Rice Starch-Templated Synthesis of Nanostructured Silica and Hematite †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology Study
2.2. Physisorption Measurements
3. Materials and Methods
3.1. Preparation of Nanostructured Silica (SiNS)
3.2. Preparation of Nanostructured Hematite (HNS)
3.3. Characterization
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zhu, H.; Jing, Y.; Pal, M.; Liu, Y.; Liu, Y.; Wang, J.; Zhang, F.; Zhao, D. Mesoporous TiO2@N-doped Carbon Composite Nanospheres Synthesized by the Direct Carbonization of surfactants After Sol–gel Process for Superior Lithium Storage. Nanoscale 2017, 9, 1539–1546. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Salavati-Niasari, M.; Masjedi-Arani, M.; Mazloom, F. An Easy Sonochemical Route for Synthesis, Characterization and Photocatalytic Performance of Nanosized FeVO4 in the Presence of Aminoacids as Green Capping Agents. J. Mater. Sci. Mater. Electron. 2018, 29, 474–485. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Y.; Abbas, Z.M.; Benicewicz, B.C. One-Pot Synthesis of Inorganic Nanoparticle Vesicles Via Surface-Initiated Polymerization-Induced Self-Assembly. Polym. Chem. 2017, 8, 370–374. [Google Scholar] [CrossRef]
- Kothary, P.; Dou, X.; Fang, Y.; Gu, Z.; Leo, S.Y.; Jiang, P. Superhydrophobic Hierarchical Arrays Fabricated by a Scalable Colloidal Lithography Approach. J. Colloid Interface Sci. 2017, 487, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, T.; Fujii, E.; Ishida, K.; Ueda, T.; Shimizu, Y. Microstructural Control of Porous In2O3 Powders Prepared by Ultrasonic-Spray Pyrolysis Employing Self-Synthesized Polymethylmethacrylate Microspheres as a Template and Their NO2-Sensing Properties. Sens. Actuators B Chem. 2017, 244, 992–1003. [Google Scholar] [CrossRef]
- Syoufian, A.; Inoue, Y.; Yada, M.; Nakashima, K. Preparation of Submicrometer-Sized Titania Hollow Spheres by Templating Sulfonated Polystyrene Latex Particles. Mater. Lett. 2007, 61, 1572–1575. [Google Scholar] [CrossRef]
- Hao, N.; Jayawardana, K.W.; Chen, X.; Yan, M. One-Step Synthesis of Amine-Functionalized Hollow Mesoporous Silica Nanoparticles as Efficient Antibacterial and Anticancer Materials. ACS Appl. Mater. Interfaces 2015, 7, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Zan, G.; Wu, Q. Biomimetic and Bioinspired Synthesis of Nanomaterials/Nanostructures. Adv. Mater. 2016, 28, 2099–2147. [Google Scholar] [CrossRef] [PubMed]
- Matmin, J.; Affendi, I.; Endud, S. Direct-Continuous Preparation of Nanostructured Titania-Silica Using Surfactant-Free Non-Scaffold Rice Starch Template. Nanomaterials 2018, 8, 514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Davis, S.A.; Mann, S. Starch Gel Templating of Spongelike Macroporous Silicalite Monoliths and Mesoporous Films. Chem. Mater. 2002, 14, 1369–1375. [Google Scholar] [CrossRef]
- Otero-de-la-Roza, A.; Mallory, J.D.; Johnson, E.R. Metallophilic Interactions from Dispersion-Corrected Density-Functional Theory. J. Chem. Phys. 2014, 140, 18A504. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, J.; Hu, Y.; Zhi, Z.; Jiang, T.; Zhang, J.; Wang, S. Development of a Novel Starch-Derived Porous Silica Monolith for Enhancing the Dissolution Rate of Poorly Water Soluble Drug. Mater. Sci. Eng. C 2012, 32, 201–206. [Google Scholar] [CrossRef]


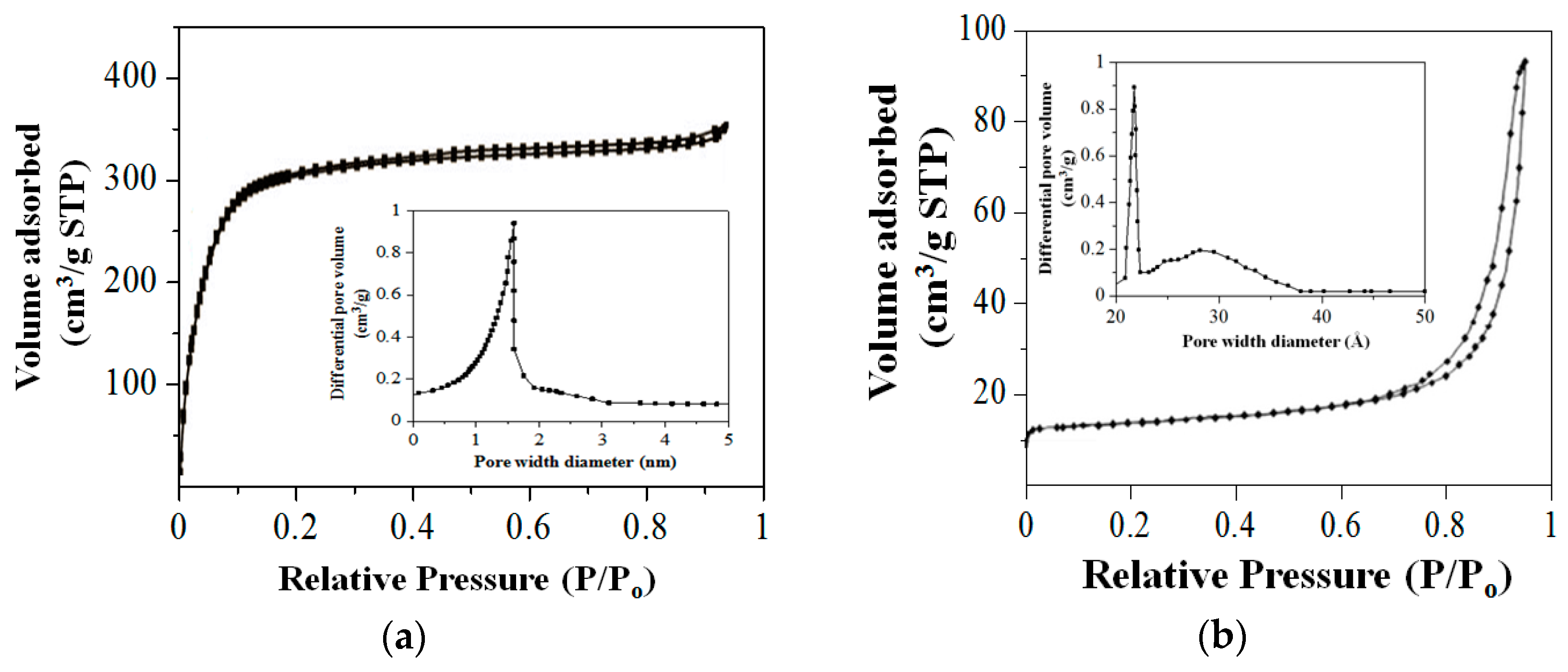
| Sample | Surface Area (m2/g) | Pore Diameter (nm) |
|---|---|---|
| SiNS | 538.74 | 1.6 |
| HNS | 20.04 | 2.2 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matmin, J. Rice Starch-Templated Synthesis of Nanostructured Silica and Hematite. Proceedings 2019, 3, 1. https://doi.org/10.3390/IOCN_2018-1-05491
Matmin J. Rice Starch-Templated Synthesis of Nanostructured Silica and Hematite. Proceedings. 2019; 3(1):1. https://doi.org/10.3390/IOCN_2018-1-05491
Chicago/Turabian StyleMatmin, Juan. 2019. "Rice Starch-Templated Synthesis of Nanostructured Silica and Hematite" Proceedings 3, no. 1: 1. https://doi.org/10.3390/IOCN_2018-1-05491





