Efficient and Eco-Friendly Mechanical Milling Preparation of Anatase/Rutile TiO2-Glucose Composite with Energy Gap Enhancement †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
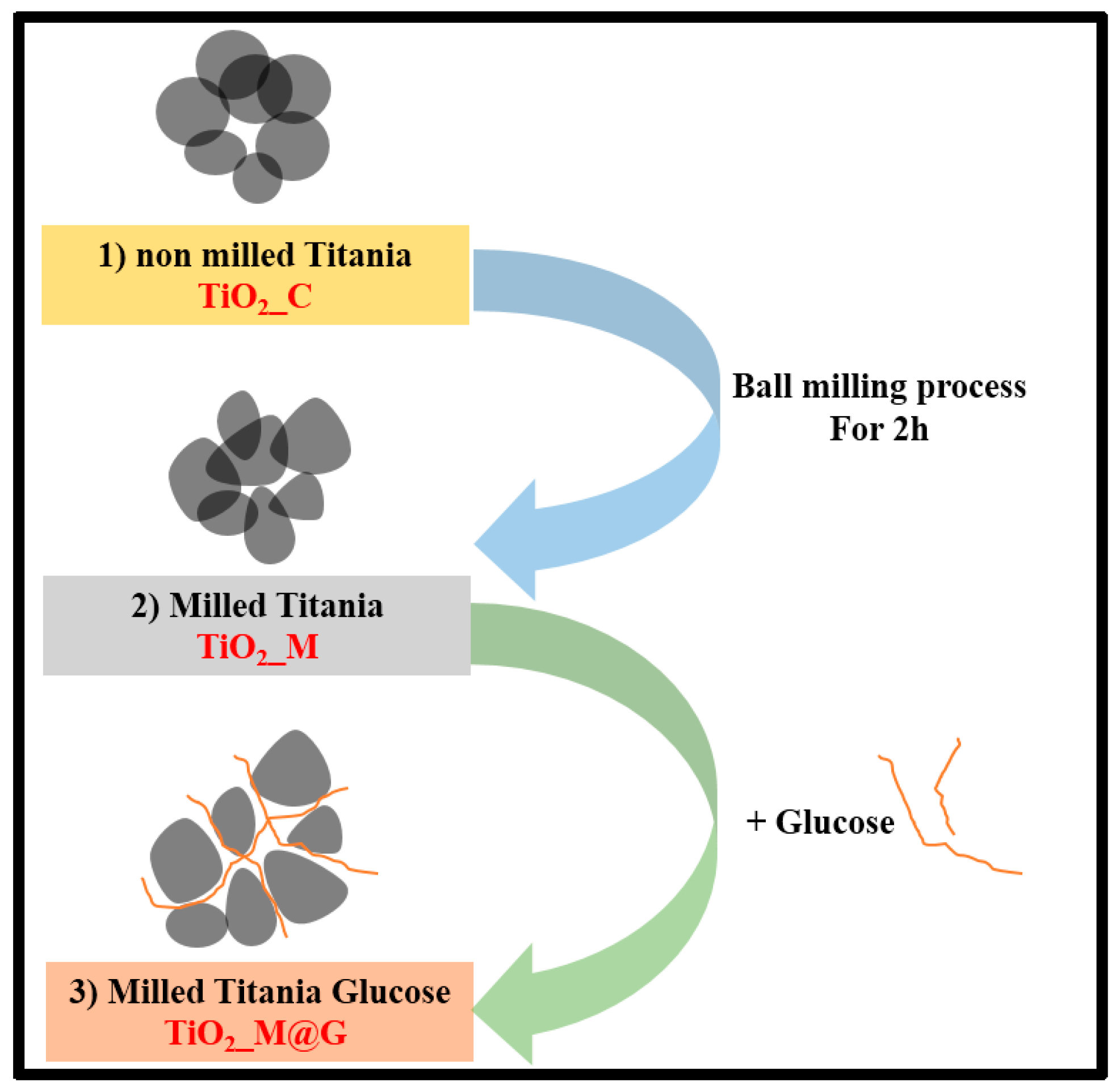
2.2. Synthesis Procedure
2.3. Characterizations and Techniques
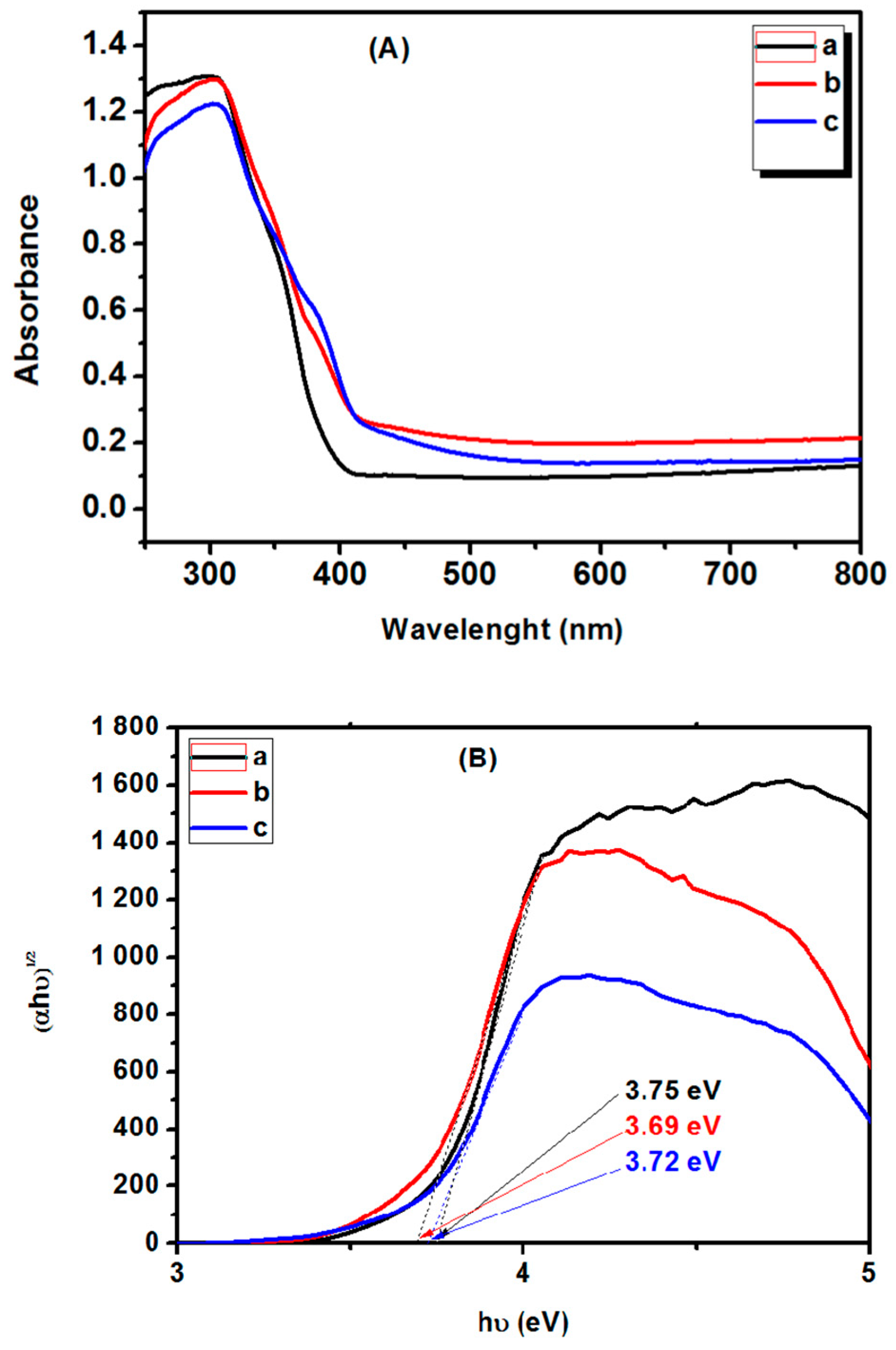
3. Results and Discussion
4. Conclusions
Conflicts of Interest
References
- Wang, Y.; Sun, C.; Zhao, X.; Cui, B.; Zeng, Z.; Wang, A.; Liu, G.; Cui, H. The Application of Nano-TiO2 Photo Semiconductors in Agriculture. Nanoscale Res. Lett. 2016, 11, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Hu, Y.; Xue, J.; Wang, X.; Liao, W. A novel co-precipitation method for preparation of Mn–Ce/TiO2 composites for NOx reduction with NH3 at low temperature. Environ. Technol. 2012, 33, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- Polat, M.; Soylu, A.M.; Erdogan, D.A.; Erguven, H.; Vovk, E.I.; Ozensoy, E. Influence of the sol-gel preparation method on the photocatalytic NO oxidation performance of TiO2/Al2O3 binary oxides. Catal. Today 2015, 241, 25–32. [Google Scholar] [CrossRef]
- Hosokawa, M.; Nogi, K.; Naito, M.; Yokoyama, T. Nanoparticle Technology Handbook, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Ren, R.; Yang, Z.; Shaw, L.L. Polymorphic transformation and powder characteristics of TiO2 during high energy milling. J. Mater. Sci. 2000, 35, 2015–2026. [Google Scholar] [CrossRef]
- Begin-Colin, S.; Girot, T.; le Caër, G.; Mocellin, A. Kinetics and Mechanisms of Phase Transformations Induced by Ball-Milling in Anatase TiO2. J. Solid State Chem. 2000, 149, 41. [Google Scholar] [CrossRef]
- Pan, X.; Ma, X. Study on the milling-induced transformation in TiO2 powder with different grain sizes. Mater. Lett. 2004, 58, 513–515. [Google Scholar] [CrossRef]
- Pan, X.; Ma, X. Phase transformations in nanocrystalline TiO2 milled in different milling atmospheres. J. Solid State Chem. 2004, 177, 4098–4103. [Google Scholar] [CrossRef]
- Uzunova-Bujnova, M.; Dimitrov, D.; Radev, D.; Bojinova, A.; Todorovsky, D. Effect of the mechanoactivation on the structure, sorption and photocatalytic properties of titanium dioxide. Mater. Chem. Phys. 2008, 110, 291–298. [Google Scholar] [CrossRef]
- Billik, P.; Plesch, G.; a, V.B.; Kuchta, L.; Valko, M.; Mazur, M. Anatase TiO2 nanocrystals prepared by mechanochemical synthesis and their photochemical activity studied by EPR spectroscopy. J. Phys. Chem. Solids 2007, 68, 1112. [Google Scholar] [CrossRef]
- Begin-Colin, S.; Gadalla, A.; Cacer, G.L.; Humbert, O.; Thomas, F.; Barres, O.; Villiéras, F.; Toma, L.F.; Bertrand, G.; Zahraa, O.; et al. On the Origin of the Decay of the Photocatalytic Activity of TiO2 Powders Ground at High Energy. J. Phys. Chem. C 2009, 113, 16589–16602. [Google Scholar] [CrossRef]
- Yin, S.; Yamaki, H.; Komatsu, M.; Zhang, Q.; Wang, J.; Tang, Q.; Saito, F.; Sato, T. Preparation of nitrogen-doped titania with high visible light induced photocatalytic activity by mechanochemical reaction of titania and hexamethylenetetramine. J. Mater. Chem. 2003, 13, 2996–3001. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Yin, S.; Sato, T.; Saito, F. Synthesis of a visible-light active TiO2-xSx photocatalyst by means of mechanochemical doping. J. Am. Ceram. Soc. 2004, 87, 1161–1163. [Google Scholar] [CrossRef]
- Chen, J.; Hirasaki, G.J.; Flaum, M. Effect of OBM on wettability and NMR responses. J. Pet. Sci. Eng. 2006, 52, 161–171. [Google Scholar] [CrossRef]
- Flood, C.; Cosgrove, T.; Espidel, Y.; Welfare, E.; Howell, I.; Revell, P. Fourier-Transform Carr-Purcell- Meiboom-Gill NMR Experiments on Polymers in Colloidal Dispersions: How Many Polymer Molecules per Particle? Langmuir 2008, 24, 7875–7880. [Google Scholar] [CrossRef]
- Madhusudan, B.M.; Raju, H.P.; Ghanaraja, S. Micro Structrual Characterization and Analysis of Ball Milled Silicon Carbide. AIP Conf. Proc. 2018, 1943, 020122. [Google Scholar] [CrossRef]
- Kim, J.; Chang, J.H.; Jeong, B.Y.; Lee, J.H. Comparison of Milling Modes as a Pretreatment Method for Cellulosic Biofuel Production Hyeon. J. Clean Energy Technol. 2013, 1, 45–48. [Google Scholar] [CrossRef]
- Damonte, L.C.; Zelis, L.A.M.; Soucase, B.M.; Fenollosa, M.A.H. Nanoparticles of ZnO obtained by mechanical milling. Powder Technol. 2004, 148, 15–19. [Google Scholar] [CrossRef]
- Lemine, O.M.; Louly, M.A.; Al-Ahmari, A.M. Planetary milling parameters optimization for the production of ZnO nanocrystalline. Int. J. Phys. Sci. 2010, 5, 2721–2729. [Google Scholar]
- Molladavoudi, A.; Amirkhanlou, S.; Shamanian, M.; Ashrafizadeh, F. The production of nanocrystalline cobalt titanide intermetallic compound via mechanical alloying. Intermetallics 2012, 29, 104–109. [Google Scholar] [CrossRef]
- Lu, K.; Zhao, K.J. Equiaxed zinc oxide nanoparticle synthesis. Chem. Eng. J. 2010, 160, 788–793. [Google Scholar] [CrossRef]
- Ozcan, S.; Can, M.M.; Ceylan, A. Single step synthesis of nanocrystalline ZnO via wet-milling. Mater. Lett. 2010, 64, 2447–2449. [Google Scholar] [CrossRef]
- Glushenkov, A.M.; Zhang, H.Z.; Chen, Y. Reactive ball milling to produce nanocrystalline ZnO. Mater. Lett. 2008, 62, 4047–4049. [Google Scholar] [CrossRef]
- Dulian, P.; Buras, M.; Żukowski, W. Modyfication of photocatalytic properties of titanium dioxide by mechanochemical method. Polish J. Chem. Technol. 2018, 3, 68–71. [Google Scholar] [CrossRef]
- Aysin, B.; Ozturk, A.; Park, J. Silver-loaded TiO2 powders prepared through mechanical ball milling. Ceram. Int. 2013, 39, 7119–7126. [Google Scholar] [CrossRef]
- Khan, H.; Swat, I.K. Fe3+-doped Anatase TiO2 with d–d Transition, Oxygen Vacancies and Ti3+ Centers: Synthesis, Characterization, UV–vis Photocatalytic and Mechanistic Studies. Ind. Eng. Chem. Res. 2016, 55, 6619–6633. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellouzi, I.; Oualid, H.A. Efficient and Eco-Friendly Mechanical Milling Preparation of Anatase/Rutile TiO2-Glucose Composite with Energy Gap Enhancement. Proceedings 2019, 3, 3. https://doi.org/10.3390/IOCN_2018-1-05497
Ellouzi I, Oualid HA. Efficient and Eco-Friendly Mechanical Milling Preparation of Anatase/Rutile TiO2-Glucose Composite with Energy Gap Enhancement. Proceedings. 2019; 3(1):3. https://doi.org/10.3390/IOCN_2018-1-05497
Chicago/Turabian StyleEllouzi, Imane, and Hicham Abou Oualid. 2019. "Efficient and Eco-Friendly Mechanical Milling Preparation of Anatase/Rutile TiO2-Glucose Composite with Energy Gap Enhancement" Proceedings 3, no. 1: 3. https://doi.org/10.3390/IOCN_2018-1-05497




