Nano Propolis, Zinc Oxide Nanoparticles, and Their Composites: A Novel Green Synthesis with Synergistic Antioxidant and Anticancer Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
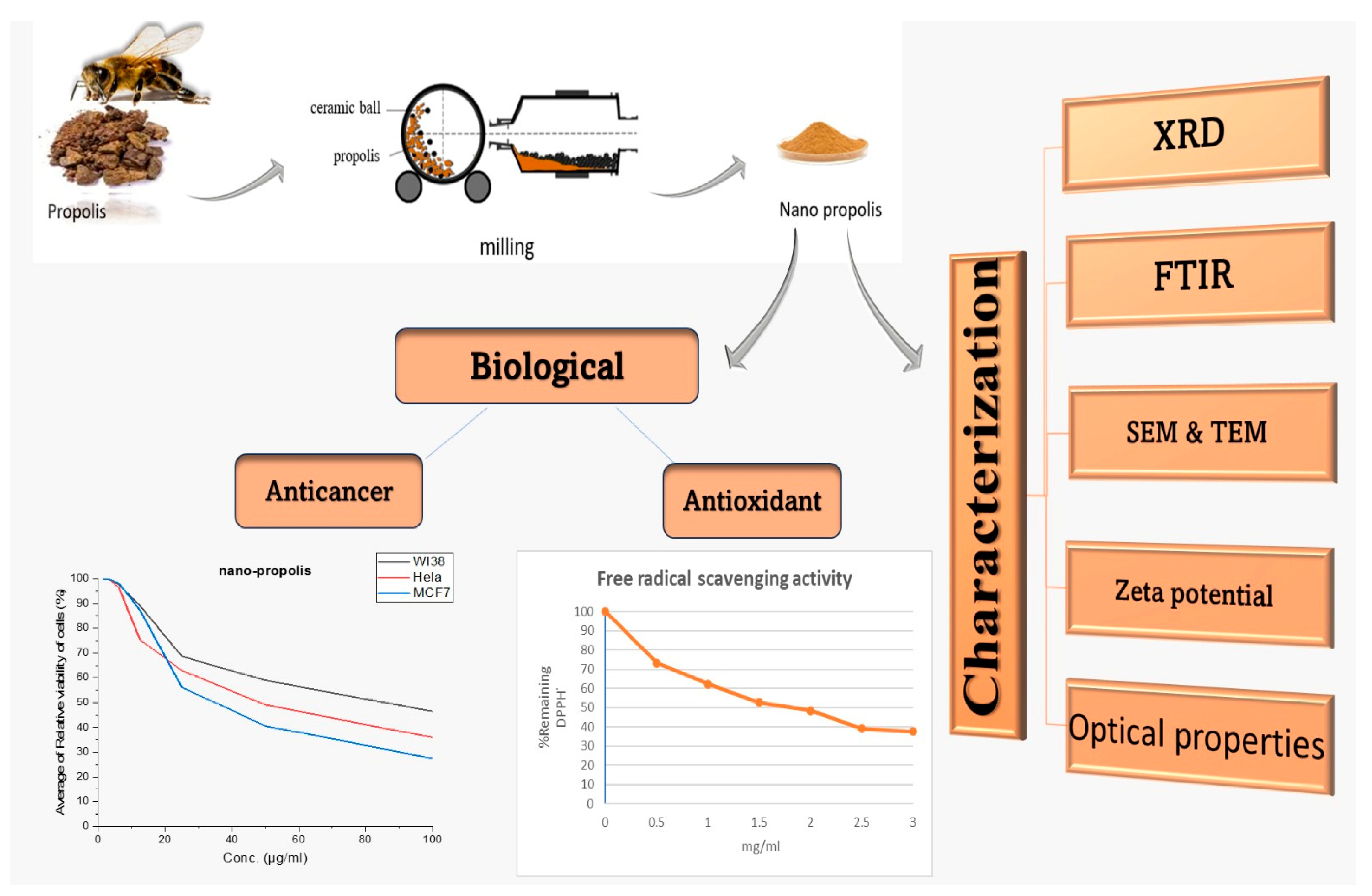
2.2. Preparation of Nanoparticles
2.2.1. Propolis Nanoparticles Preparation
2.2.2. Green Synthesis of Zn Oxide Nanoparticles Using Propolis
2.2.3. Zinc Oxide–Propolis Nanocomposite (ZnO-P NCs) Preparation
2.3. Characterization
2.4. Total Phenolic and Flavonoid Contents Determination
2.5. Antioxidant Activity (DPPH Assay)
2.6. Anticancer Assay (MTT Assay)
3. Results
3.1. Characterization
- X-ray Diffraction characteristics
- Fourier transforms infrared spectroscopy
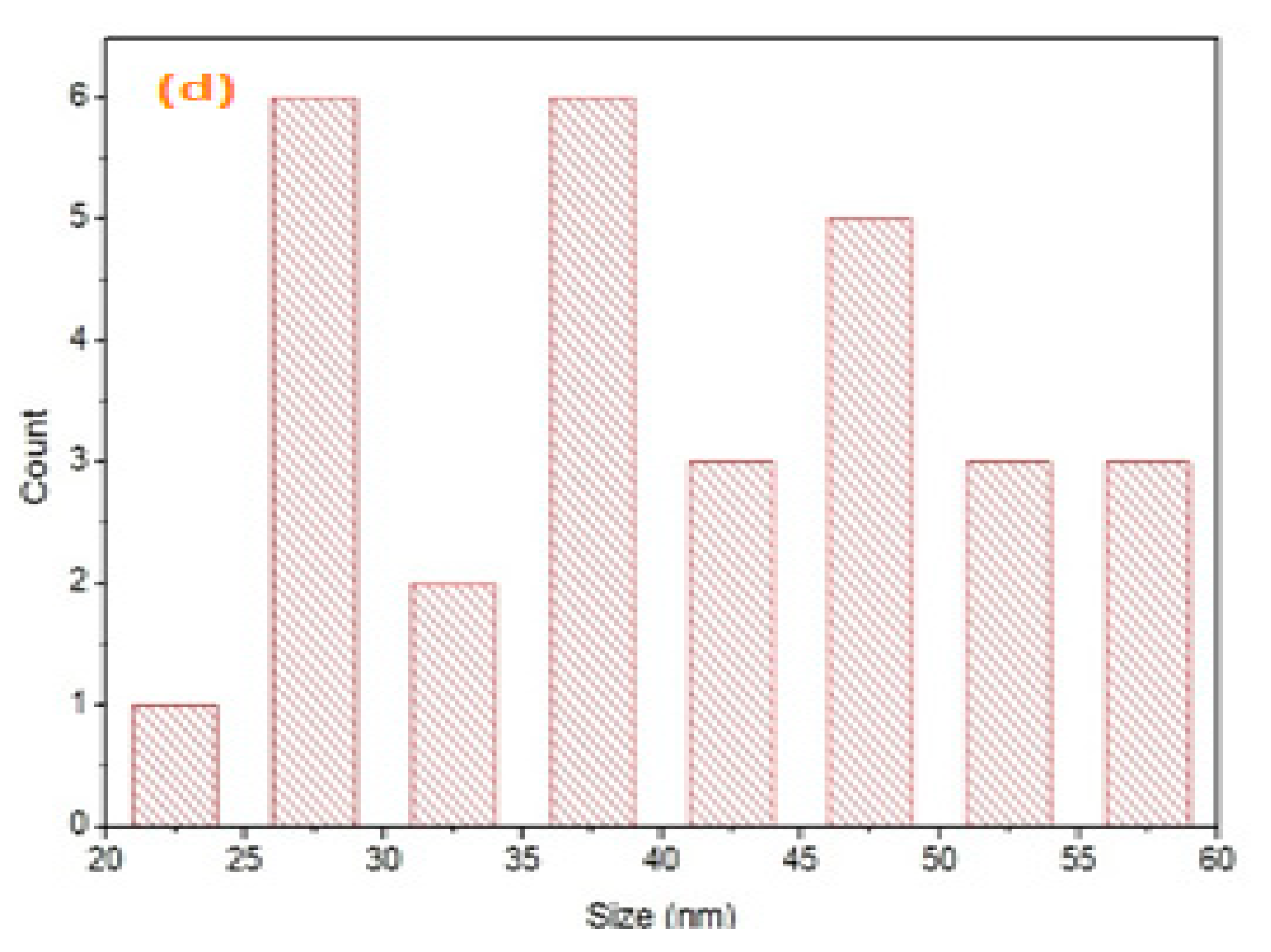
- Scanning electron microscopy and transmission electron microscopy
- Zeta potential
- Optical properties
3.2. Biological Activities
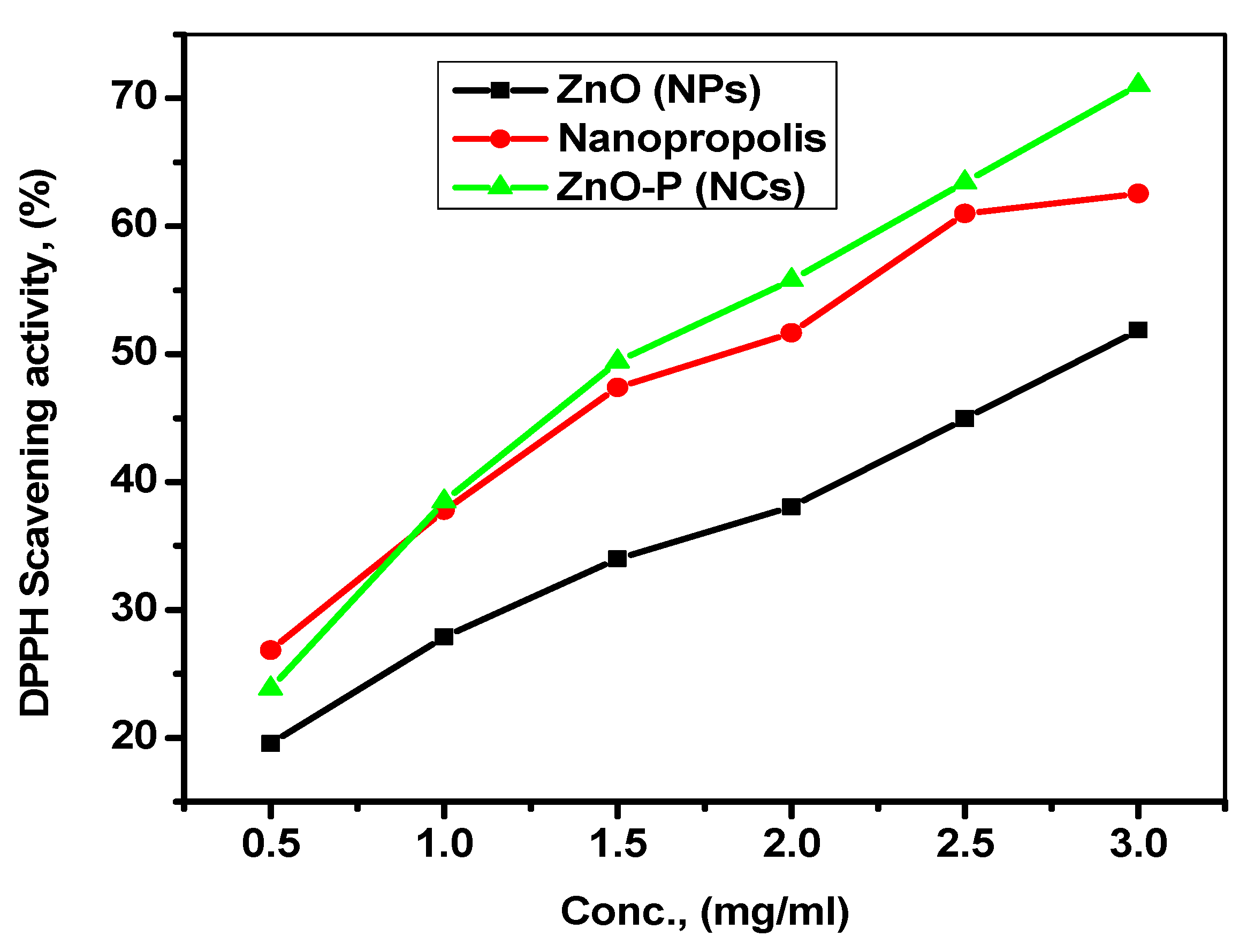
- Antioxidant activity
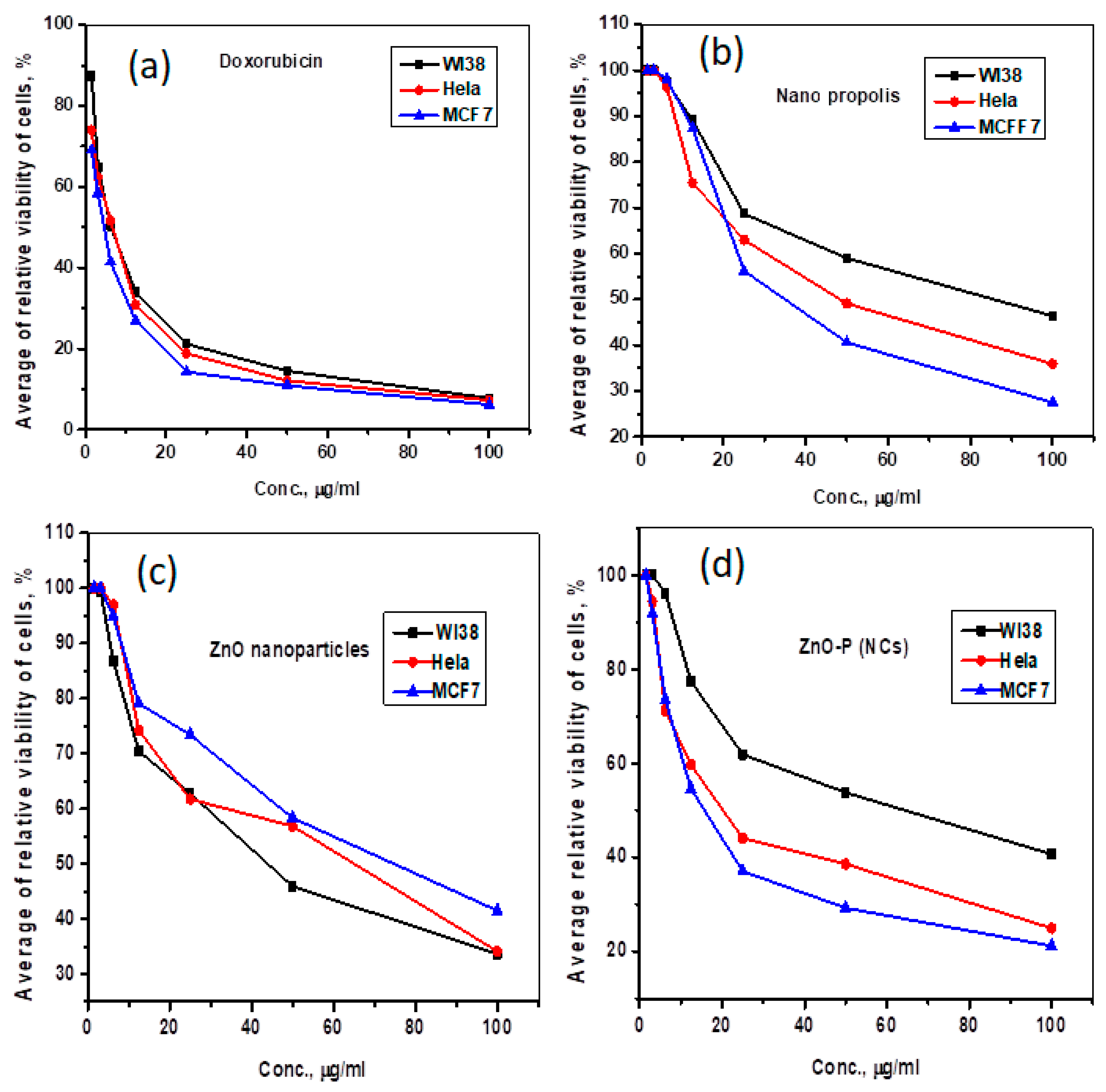
- Cytotoxic activity
- Comparison of the DPPH free radical scavenging activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luechinger, N.A.; Grass, R.N.; Athanassiou, E.K.; Stark, W.J. Bottom-up fabrication of metal/metal nanocomposites from nanoparticles of immiscible metals. Chem. Mater. 2010, 22, 155–160. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Hu, Q.H.; Max Lu, G.Q. Magnetic Nanocomposites: Magnetic Nanocomposites with Mesoporous Structures: Synthesis and Applications (Small 4/2011). Small 2011, 7, 418. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Sudheer, S.; Bai, R.G.; Muthoosamy, K.; Tuvikene, R.; Gupta, V.K.; Manickam, S. Biosustainable production of nanoparticles via mycogenesis for biotechnological applications: A critical review. Environ. Res. 2022, 204, 111963. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. “Green” synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Andhari, S.S.; Wavhale, R.D.; Dhobale, K.D.; Tawade, B.V.; Chate, G.P.; Patil, Y.N.; Khandare, J.J.; Banerjee, S.S. Self-Propelling Targeted Magneto-Nanobots for Deep Tumor Penetration and pH-Responsive Intracellular Drug Delivery. Sci. Rep. 2020, 10, 4703. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Surek, M.; Fachi, M.M.; de Fátima Cobre, A.; de Oliveira, F.F.; Pontarolo, R.; Crisma, A.R.; de Souza, W.M.; Felipe, K.B. Chemical composition, cytotoxicity, and antibacterial activity of propolis from Africanized honeybees and three different Meliponini species. J. Ethnopharmacol. 2021, 269, 113662. [Google Scholar] [CrossRef]
- Kolaylı, S.; Birinci, C.; Kara, Y.; Ozkok, A.; Samancı, A.E.T.; Sahin, H.; Yildiz, O. A melissopalynological and chemical characterization of Anatolian propolis and an assessment of its antioxidant potential. Eur. Food Res. Technol. 2023, 249, 1213–1233. [Google Scholar] [CrossRef]
- Rocha, V.M.; Portela, R.D.; dos Anjos, J.P.; de Souza, C.O.; Umsza-Guez, M.A. Stingless bee propolis: Composition, biological activities and its applications in the food industry. Food Prod. Process. Nutr. 2023, 5, 29. [Google Scholar] [CrossRef]
- Ngaini, Z.; Hussain, H.; Kelabo, E.S.; Wahi, R.; Farooq, S. Chemical profiling, biological properties and environmental contaminants of stingless bee honey and propolis. J. Apic. Res. 2023, 62, 131–147. [Google Scholar] [CrossRef]
- Berretta, A.A.; Zamarrenho, L.G.; Correa, J.A.; De Lima, J.A.; Borini, G.B.; Ambrósio, S.R.; Barud, H.d.S.; Bastos, J.K.; De Jong, D. Development and Characterization of New Green Propolis Extract Formulations as Promising Candidates to Substitute for Green Propolis Hydroalcoholic Extract. Molecules 2023, 28, 3510. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, M. Biological activity of bee propolis in health and disease. Asian Pacific J. Cancer Prev. 2006, 7, 22–31. [Google Scholar]
- Marcucci, M.C. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- Braakhuis, A. Evidence on the health benefits of supplemental propolis. Nutrients 2019, 11, 2705. [Google Scholar] [CrossRef]
- Bankova, V.; Boudourova-Krasteva, G.; Frete, X.; Popov, S.; Sforcin, J.M.; Maimoni-Rodella, R.; Kujumgiev, A. Phytochemical Evidence for the Plant Origin of Brazilian Propolis from São Paulo State. Z. Fur Naturforsch.—Sect. C J. Biosci. 1999, 54, 401–405. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, P.; Zhang, H.; Zhu, Q.; Liao, C.; Jiang, G. Analysis, occurrence, toxicity and environmental health risks of synthetic phenolic antioxidants: A review. Environ. Res. 2021, 201, 111531. [Google Scholar] [CrossRef]
- Bonamigo, T.; Campos, J.F.; Alfredo, T.M.; Balestieri, J.B.P.; Cardoso, C.A.L.; Paredes-Gamero, E.J.; De Picoli Souza, K.; Dos Santos, E.L. Antioxidant, Cytotoxic, and Toxic Activities of Propolis from Two Native Bees in Brazil: Scaptotrigona depilis and Melipona quadrifasciata anthidioides. Oxid. Med. Cell. Longev. 2017, 2017, 1038153. [Google Scholar] [CrossRef] [PubMed]
- Fabris, S.; Bertelle, M.; Astafyeva, O.; Gregoris, E.; Zangrando, R.; Gambaro, A.; Lima, G.P.P.; Stevanato, R. Antioxidant Properties and Chemical Composition Relationship of Europeans and Brazilians Propolis. Pharmacol. Pharm. 2013, 4, 46–51. [Google Scholar] [CrossRef]
- Xuan, H.; Li, Z.; Yan, H.; Sang, Q.; Wang, K.; He, Q.; Wang, Y.; Hu, F. Antitumor activity of Chinese propolis in human breast cancer MCF-7 and MDA-MB-231 cells. Evid.-Based Complement. Altern. Med. 2014, 2014, 280120. [Google Scholar] [CrossRef] [PubMed]
- Singla, S.; Kumar, N.; Kaur, J. In vivo studies on the protective effect of propolis on doxorubicin-induced toxicity in liver of male rats. Toxicol. Int. 2014, 21, 191. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.M.; Donia, T.; Abu-Khudir, R.; Ramadan, H.; Ali, E.M.M.; Mohamed, T.M. Propolis Potentiates Methotrexate Anticancer Mechanism and Reduces its Toxic Effects. Nutr. Cancer 2020, 72, 460–480. [Google Scholar] [CrossRef] [PubMed]
- Benguedouar, L.; Lahouel, M.; Gangloff, S.; Durlach, A.; Grange, F.; Bernard, P.; Antonicelli, F. Algerian ethanolic extract of Propolis and galangin decreased melanoma tumour progression in C57BL6 mice. Ann. Dermatol. Venereol. 2015, 142, S294. [Google Scholar] [CrossRef]
- Demir, S.; Aliyazicioglu, Y.; Turan, I.; Misir, S.; Mentese, A.; Yaman, S.O.; Akbulut, K.; Kilinc, K.; Deger, O. Antiproliferative and proapoptotic activity of Turkish propolis on human lung cancer cell line. Nutr. Cancer 2016, 68, 165–172. [Google Scholar] [CrossRef]
- Rouibah, H.; Kebsa, W.; Lahouel, M.; Zihlif, M.; Ahram, M.; Aburmaileh, B.; Mansour Al Shhab, M.A.; Al-Ameer, H.J.; Mustafa, E. Algerian propolis: Between protection of normal cells and potentialisation of the anticancer effects of doxorubicin against breast cancer cells via P-glycoprotein inhibition and cell cycle arrest in the S phase. J. Physiol. Pharmacol. 2021, 72, 2. [Google Scholar] [CrossRef]
- Diva, A.N.; Pratami, D.K.; Wijanarko, A.; Hermansyah, H.; Sahlan, M. Effect of ethanolic propolis extract from Tetragonula biroi bees on the growth of human cancer cell lines HeLa and MCF-7. In 3RD BIOMEDICAL ENGINEERING’S RECENT PROGRESS IN BIOMATERIALS, DRUGS DEVELOPMENT, AND MEDICAL DEVICES, Proceedings of the International Symposium of Biomedical Engineering (ISBE) 2018, Jakarta, Indonesia, 6–8 August 2018; AIP Conference Proceedings; AIP (ACP): College Park, MD, USA, 2019. [Google Scholar]
- Turan, I.; Demir, S.; Misir, S.; Kilinc, K.; Mentese, A.; Aliyazicioglu, Y.; Deger, O. Cytotoxic effect of Turkish propolis on liver, colon, breast, cervix and prostate cancer cell lines. Trop. J. Pharm. Res. 2015, 14, 777. [Google Scholar] [CrossRef]
- Forma, E.; Bryś, M. Anticancer activity of propolis and its compounds. Nutrients 2021, 13, 2594. [Google Scholar] [CrossRef]
- Javed, S.; Mangla, B.; Ahsan, W. From propolis to nanopropolis: An exemplary journey and a paradigm shift of a resinous substance produced by bees. Phyther. Res. 2022, 36, 2016–2041. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.; Sharma, D.; Kalia, K.; Tekade, R.K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review. Acta Biomater. 2020, 101, 43–68. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Z.; Chen, H.; Gao, Y. Nanoparticle-based drug delivery systems for controllable photodynamic cancer therapy. Eur. J. Pharm. Sci. 2020, 144, 105213. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Singh, T.A.; Sharma, A.; Tejwan, N.; Ghosh, N.; Das, J.; Sil, P.C. A state of the art review on the synthesis, antibacterial, antioxidant, antidiabetic and tissue regeneration activities of zinc oxide nanoparticles. Adv. Colloid Interface Sci. 2021, 295, 102495. [Google Scholar] [CrossRef]
- Hermansyah, D.; Zulhendri, F.; Perera, C.O.; Firsty, N.N.; Chandrasekaran, K.; Abdulah, R.; Herman, H.; Lesmana, R. The Potential Use of Propolis as an Adjunctive Therapy in Breast Cancers. Integr. Cancer Ther. 2022, 21, 15347354221096868. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef]
- Katwal, G.; Paulose, M.; Rusakova, I.A.; Martinez, J.E.; Varghese, O.K. Rapid Growth of Zinc Oxide Nanotube-Nanowire Hybrid Architectures and Their Use in Breast Cancer-Related Volatile Organics Detection. Nano Lett. 2016, 16, 3014–3021. [Google Scholar] [CrossRef]
- Firdous, S. Development and imaging of zinc oxide nanorods as a photosensitizer for the diagnosis and treatment of cancer using lasers. Laser Phys. Lett. 2018, 15, 095604. [Google Scholar] [CrossRef]
- Hatami, R.; Javadi, A.; Jafarizadeh-Malmiri, H. Effectiveness of six different methods in green synthesis of selenium nanoparticles using propolis extract: Screening and characterization. Green Process. Synth. 2020, 9, 685–692. [Google Scholar] [CrossRef]
- Bankova, V.S.; De Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Hajizadeh, Y.S.; Harzandi, N.; Babapour, E.; Yazdanian, M.; Ranjbar, R. Green Synthesize and Characterization of Copper Nanoparticles Using Iranian Propolis Extracts. Adv. Mater. Sci. Eng. 2022, 2022, 8100440. [Google Scholar] [CrossRef]
- Priyadarshini, J.F.; Sivakumari, K.; Selvaraj, R.; Ashok, K.; Jayaprakash, P.; Rajesh, S. Green synthesis of silver nanoparticles from propolis. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2018, 4, 23–36. [Google Scholar]
- Barsola, B.; Kumari, P. Green synthesis of nano-propolis and nanoparticles (Se and Ag) from ethanolic extract of propolis, their biochemical characterization: A review. Green Process. Synth. 2022, 11, 659–673. [Google Scholar] [CrossRef]
- Bayrami, M.; Bayrami, A.; Habibi-Yangjeh, A.; Shafeeyan, M.S.; Feizpoor, S.; Arvanagh, F.M.; Nourani, M.R.; Taheri, R.A. Biologically-synthesised ZnO/CuO/Ag nanocomposite using propolis extract and coated on the gauze for wound healing applications. IET Nanobiotechnol. 2020, 14, 548–554. [Google Scholar] [CrossRef]
- Li, W.; Zhao, D.; Li, W.; Wen, R.; Liu, X.; Liu, L.; Li, T.; Fan, L. Chemorobust dye-encapsulated framework as dual-emission self-calibrating ratiometric sensor for intelligent detection of toluene exposure biomarker in urine. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2023, 296, 122637. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, D.; Li, W.; Zhang, H.; Li, B.; Hu, T.; Fan, L. Rod-shaped units based cobalt(II) organic framework as an efficient electrochemical sensor for uric acid detection in serum. Microchem. J. 2023, 185, 108154. [Google Scholar] [CrossRef]
- Yin, J.; Li, W.; Li, W.; Liu, L.; Zhao, D.; Liu, X.; Hu, T.; Fan, L. Heterometallic ZnHoMOF as a Dual-Responsive Luminescence Sensor for Efficient Detection of Hippuric Acid Biomarker and Nitrofuran Antibiotics. Molecules 2023, 28, 6274. [Google Scholar] [CrossRef]
- Zhao, D.; Li, W.; Wen, R.; Lei, N.; Li, W.; Liu, X.; Zhang, X.; Fan, L. Eu(III)-Functionalized MOF-Based Dual-Emission Ratiometric Sensor Integrated with Logic Gate Operation for Efficient Detection of Hippuric Acid in Urine and Serum. Inorg. Chem. 2023, 62, 2715–2725. [Google Scholar] [CrossRef]
- Rezk, N.; Abdelsattar, A.S.; Makky, S.; Hussein, A.H.; Kamel, A.G.; El-Shibiny, A. New formula of the green synthesised Au@Ag core@shell nanoparticles using propolis extract presented high antibacterial and anticancer activity. AMB Express 2022, 12, 108. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Rhim, J.W. Development of multifunctional pullulan/chitosan-based composite films reinforced with ZnO nanoparticles and propolis for meat packaging applications. Foods 2021, 10, 2789. [Google Scholar] [CrossRef]
- Mocanu, A.; Isopencu, G.; Busuioc, C.; Popa, O.M.; Dietrich, P.; Socaciu-Siebert, L. Bacterial cellulose films with ZnO nanoparticles and propolis extracts: Synergistic antimicrobial effect. Sci. Rep. 2019, 9, 17687. [Google Scholar] [CrossRef]
- Matei, P.M.; Martín-Ramos, P.; Sánchez-Báscones, M.; Hernández-Navarro, S.; Correa-Guimaraes, A.; Navas-Gracia, L.M.; Rufino, C.A.; Ramos-Sánchez, M.C.; Martín-Gil, J. Synthesis of chitosan oligomers/propolis/silver nanoparticles composite systems and study of their activity against diplodia seriata. Int. J. Polym. Sci. 2015, 2015, 864729. [Google Scholar] [CrossRef]
- Singh, R.P.; Shukla, V.K.; Yadav, R.S.; Sharma, P.K.; Singh, P.K.; Pandey, A.C. Biological approach of zinc oxide nanoparticles formation and its characterization. Adv. Mater. Lett. 2011, 2, 313–317. [Google Scholar] [CrossRef]
- Moaty, S.A.A.; Farghali, A.A.; Khaled, R. Preparation, characterization and antimicrobial applications of Zn-Fe LDH against MRSA. Mater. Sci. Eng. C 2016, 68, 184–193. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Hu, C.; Yuan, Y.V.; Kitts, D.D. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem. Toxicol. 2007, 45, 2219–2227. [Google Scholar] [CrossRef]
- Fidrianny, I.; Harnovi, M.; Insanu, M. Evaluation of antioxidant activities from various extracts of sweet orange peels using DPPH, FRAP assays and correlation with phenolic, flavonoid, carotenoid content. Asian J. Pharm. Clin. Res. 2014, 7, 186–190. [Google Scholar]
- Maharani, A.; Rini, R.; Sugiman, S. Pengaruh Penggunaan Media Interaktif Animasi Terhadap Minat Belajar Matematika Peserta Didik. Pedagog. J. Pendidik. Dasar 2019, 6. [Google Scholar]
- Fakhari, S.; Jamzad, M.; Kabiri Fard, H. Green synthesis of zinc oxide nanoparticles: A comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef]
- PD, D.A.; Plashintania, D.R.; Putri, R.M.; Wibowo, I.; Ramli, Y.; Herdianto, S.; Indarto, A. Synthesis of zinc oxide nanoparticles using methanol propolis extract (Pro-ZnO NPs) as antidiabetic and antioxidant. PLoS ONE 2023, 18, e0289125. [Google Scholar] [CrossRef]
- Hilo, D.H.; Al-garawi, Z.S.; Ismail, A.H. Green synthesis of ZnO NPs using ginger extract and assessment of their antioxidant activity. Egypt. J. Chem. 2023, 66, 111–117. [Google Scholar] [CrossRef]
- Corciovă, A.C.; Mircea, C.; Burlec, A.; Cioancă, O.C.; Tuchiluş, C.T.; Fifere, A.; Lungoci, A.; Lungoci, A.L.; Marangoci, N.; Hăncianu, M. Antioxidant, Antimicrobial and Photocatalytic Activities of Silver Nanoparticles Obtained by Bee Propolis Extract Assisted Biosynthesis. Farmacia 2019, 67, 482–489. [Google Scholar] [CrossRef]
- Zin, N.B.M.; Azemin, A.; Rodi, M.M.M.; Rashid, Z.M.; Mohd, K.S. Application of FTIR fingerprints coupled with chemometric for comparison of stingless bee propolis from different extraction methods. Malays. J. Fundam. Appl. Sci. 2019, 15, 350–355. [Google Scholar] [CrossRef]
- Svečnjak, L.; Marijanović, Z.; Okińczyc, P.; Kuś, P.M.; Jerković, I. Mediterranean propolis from the adriatic sea islands as a source of natural antioxidants: Comprehensive chemical biodiversity determined by GC-MS, ftiratr, UHPLC-DAD-QQTOF-MS, DPPH and FRAP assay. Antioxidants 2020, 9, 337. [Google Scholar] [CrossRef]
- Mahalakshmi, S.; Hema, N.; Vijaya, P.P. In Vitro Biocompatibility and Antimicrobial activities of Zinc Oxide Nanoparticles (ZnO NPs) Prepared by Chemical and Green Synthetic Route—A Comparative Study. BioNanoScience 2019, 10, 112–121. [Google Scholar]
- Mahmoud, R.; Moaty, S.A.; Mohamed, F.; Farghali, A. Comparative Study of Single and Multiple Pollutants System Using Ti-Fe Chitosan LDH Adsorbent with High Performance in Wastewater Treatment. J. Chem. Eng. Data 2017, 62, 3703–3722. [Google Scholar] [CrossRef]
- Yousaf, S.; Zulfiqar, S.; Shahi, M.N.; Warsi, M.F.; Al-Khalli, N.F.; Aly Aboud, M.F.; Shakir, I. Tuning the structural, optical and electrical properties of NiO nanoparticles prepared by wet chemical route. Ceram. Int. 2020, 46, 3750–3758. [Google Scholar] [CrossRef]
- Gupta, K.; Jana, P.C.; Meikap, A.K. Optical and electrical transport properties of polyaniline-silver nanocomposite. Synth. Met. 2010, 160, 1566–1573. [Google Scholar] [CrossRef]
- Atta, A.; Abdelhamied, M.M.; Abdelreheem, A.M.; Berber, M.R. Flexible methyl cellulose/polyaniline/silver composite films with enhanced linear and nonlinear optical properties. Polymers 2021, 13, 1225. [Google Scholar] [CrossRef]
- Mosnáčková, K.; Chehimi, M.M.; Fedorko, P.; Omastová, M. Polyamide grafted with polypyrrole: Formation, properties, and stability. Chem. Pap. 2013, 67, 979–994. [Google Scholar] [CrossRef]
- Moţ, A.C.; Silaghi-Dumitrescu, R.; Sârbu, C. Rapid and effective evaluation of the antioxidant capacity of propolis extracts using DPPH bleaching kinetic profiles, FT-IR and UV-vis spectroscopic data. J. Food Compos. Anal. 2011, 24, 516–522. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Abd El Hady, F.K. Egyptian propolis: 3. Antioxidant, antimicrobial activities and chemical composition of propolis from reclaimed lands. Z. Fur Naturforsch.—Sect. C J. Biosci. 2002, 57, 395–402. [Google Scholar] [CrossRef]
- Shehata, M.G.; Ahmad, F.T.; Badr, A.N.; Masry, S.H.; El-Sohaimy, S.A. Chemical analysis, antioxidant, cytotoxic and antimicrobial properties of propolis from different geographic regions. Ann. Agric. Sci. 2020, 65, 209–217. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- El Sohaimy, S.; Masry, S. Phenolic Content, Antioxidant and Antimicrobial Activities of Egyptian and Chinese Propolis. Am. Eurasian J. Agric. Environ. Sci. 2014, 14, 1116–1124. [Google Scholar] [CrossRef]
- Bisht, G.; Rayamajhi, S. ZnO Nanoparticles: A Promising Anticancer Agent. Nanobiomedicine 2016, 3, 9. [Google Scholar] [CrossRef]
- Patel, S. Emerging Adjuvant Therapy for Cancer: Propolis and its Constituents. J. Diet. Suppl. 2016, 13, 245–268. [Google Scholar] [CrossRef]
- Ali, A.; Ambreen, S.; Maqbool, Q.; Naz, S.; Shams, M.F.; Ahmad, M.; Phull, A.R.; Zia, M. Zinc impregnated cellulose nanocomposites: Synthesis, characterization and applications. J. Phys. Chem. Solids 2016, 98, 174–182. [Google Scholar] [CrossRef]
- Prakash, O.; Belal, B.; Dhanik, J.; Verma, A.; Joshi, H.C.; Vivekanand, V. Biological Activities of Metal Complexes with Rutin and Bio-Conjugate of Citrus Extract. Univers. J. Chem. 2020, 7, 1–24. [Google Scholar] [CrossRef]
- Ali, S.; Sudha, K.G.; Thirumalaivasan, N.; Ahamed, M.; Pandiaraj, S.; Rajeswari, V.D.; Vinayagam, Y.; Thiruvengadam, M.; Govindasamy, R. Green Synthesis of Magnesium Oxide Nanoparticles by Using Abrus precatorius Bark Extract and Their Photocatalytic, Antioxidant, Antibacterial, and Cytotoxicity Activities. Bioengineering 2023, 10, 302. [Google Scholar] [CrossRef]
- Ramamurthy, C.H.; Padma, M.; Mariya Samadanam, I.D.; Mareeswaran, R.; Suyavaran, A.; Kumar, M.S.; Premkumar, K.; Thirunavukkarasu, C. The extra cellular synthesis of gold and silver nanoparticles and their free radical scavenging and antibacterial properties. Colloids Surf. B Biointerfaces 2013, 102, 808–815. [Google Scholar] [CrossRef]
- Das, D.; Nath, B.C.; Phukon, P.; Dolui, S.K. Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles. Colloids Surf. B Biointerfaces 2013, 101, 430–433. [Google Scholar] [CrossRef]












| Materials/Band Position (cm−1) | |||
|---|---|---|---|
| Propolis | ZnO | ZnO-P NCs | Characteristic Group |
| 3371 | 3140 | 3371 | O-H and NH |
| 2925.4 | 2925.4 | CH2 | |
| 2854.18 | 2854.18 | CH3 | |
| 1631 | 1644 | C=C | |
| 1247.8 | 1558 | 1370.2 | C=O |
| 1440 | C-H | ||
| 688 | 667.8 | Zn-O | |
| 454 | 458.19 | Zn-O | |
| Sample | DPPH % Scavenging Activity | IC50 (mg mL−1) |
|---|---|---|
| Nano propolis | 51.85 | 1.7 |
| ZnO NPs | 62.56 | 2.75 |
| ZnO-P NCs | 71.02 | 1.45 |
| Compound | In Vitro Cytotoxicity IC50 (µg/mL) | ||
|---|---|---|---|
| WI38 | Hela | MCF7 | |
| DOX | 6.72 ± 0.5 | 5.57 ± 0.4 | 4.17 ± 0.2 |
| Nano propolis | 75.48 ± 3.9 | 49.01 ± 2.8 | 38.96 ± 2.3 |
| ZnO NPs | 43.10 ± 2.6 | 52.36 ± 3.0 | 68.80 ± 3.5 |
| ZnO-P NCs | 57.09 ± 3.3 | 23.77 ± 1.9 | 18.17 ± 1.4 |
| Sample | DPPH % Scavenging Activity | Ref. |
|---|---|---|
| Nano propolis | 51.85 | This work |
| ZnO NPs | 62.56 | This work |
| ZnO-P NCs | 71.02 | This work |
| Cellulose | 4.29 | [80] |
| ZnO NPs | 6.75 | [80] |
| ZnO-Cel | 14.85 | [80] |
| Rut-Zn | 41.92 | [81] |
| Cit-Zn | 31.33 | [81] |
| MgO NPs | 43.16 | [82] |
| Gold | 50 | [83] |
| Silver | 55 | [83] |
| Copper oxide | 85 (Conc. = 37.5 mg/mL) | [84] |
| Nickel oxide | 90 (Conc. = 33.3 mg/mL) | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salama, S.A.; Atta, R.R.; Khalil, E.M.; Abdelaleim, Y.F.; Abd-Eltawab, S.; Farghali, A.A.; Essam, D.; Alkhalifah, D.H.M.; Hozzein, W.N.; Mahmoud, R. Nano Propolis, Zinc Oxide Nanoparticles, and Their Composites: A Novel Green Synthesis with Synergistic Antioxidant and Anticancer Properties. J. Compos. Sci. 2023, 7, 480. https://doi.org/10.3390/jcs7110480
Salama SA, Atta RR, Khalil EM, Abdelaleim YF, Abd-Eltawab S, Farghali AA, Essam D, Alkhalifah DHM, Hozzein WN, Mahmoud R. Nano Propolis, Zinc Oxide Nanoparticles, and Their Composites: A Novel Green Synthesis with Synergistic Antioxidant and Anticancer Properties. Journal of Composites Science. 2023; 7(11):480. https://doi.org/10.3390/jcs7110480
Chicago/Turabian StyleSalama, Shaimaa A., Ramadan R. Atta, Ensaf M. Khalil, Yasser F. Abdelaleim, Samah Abd-Eltawab, Ahmed A. Farghali, Doaa Essam, Dalal Hussien M. Alkhalifah, Wael N. Hozzein, and Rehab Mahmoud. 2023. "Nano Propolis, Zinc Oxide Nanoparticles, and Their Composites: A Novel Green Synthesis with Synergistic Antioxidant and Anticancer Properties" Journal of Composites Science 7, no. 11: 480. https://doi.org/10.3390/jcs7110480






