Control of Tungiasis in Absence of a Roadmap: Grassroots and Global Approaches
Abstract
:1. Introduction
2. Life Cycle
3. Epidemiology
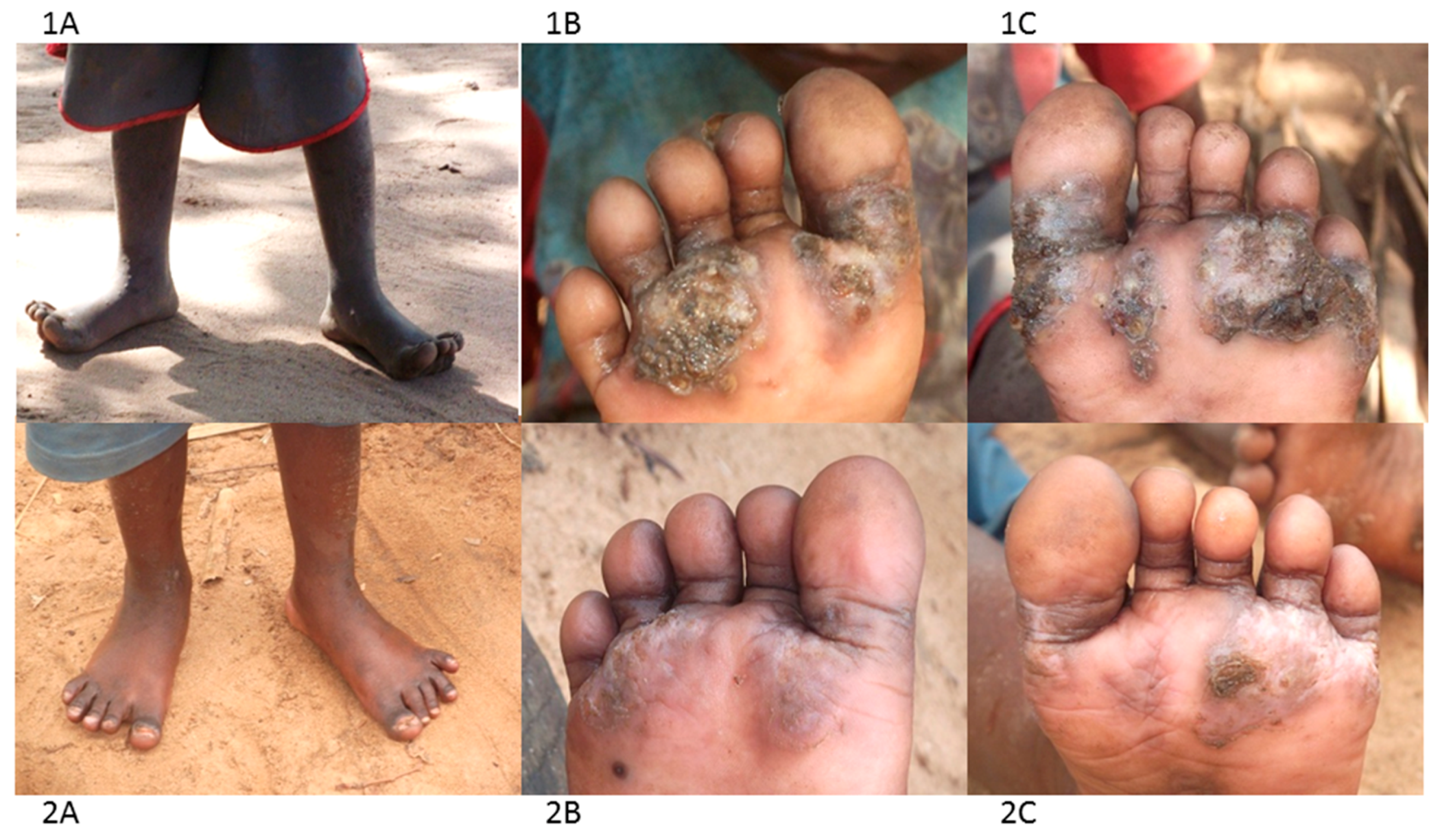
4. Morbidity
5. Prevention
6. Treatment
7. Control of Off-Host Stages
8. Control of Animal Tungiasis
9. Digital Mapping Technologies
10. A New Grassroots Approach
11. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nagy, N.; Abari, E.; D’Haese, D.; Calheiros, C.; Heukelbach, J.; Mencke, N.; Feldmeier, H.; Mehlhorn, H. Investigations on the life cycle and morphology of Tunga penetrans in Brazil. Parasitol. Res. 2007, 101 (Suppl. 2), S233–S242. [Google Scholar] [CrossRef] [PubMed]
- Heukelbach, J.; de Oliveira, F.; Hesse, G.; Feldmeier, H. Tungiasis: A neglected health problem of poor communities. Trop. Med. Int. Health 2001, 6, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Heukelbach, J.; Ugbomoiko, U. Tungiasisin the past and present: A dire need for intervention. Niger. J. Parasitol. 2007, 28, 1–5. [Google Scholar]
- Chadee, D. Tungiasis among five communities in south-western Trinidad, West Indies. Ann. Trop. Med. Parasitol. 1998, 92, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Ratonovato, J.; Randriambelosoa, J.; Robert, V. Tunga penetrans (Insecta, Siphonaptera,Tungidae) à Madagascar: Une nuisance négligée. Rev. Méd. Vét. 2008, 159, 551–556. [Google Scholar]
- Ugbomoiko, U.S.; Ofoezie, I.E.; Heukelbach, J. Tungiasis: High prevalence, parasite load, and morbidity in arural community in Lagos State, Nigeria. Int. J. Dermatol. 2007, 46, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Wilcke, T.; Heukelbach, J.; Moura, R.C.S.; Kerr-Pontes, L.R.S.; Feldmeier, H. High prevalence of tungiasis in a poor neighbourhood in Fortaleza, Northeast Brazil. Acta Trop. 2002, 83, 255–258. [Google Scholar] [CrossRef]
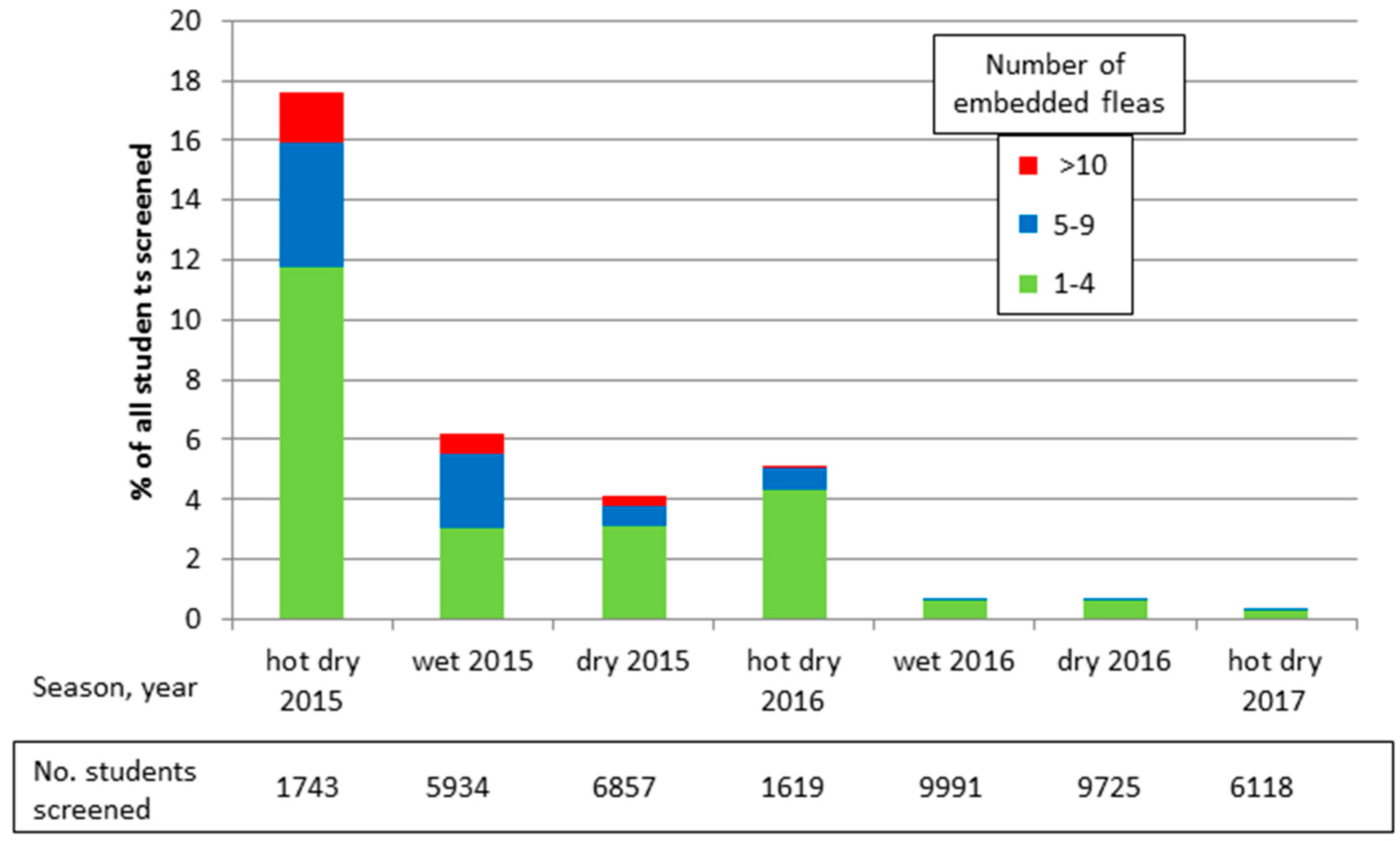
- Heuklbach, J.; Wilcke, T.; Harms, G.; Feldmeier, H. Seasonal Variation of tungiasis in an endemic community. Am. J. Trop. Med. Hyg. 2005, 72, 145–149. [Google Scholar]
- Bezerra, S. Tungiasis—An unusual case of severe infestation. Int. J. Dermatol. 1994, 33, 725. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A. Generalized tungiasis treated with thiabendazole. Arch. Dermatol. 1981, 117, 127. [Google Scholar] [CrossRef]
- Feldmeier, H.; Charité University Medicine, Berlin, Germany. Personal observation. 2017.
- Ugbomoiko, U.S.; Ariza, L.; Ofoezie, I.E.; Heukelbach, J. Risk factors for tungiasis in Nigeria: Identification of targets for effective intervention. PLoS Negl. Trop. Dis. 2007, 1, e87. [Google Scholar] [CrossRef] [PubMed]
- Muehlen, M.; Feldmeier, H.; Wilcke, T.; Winter, B.; Heukelbach, J. Identifying risk factors for tungiasis and heavy infestation in a resource-poor community in Northeast Brazil. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Njau, N.; Wanzala, P.; Mutugi, M.; Ariza, L.; Heukelbach, J. Tungiasis (jigger infestation) in rural Kenya, an emerging infectious disease. Retrovirology 2012, 9 (Suppl. 1), P37. [Google Scholar] [CrossRef]
- Cooper, J. Tunga penetrans infestation in pigs. Vet. Rec. 1976, 98, 472. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J. An outbreak of Tunga penetrans in a pig herd. Vet. Rec. 1967, 80, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Pampiglione, S.; Fioravanti, M.; Gustinelli, A.; Onore, G.; Mantovani, A. Sand flea (Tunga spp.) infections in humans and domestic animals: State of the art. Med. Vet. Entomol. 2009, 23, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Heukelbach, J.; Costa, A.; Wilcke, T.; Mencke, N.; Feldmeier, H. The animal reservoir of Tunga penetrans in severely affected communities of north-east Brazil. Med. Vet. Entomol. 2004, 18, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Pilger, D.; Schwalfenberg, S.; Heukelbach, J.; Witt, L.; Mehlhorn, H.; Mencke, N.; Feldmeier, H. Investigations on the biology, epidemiology, pathology, and control of Tunga penetrans in Brazil: VII. The importance of animal reservoirs for human infestation. Parasitol. Res. 2008, 102, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Feldmeier, H.; Charité University Medicine, Berlin, Germany. Personal observation. 2015.
- Feldmeier, H.; Kehr, J.; Poggensee, G.; Heukelbach, J. High exposure to Tunga penetrans (Linnaeus, 1758) correlates with intensity of infestation. Mem. Inst. Oswaldo Cruz. 2006, 10, 65–69. [Google Scholar] [CrossRef]
- Eisele, M.; Heukelbach, J.; Marck, E.V.; Mehlhorn, H.; Meckes, O.; Franck, S.; Feldmeier, H. Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil: I. Natural history of tungiasis in man. Parasitol. Res. 2003, 90, 87–99. [Google Scholar] [PubMed]
- Joseph, J.; Bazile, J.; Mutter, J.; Shin, S.; Ruddle, A.; Ivers, L.; Lyon, E.; Farmer, P. Tungiasis in rural Haiti: A community-based response. Trans. R. Soc. Trop. Med. Hyg. 2006, 100, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Tonge, B. Tetanus from chigger flea sores. J. Trop. Pediatr. 1989, 35, 94. [Google Scholar] [CrossRef] [PubMed]
- Feldmeier, H.; Eisele, M.; Saboia-Moura, R.; Heukelbach, J. Severe tungiasis in underprivileged communities: Case series from Brazil. Emerg. Infect. Dis. 2003, 9, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Feldmeier, H.; Heukelbach, J.; Eisele, M.; Sousa, A.; Barbosa, L.; Carvalho, C. Bacterial superinfection in human tungiasis. Trop. Med. Int. Health 2002, 7, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Weise, S.; Elson, L.; Feldmeier, H. Tungiasis-related life quality impairment in children living in rural Kenya. PLoS Negl. Trop. Dis. 2017, in press. [Google Scholar]
- Ngunjiri, J.; Keiyoro, P.N.; Mwanda, W. Impact of tungiasis on acquisition of basic education among children aged 5–14 years. Int. J. Sci. Res. Innov. Technol. 2015, 2, 128–142. [Google Scholar]
- Muhoro, B.; Odhiambo, S.; Mbithi, M. Effect of jigger infestation on agricultural productivity: A case study of Murarandia Division. Int. J. Econ. 2016, 1, 1–11. [Google Scholar]
- Mutebi, F.; Krücken, J.; Feldmeier, H.; Waiswa, C.; Mencke, N.; Sentongo, E. Animal reservoirs of zoonotic tungiasis in endemic rural villages of Uganda. PLoS Negl. Trop. Dis. 2015, 9, e0004126. [Google Scholar] [CrossRef] [PubMed]
- Mutebi, F.; Krücken, J.; Feldmeier, H.; Waiswa, C.; Mencke, N.; Samson-Himmelstjerna, G. Tungiasis-associated morbidity in pigs and dogs in endemic villages of Uganda. Parasites Vectors 2016, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Mutebi, F.; von Samson-Himmelstjerna, G.; Feldmeier, H.; Waiswa, C.; Bukeka Muhindo, J.; Krucken, J. Successful treatment of severe tungiasis in pigs using a topical aerosol containing chlorfenvinphos, dichlorphos andgentian violet. PLoS Negl. Trop. Dis. 2016, 10, e0005056. [Google Scholar] [CrossRef] [PubMed]
- Feldmeier, H.; Kehr, J.; Heukelbach, J. A plant-based repellent protects against Tunga penetrans infestation and sand flea disease. Acta Trop. 2006, 99, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Buckendahl, J.; Heukelbach, J.; Ariza, L.; Kehr, J.; Seidenschwang, M.; Feldmeier, H. Control of tungiasis through intermittent application of a plant-based repellent: An intervention study in a resource-poor community in Brazil. PLoS Negl. Trop. Dis. 2010, 4, e879. [Google Scholar] [CrossRef] [PubMed]
- Thielecke, M.; Raharimanga, V.; Rogier, C.; Stauss-Grabo, M.; Richard, V.; Feldmeier, H. Prevention of tungiasis and tungiasis-associated morbidity using the plant-based repellent Zanzarin: A randomized, controlled field study in rural Madagascar. PLoS Negl. Trop. Dis. 2013, 7, e2426. [Google Scholar] [CrossRef] [PubMed]
- Veraldi, S.; Valsecchi, M. Imported tungiasis: A report of 19 cases and review of the literature. Int. J. Dermatol. 2007, 46, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Winter, B.; Oliveira, F.; Wilcke, T.; Heukelbach, J.; Feldmeier, H. Tungiasis-related knowledge and treatment practices in two endemic communities in northeast Brazil. J. Infect. Dev. Ctries. 2009, 3, 458–466. [Google Scholar] [PubMed]
- Kamau, T.; House, S. The potential risk of HIV infection and transmission of other blood-borne pathogens through the sharing of needles and pins among people infested with jiggers in Kenya. Int. J. Health Sci. Res. 2014, 4, 278–285. [Google Scholar]
- Feldmeier, H.; Sentongo, E.; Krantz, I. Tungiasis (sand flea disease): A parasitic disease with intriguing challenges for public health. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 32, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Heukelbach, J.; Bonow, I.; Witt, L.; Feldmeier, H.; Fischer, P. High infection rate of Wolbachia endobacteria in the sand flea Tunga penetrans from Brazil. Acta Trop. 2004, 92, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Elson, L.; Dabaso Tujengane CBO, Watamu, Kenya. Personal observation. 2015.
- Elson, L.; Dabaso Tujengane CBO, Watamu, Kenya. Personal observation. 2017.
- Maunguja, A.B. Assessment of plant diversity and utilization of wild medicinal species by households proximate to Arabuko Sokoke Forest in Kilifi County of Kenya. Master’s Thesis, University of Nairobi, Nairobi, Kenya, 2016. [Google Scholar]
- Thielecke, M.; Nordin, P.; Ngomi, N.; Feldmeier, H. Treatment of tungiasis with dimeticone: A proof-of-principle study in rural Kenya. PLoS Negl. Trop. Dis. 2014, 8, e3058. [Google Scholar] [CrossRef] [PubMed]
- Nordin, P.; Thielecke, M.; Ngomi, N.; Mudanga, G.; Krantz, I.; Feldmeier, H. Treatment of tungiasis with a two-component dimeticone: A comparisonbetween moistening the whole foot and directly targeting the embedded sand fleas. Trop. Med.Health 2017, 45, 6. [Google Scholar] [CrossRef] [PubMed]
- Nair, B. Final report on the safety assessment of stearoxy dimethicone. Int. J. Toxicol. 2003, 22 (Suppl. 2), 11–35. [Google Scholar] [PubMed]
- Linardi, P.; Calheiros, C.M.; Campelo-Junior, E.; Duarte, E.; Heukelbach, J.; Feldmeier, H. Occurrence of the off-host life stages of Tunga penetrans (Siphonaptera) in various environments in Brazil. Ann. Trop. Med. Par. 2010, 104, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, U.; Elson, L.; Feldmeier, H. Location of off-host stages of Tunga penetrans in Kenya and Uganda. 2017; manuscript in preparation. [Google Scholar]
- Datar, G.; Earthenable, Kigali, Rwanda. Personal communication, 2017.
- Klimpel, S.; Mehlhorn, H.; Heukelbach, J.; Feldmeier, H.; Mencke, N. Field trial of the efficacy of a combination of imidacloprid and permethrin against Tunga penetrans (sand flea, jigger flea) in dogs in Brazil. Parasitol. Res. 2005, 97, S113–S120. [Google Scholar] [CrossRef] [PubMed]
- Brooker, S.; Hotez, P.J.; Bundy, D.A.P. The Global Atlas of Helminth Infection: Mapping the way forward in neglected tropical disease control. PLoS Negl. Trop. Dis. 2010, 4, e779. [Google Scholar] [CrossRef] [PubMed]
- Brooker, S.; Utzinger, J. Integrated disease mapping in a polyparasitic world. Geospat. Health 2007, 1, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.; Eisen, R.J. Need for improved methods to collect and present spatial epidemiologic data for vectorborne diseases. Emerg. Infect. Dis. 2007, 13, 1816–1820. [Google Scholar] [CrossRef] [PubMed]
- Flueckiger, R.M.; Nikolay, B.; Gelderblom, H.C.; Smith, J.L.; Haddad, D.; Tack, W.; Hendrickx, G.; Addiss, D.; Cano, J.; Hatcher, D.R.; et al. integrating data and resources on neglected tropical diseases for better planning: The NTD Mapping Tool (NTDmap.org). PLoS Negl. Trop. Dis. 2015, 9, e0003400. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.I.; Snow, R.W. The Malaria Atlas Project: Developing global maps of malaria risk. PLoS Med. 2006, 3, e473. [Google Scholar] [CrossRef] [PubMed]
- Hürlimann, E.; Schur, N.; Boutsika, K.; Stensgaard, A.S.; Himpsl, M.L.D.; Ziegelbauer, K.; Laizer, N.; Camenzind, L.; Di Pasquale, A.; Ekpo, U.F.; et al. Toward an open-access global database for mapping, control, and surveillance of neglected tropical diseases. PLoS Negl. Trop. Dis. 2011, 5, e1404. [Google Scholar] [CrossRef] [PubMed]
- Mann, R. This wormy world. Available online: http://www.thiswormyworld.org/news-blogs/blogs/mapping-trachoma-data-collection-and-approval (accessed on 15 January 2017).
- Smith, J.L.; Flueckiger, R.M.; Hooper, P.J.; Polack, S.; Cromwell, E.A.; Palmer, S.L.; Emerson, P.M.; Mabey, D.C.W.; Solomon, A.W.; Haddad, D.; et al. The geographical distribution and burden of trachoma in Africa. PLoS Negl. Trop. Dis. 2013, 7, e2359. [Google Scholar] [CrossRef]
- Cromley, E.K.; McLafferty, S. GIS and Public Health; The Guilford Press: New York, NY, USA, 2012. [Google Scholar]
- Committee on Environmental Epidemiology. Environmental Epidemiology: Use of the Gray Literature and Other Data in Environmental Epidemiology; National Research Council, Ed.; National Academies Press: Washington, DC, USA, 1997. [Google Scholar]
- Jones, C.B.; Purves, R.S. Web-Based GIS. In The Handbook of Geographic Information Science; Fotheringham, S.A., Wilson, J.P., Eds.; Blackwell: Malden, MA, USA, 2008. [Google Scholar]
- Fu, P.; Sun, J. Web GIS: Principles and Applications; Esri Press: Redlands, CA, USA, 2010. [Google Scholar]
- Wright, K.A. Web GIS as a DIsease Management Workspace: Enabling Advocacy at Multiple Scales Across Multiple Continents with the Case of Tungiasis. Master’s thesis, University of Southern California, Los Angeles, CA, USA, 2017. [Google Scholar]
- Wiese, S.; Thielecke, M.; Nordin, P.; Elson, L.; Corbett, M.; Mukon, G. Control of tungiasis through community participation: Self-diagnosis as a means to target prevention and treatment. 2017; manuscript in preparation. [Google Scholar]
- Board on Science and Technology for International Development (BOSTID). Neem: A Tree for Solving Global Problems; National Research Council, Policy and Global Affairs, Office of International Affairs, Ed.; National Academic Press: Washington, DC, USA, 1992. [Google Scholar]
- Abdel-Ghaffar, F.; Semmler, M. Efficacy of neem seed extract shampoo on head lice of naturally infected humans in Egypt. Parasitol. Res. 2007, 100, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghaffar, F.; Al-Quraishy, S.; Sobhy, H.; Semmler, M. Neem seed extract shampoo, Wash Away Louse, an effective plant agent against sarcoptes scabiei mites infesting dogs in Egypt. Parasitol. Res. 2008, 104, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Shi, D.; Yin, Z.; Guo, J.; Jia, R.; Xu, J.; Song, X.; Lv, C.; Fan, Q.; Liang, X.; et al. Acaricidal activity of petroleum ether extract of neem (Azadirachta indica) oil and its four fractions separated by column chromatography against Sarcoptes scabiei var. cuniculi larvae in vitro. Exp. Parasitol. 2012, 130, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Tabassam, S.; Iqbal, Z.; Jabbar, A.; Sindhu, Z.; Chattha, A. Efficacy of crude neem seed kernel extracts against natural infestation of Sarcoptes scabiei var. ovis. J. Ethnopharmacol. 2008, 115, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Ghosh, S.; Mandal, D.; Azhahianambi, P.; Singhal, P.; Pandey, N.; Azhahianambi, P.; Swarupet, D.; Lopez-Ferber, M. Efficacy of Azadirachta indica extracts against Boophilus microplus. Parasitol. Res. 2008, 104, 149–153. [Google Scholar] [CrossRef] [PubMed]
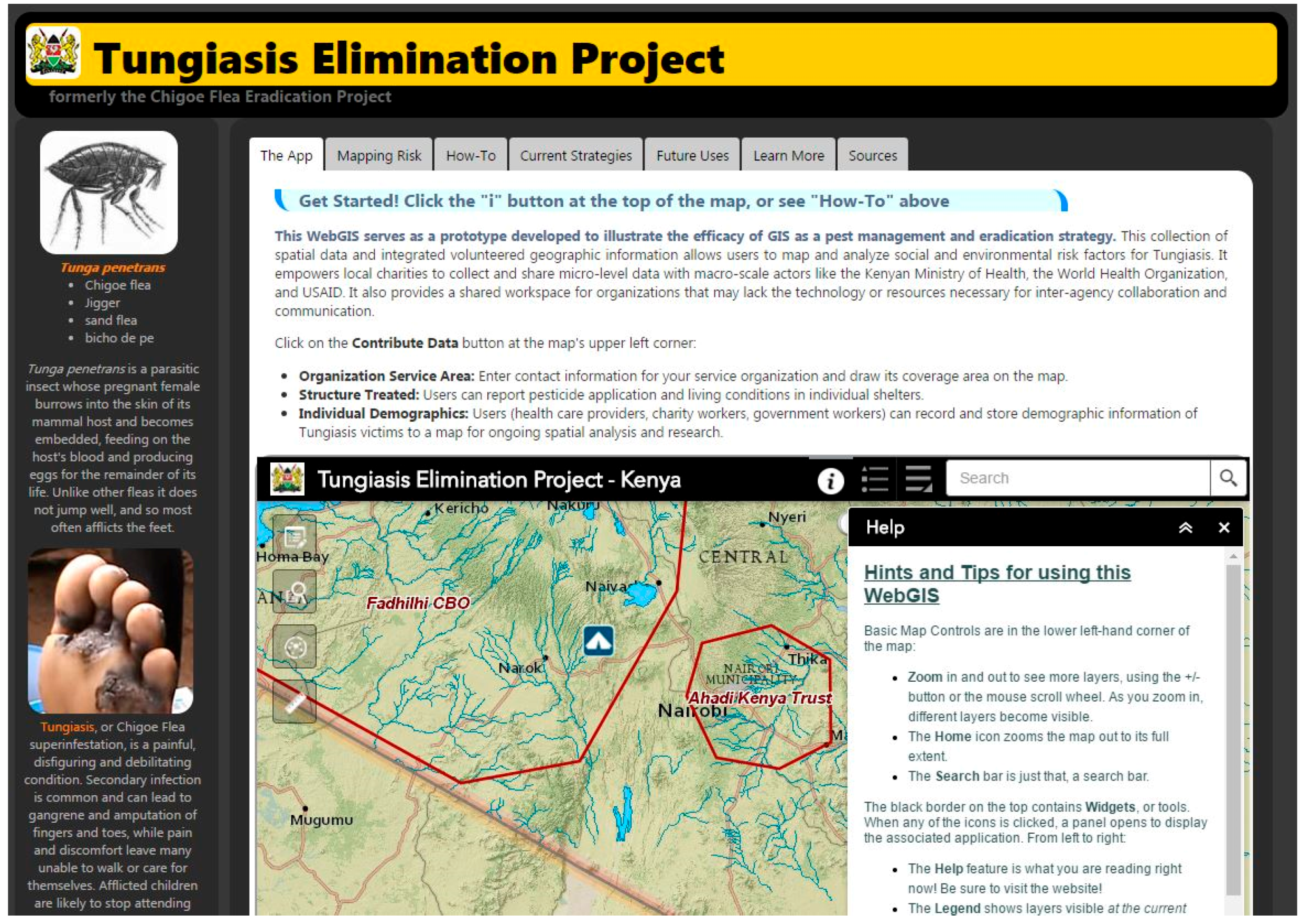
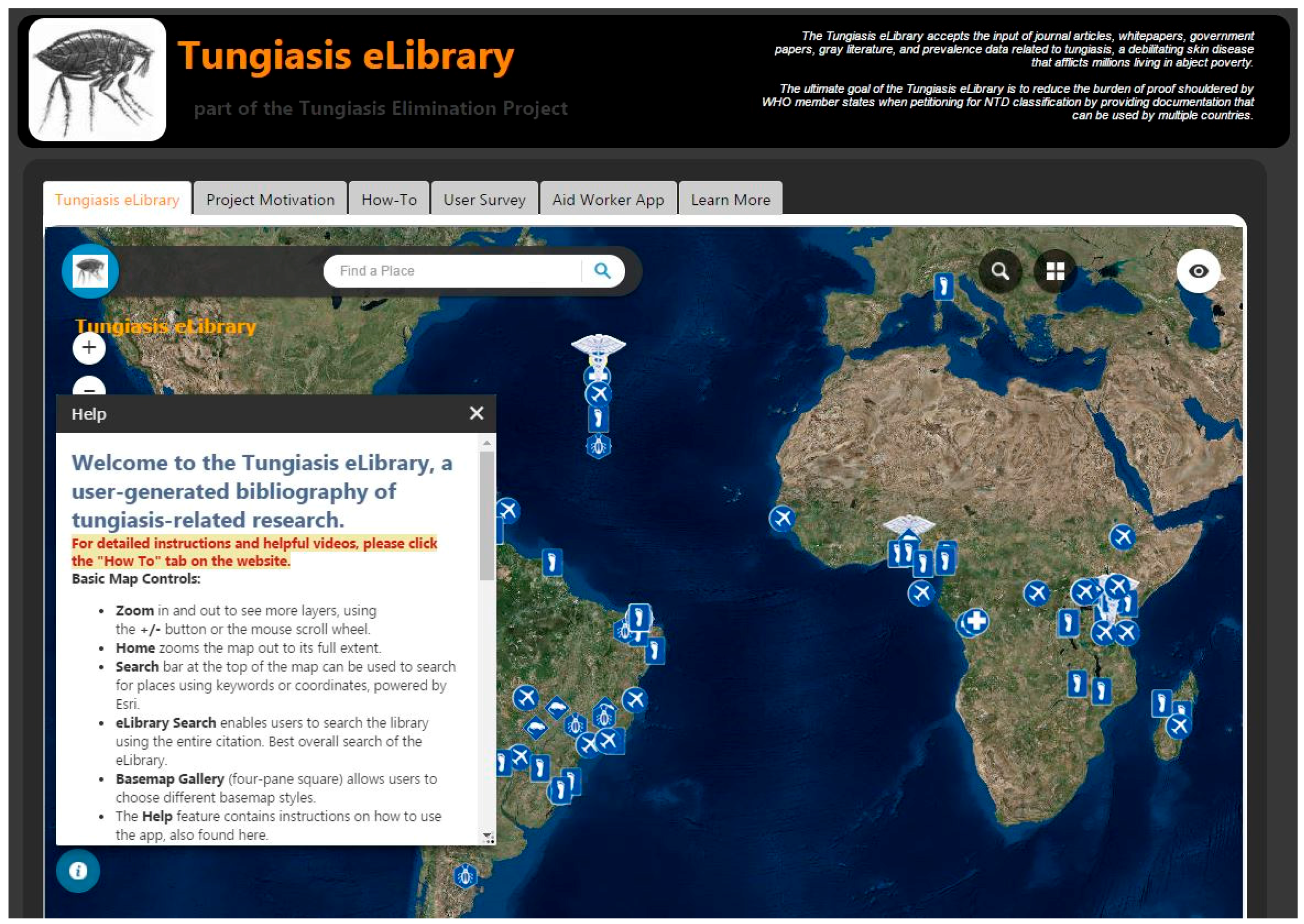
| Tungiasis Elimination Project Web Page |
|---|
| http://www-scf.usc.edu/~kawright/Chigoe/ChigoeEradication.html |
| Tungiasis Elimination Project Map URL |
| http://uscssi.maps.arcgis.com/apps/webappviewer/index.html?id=4563a0de487046eb8c74eccfedc94ba0 |
| Tungiasis eLibrary Web Page |
| http://www-scf.usc.edu/~kawright/Chigoe/TungiasiseLibrary.html |
| Tungiasis eLibrary Map URL |
| http://uscssi.maps.arcgis.com/apps/webappviewer/index.html?id=3f0a38ccd8c44ba7a6c4d58c4f24f6c8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elson, L.; Wright, K.; Swift, J.; Feldmeier, H. Control of Tungiasis in Absence of a Roadmap: Grassroots and Global Approaches. Trop. Med. Infect. Dis. 2017, 2, 33. https://doi.org/10.3390/tropicalmed2030033
Elson L, Wright K, Swift J, Feldmeier H. Control of Tungiasis in Absence of a Roadmap: Grassroots and Global Approaches. Tropical Medicine and Infectious Disease. 2017; 2(3):33. https://doi.org/10.3390/tropicalmed2030033
Chicago/Turabian StyleElson, Lynne, Katherine Wright, Jennifer Swift, and Herman Feldmeier. 2017. "Control of Tungiasis in Absence of a Roadmap: Grassroots and Global Approaches" Tropical Medicine and Infectious Disease 2, no. 3: 33. https://doi.org/10.3390/tropicalmed2030033




