev. Anal. Chem. 2017, 47, 474–489. [Google Scholar] [CrossRef]
- Mammone, F.R.; Rotundo, P.; Ferretti, R.; Puxeddu, M.; Silvestri, R.; Cirilli, R. Chemo- and Enantio-Selective Reversed-Phase HPLC Analysis of Rosuvastatin Using a Cellulose-Based Chiral Stationary Phase in Gradient Elution Mode. J. Pharm. Biomed. Anal. 2023, 225, 115239. [Google Scholar] [CrossRef]
- Bozhanov, S.; Maslarska, V. Spectroscopic and high-performance liquid chromatography methods for determination of statins. Pharmacia 2016, 63, 14. [Google Scholar]
- Dastaran, R.; Sheibani, A.; Shishehbore, M.R.; Saeedi-Sourck, H. Continuous Wavelet Transform for Simultaneous Determination of Atorvastatin and Rosuvastatin in Combined Pharmaceutical Formulations. J. Appl. Spectrosc. 2022, 88, 1195–1201. [Google Scholar] [CrossRef]
- Pandit, U.J.; Naikoo, G.A.; Sheikh, M.U.D.; Khan, G.A.; Raj, K.K.; Limaye, S.N. Electrochemical Determination of an Anti-Hyperlipidimic Drug Pitavastatin at Electrochemical Sensor Based on Electrochemically Pre-Treated Polymer Film Modified GCE. J. Pharm. Anal. 2017, 7, 258–264. [Google Scholar] [CrossRef]
- Silva, T.A.; Zanin, H.; Vicentini, F.C.; Corat, E.J.; Fatibello-Filho, O. Electrochemical Determination of Rosuvastatin Calcium in Pharmaceutical and Human Body Fluid Samples Using a Composite of Vertically Aligned Carbon Nanotubes and Graphene Oxide as the Electrode Material. Sens. Actuators B Chem. 2015, 218, 51–59. [Google Scholar] [CrossRef]
- Kokilambigai, K.S.; Seetharaman, R.; Lakshmi, K.S. Critical Review on the Analytical Techniques for the Determination of the Oldest Statin—Atorvastatin—In Bulk, Pharmaceutical Formulations and Biological Fluids. Crit. Rev. Anal. Chem. 2017, 47, 538–555. [Google Scholar] [CrossRef]
- Patil, V.B.; Malode, S.J.; Tuwar, S.M.; Shetti, N.P. Graphene Sheet-Based Electrochemical Sensor with Cationic Surfactant for Sensitive Detection of Atorvastatin. Sens. Int. 2022, 3, 100198. [Google Scholar] [CrossRef]
- Monteiro, M.C.; Winiarski, J.P.; Santana, E.R.; Szpoganicz, B.; Vieira, I.C. Ratiometric Electrochemical Sensor for Butralin Determination Using a Quinazoline-Engineered Prussian Blue Analogue. Materials 2023, 16, 1024. [Google Scholar] [CrossRef]
- Rebelo, P.; Pacheco, J.G.; Voroshylova, I.V.; Melo, A.; Cordeiro, M.N.D.S.; Delerue-Matos, C. A Simple Electrochemical Detection of Atorvastatin Based on Disposable Screen-Printed Carbon Electrodes Modified by Molecularly Imprinted Polymer: Experiment and Simulation. Anal. Chim. Acta 2022, 1194, 339410. [Google Scholar] [CrossRef]
- Kalate Bojdi, M.; Behbahani, M.; Ranjbari Koloyi, S. Application of a New Electrochemical Sensor Based on Carbon Paste Electrode with NiFe2O4 and Cross-Linked Chitosan Composite for Trace Detection of Atorvastatin. Appl. Chem. 2022, 17, 169–188. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Karimi, F.; Alizadeh, M.; Sanati, A.L. Electrochemical Sensors, a Bright Future in the Fabrication of Portable Kits in Analytical Systems. Chem. Rec. 2020, 20, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Lima, H.R.S.; da Silva, J.S.; de Oliveira Farias, E.A.; Teixeira, P.R.S.; Eiras, C.; Nunes, L.C.C. Electrochemical Sensors and Biosensors for the Analysis of Antineoplastic Drugs. Biosens. Bioelectron. 2018, 108, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; He, B.; Gao, B. Emerging Electrochemical Sensors for Life Healthcare. Eng. Regen. 2021, 2, 175–181. [Google Scholar] [CrossRef]
- Stefano, J.S.; Orzari, L.O.; Silva-Neto, H.A.; de Ataíde, V.N.; Mendes, L.F.; Coltro, W.K.T.; Longo Cesar Paixão, T.R.; Janegitz, B.C. Different Approaches for Fabrication of Low-Cost Electrochemical Sensors. Curr. Opin. Electrochem. 2022, 32, 100893. [Google Scholar] [CrossRef]
- Nhu-Trang, T.-T.; Nguyen, H.-N.; Thach, T.-X.; Nguyen, H.-N.; Nguyen, C.-H.; Do, M.H. A Simple Electrochemical Method for Determination of Rosuvastatin Calcium in Cholesterol-Lowering Medical Products. AIP Conf. Proc. 2022, 2610, 060002. [Google Scholar] [CrossRef]
- Karadurmus, L.; Kurbanoglu, S.; Uslu, B.; Ozkan, S.A. An Efficient, Simultaneous Electrochemical Assay of Rosuvastatin and Ezetimibe from Human Urine and Serum Samples. Methods Protoc. 2022, 5, 90. [Google Scholar] [CrossRef]
- Silva, T.A.; Pereira, G.F.; Fatibello-Filho, O.; Eguiluz, K.I.B.; Salazar-Banda, G.R. Square-Wave Voltammetric Determination of Rosuvastatin Calcium in Pharmaceutical and Biological Fluid Samples Using a Cathodically Pretreated Boron-Doped Diamond Electrode. Diam. Relat. Mater. 2015, 58, 103–109. [Google Scholar] [CrossRef]
- Parsa, A.; Heli, H. Electrodeposition of Nickel Wrinkled Nanostructure from Choline Chloride:Urea Deep Eutectic Solvent (Reline) and Application for Electroanalysis of Simvastatin. Microchem. J. 2020, 152, 104267. [Google Scholar] [CrossRef]
- Ashrafi, A.M.; Richtera, L. Preparation and Characterization of Carbon Paste Electrode Bulk-Modified with Multiwalled Carbon Nanotubes and Its Application in a Sensitive Assay of Antihyperlipidemic Simvastatin in Biological Samples. Molecules 2019, 24, 2215. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Cellat, K.; Arıkan, K.; Savk, A.; Karimi, F.; Şen, F. Palladium–Nickel Nanoparticles Decorated on Functionalized-MWCNT for High Precision Non-Enzymatic Glucose Sensing. Mater. Chem. Phys. 2020, 250, 123042. [Google Scholar] [CrossRef]
- Piovesan, J.V.; de Lima, C.A.; Santana, E.R.; Spinelli, A. Voltammetric Determination of Condensed Tannins with a Glassy Carbon Electrode Chemically Modified with Gold Nanoparticles Stabilized in Carboxymethylcellulose. Sens. Actuators B Chem. 2017, 240, 838–847. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef] [PubMed]
- Amouzadeh Tabrizi, M.; Acedo, P. An Electrochemical Impedance Spectroscopy-Based Aptasensor for the Determination of SARS-CoV-2-RBD Using a Carbon Nanofiber–Gold Nanocomposite Modified Screen-Printed Electrode. Biosensors 2022, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical Impedance Spectroscopy. Nat. Rev. Methods Primers 2021, 1, 41. [Google Scholar] [CrossRef]
- Fan, Y.; Shi, S.; Ma, J.; Guo, Y. Smartphone-Based Electrochemical System with Multi-Walled Carbon Nanotubes/Thionine/Gold Nanoparticles Modified Screen-Printed Immunosensor for Cancer Antigen 125 Detection. Microchem. J. 2022, 174, 107044. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Tyrosinase-Based Biosensor—A New Tool for Chlorogenic Acid Detection in Nutraceutical Formulations. Materials 2022, 15, 3221. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, I.M.; Apetrei, C. Voltammetric Determination of Melatonin Using a Graphene-Based Sensor in Pharmaceutical Products. IJN 2016, 2016, 1859. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001; ISBN 978-0-471-04372-0. [Google Scholar]
- Burestedt, E.; Narvaez, A.; Ruzgas, T.; Gorton, L.; Emnéus, J.; Domínguez, E.; Marko-Varga, G. Rate-Limiting Steps of Tyrosinase-Modified Electrodes for the Detection of Catechol. Anal. Chem. 1996, 68, 1605–1611. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Carbonaceous Nanomaterials Employed in the Development of Electrochemical Sensors Based on Screen-Printing Technique—A Review. Catalysts 2020, 10, 680. [Google Scholar] [CrossRef]
- García-Carmona, L.; Moreno-Guzmán, M.; Sierra, T.; González, M.C.; Escarpa, A. Filtered Carbon Nanotubes-Based Electrodes for Rapid Sensing and Monitoring of L-Tyrosine in Plasma and Whole Blood Samples. Sens. Actuators B Chem. 2018, 259, 762–767. [Google Scholar] [CrossRef]
- Olivares, F.; Peón, F.; Henríquez, R.; del Río, R.S. Strategies for Area-Selective Deposition of Metal Nanoparticles on Carbon Nanotubes and Their Applications: A Review. J. Mater. Sci. 2022, 57, 2362–2387. [Google Scholar] [CrossRef]
- Darroudi, A.; Nazari, S.; Marashi, S.A.; Nazar Abad, M.K. Determination of Simvastatin by Voltammetry Method at Screen-Printed Electrode Modified by Graphene Oxide Nanosheets and Sodium Dodecyl Sulfate. J. Electrochem. Soc. 2022, 169, 026501. [Google Scholar] [CrossRef]
- El-Wekil, M.M.; Abdelhady, K.K.; Abdel Salam, R.A.; Hadad, G.M.; Ali, R. Facile Synthesis of Novel Nanocomposite Prepared from Spinel Copper Ferrite and Reduced Graphene Oxide in the Presence of Anti-Fouling Agent Diethyl Ammonium Acid Sulphate for Ultrasensitive Detection of Rosuvastatin in Human Plasma. Microchem. J. 2019, 147, 1133–1140. [Google Scholar] [CrossRef]
- Rus, I.; Tertiș, M.; Barbălată, C.; Porfire, A.; Tomuță, I.; Săndulescu, R.; Cristea, C. An Electrochemical Strategy for the Simultaneous Detection of Doxorubicin and Simvastatin for Their Potential Use in the Treatment of Cancer. Biosensors 2021, 11, 15. [Google Scholar] [CrossRef]
- Laviron, E.; Roullier, L.; Degrand, C. A Multilayer Model for the Study of Space Distributed Redox Modified Electrodes: Part II. Theory and Application of Linear Potential Sweep Voltammetry for a Simple Reaction. J. Electroanal. Chem. Interfacial Electrochem. 1980, 112, 11–23. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Rodriguez-Mendez, M.L.; Apetrei, C.; de Saja, J.A. Fish Freshness Monitoring Using an E-Tongue Based on Polypyrrole Modified Screen-Printed Electrodes. IEEE Sens. J. 2013, 13, 2548–2554. [Google Scholar] [CrossRef]
- Fayazfar, H.; Afshar, A.; Dolati, A.; Ghalkhani, M. Modification of Well-Aligned Carbon Nanotubes with Dihexadecyl Hydrogen Phosphate: Application to Highly Sensitive Nanomolar Detection of Simvastatin. J. Appl. Electrochem. 2014, 44, 263–277. [Google Scholar] [CrossRef]
- El-Zahry, M.R.; Ali, M.F.B. Enhancement Effect of Reduced Graphene Oxide and Silver Nanocomposite Supported on Poly Brilliant Blue Platform for Ultra-Trace Voltammetric Analysis of Rosuvastatin in Tablets and Human Plasma. RSC Adv. 2019, 9, 7136–7146. [Google Scholar] [CrossRef]
- Coruh, Ö.; Özkan, S.A. Determination of the Antihyperlipidemic Simvastatin by Various Voltammetric Techniques in Tablets and Serum Samples. Die Pharm.-Int. J. Pharm. Sci. 2006, 61, 285–290. [Google Scholar]
- Salih, O.; Samein, L.H.; Ali, W. Formulation and in Vitro Evaluation of Rosuvastatin Calcium Niosomes. Int. J. Pharm. Pharm. Sci. 2013, 5, 525. [Google Scholar]
- Mallah, M.A.; Sherazi, S.T.H.; Bhanger, M.I.; Mahesar, S.A.; Bajeer, M.A. A Rapid Fourier-Transform Infrared (FTIR) Spectroscopic Method for Direct Quantification of Paracetamol Content in Solid Pharmaceutical Formulations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 141, 64–70. [Google Scholar] [CrossRef] [PubMed]





| Sensor | I vs. v | R2 | log (I vs. v) | R2 |
|---|---|---|---|---|
| Rosuvastin | ||||
| SPCE | I(μA) = 96.099v(V/s) + 4.0549 | 0.9946 | log I(μA) = 0.9312 log v(V/s) + 1.8259 | 0.967 |
| AuNP-MWCNTs/SPCE | I(μA) = 218.11v(V/s) + 9.0463 | 0.9954 | log I(μA) = 0.8871 log v(V/s) + 2.066 | 0.977 |
| Simvastatin | ||||
| SPCE | I(μA) = 76.199v(V/s) + 2.0539 | 0.9955 | log I(μA) = 0.4512 log v(V/s)+ 1.0524 | 0.968 |
| AuNP-MWCNTs/SPCE | I(μA) = 208.17v(V/s) + 8.0253 | 0.9962 | log I(μA) = 0.7861 log v(V/s) + 2.125 | 0.978 |
| Electrode | Slope (A × s/V) | Γ (mol × cm−2) |
|---|---|---|
| Rosuvastatin | ||
| SPCE | 0.000096099 | 3.512·10−11 |
| AuNP-MWCNTs/SPCE | 0.00021811 | 6.829·10−11 |
| Simvastatin | ||
| SPCE | 0.000076199 | 6.969·10−11 |
| AuNP-MWCNTs/SPCE | 0.00020817 | 1.904·10−10 |
| Sensor/Analyte | Calibration Equation | R2 | LOD (μM) | LOQ (μM) | ||||
|---|---|---|---|---|---|---|---|---|
| RV | SV | RV | SV | RV | SV | RV | SV | |
| SPCE | I = 0.0009c + 0.365 | I = 0.001c + 0.713 | 0.986 | 0.981 | 5.37 | 4.83 | 17.9 | 16.1 |
| AuNP-MWCNTs/SPCE | I = 0.0032c + 0.666 | I = 0.0011c + 0.720 | 0.954 | 0.988 | 0.151 | 0.302 | 5.03 | 1.01 |
| Sensor | LOD (μM) | LOQ (μM) | Linearity Range (μM) | Ref. |
|---|---|---|---|---|
| RV | ||||
| GCE | 0.194 | 0.66 | 150–2500 | [26] |
| GCE | 2 | 6.6 | 5–12.5 | [27] |
| Boron-doped Diamond Electrode (BDD) | 1.04 | - | 9.40–88.8 | [28] |
| AuNP-MWCNTs/SPCE | 0.151 | 5.03 | 20–275 | This work |
| SV | ||||
| Ni/NiWN (nickel hydroxide) electrode | 4.88 | 16.3 | 50–400 | [29] |
| GCE | 0.550 | - | 2–100 | [51] |
| Carbon Paste Electrode Bulk-Modified with Multiwalled Carbon Nanotubes | 0.24 | 0.8 | 3.75–20 | [30] |
| AuNP-MWCNTs/SPCE | 0.302 | 1.01 | 50–350 | This work |
| Added Concentration (×10−5 M) | Found Concentration (×10−5 M) | Recovery (%) ± RSD (n = 5) |
|---|---|---|
| Rosuvastatin | ||
| 4 | 4.23 | 105.8 ± 0.9 |
| 7 | 6.97 | 99.6 ± 0.8 |
| 10 | 10.06 | 100.6 ± 0.9 |
| Simvastatin | ||
| 2 | 2.13 | 106.5 ± 0.9 |
| 4 | 3.97 | 99.3 ± 0.8 |
| 6 | 6.16 | 102.6 ± 0.9 |
| Stock Solution | Interferent Compound | Raport | Recovery/% |
|---|---|---|---|
| RV | Atorvastatin | 1:1 | 102 ± 1.4 |
| SV | 1:1 | 103 ± 2.2 |
| Pharmaceutical Product | Quantity Reported by Producer/mg | Detected Quantity | |||||
|---|---|---|---|---|---|---|---|
| CV Method (mg) | Coefficient of Variation (%) | FTIR Method (mg) | Coefficient of Variation (%) | p Value | t Statistic | ||
| Crestor | 10 | 9 | 2.2 | 10 | 1.7 | 0.6109 | −0.542 |
| 20 | 21 | 1.0 | 22 | 2.0 | |||
| 40 | 41 | 1.4 | 39 | 2.0 | |||
| Simvacard | 10 | 10 | 2.3 | 9 | 2.5 | ||
| 20 | 21 | 1.0 | 19 | 1.9 | |||
| 40 | 40 | 1.3 | 41 | 1.5 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roșca, R.O.; Bounegru, A.V.; Apetrei, C. Quantification of Statins in Pharmaceutical Products Using Screen-Printed Sensors Based of Multi-Walled Carbon Nanotubes and Gold Nanoparticles. Inventions 2023, 8, 111. https://doi.org/10.3390/inventions8050111
Roșca RO, Bounegru AV, Apetrei C. Quantification of Statins in Pharmaceutical Products Using Screen-Printed Sensors Based of Multi-Walled Carbon Nanotubes and Gold Nanoparticles. Inventions. 2023; 8(5):111. https://doi.org/10.3390/inventions8050111
Chicago/Turabian StyleRoșca, Ramona Oana, Alexandra Virginia Bounegru, and Constantin Apetrei. 2023. "Quantification of Statins in Pharmaceutical Products Using Screen-Printed Sensors Based of Multi-Walled Carbon Nanotubes and Gold Nanoparticles" Inventions 8, no. 5: 111. https://doi.org/10.3390/inventions8050111



.jpg)




