Cu-Doped Porous ZnO-ZnAl2O4 Nanocomposites Synthesized by Polymer-Salt Method for Photocatalytic Water Purification
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
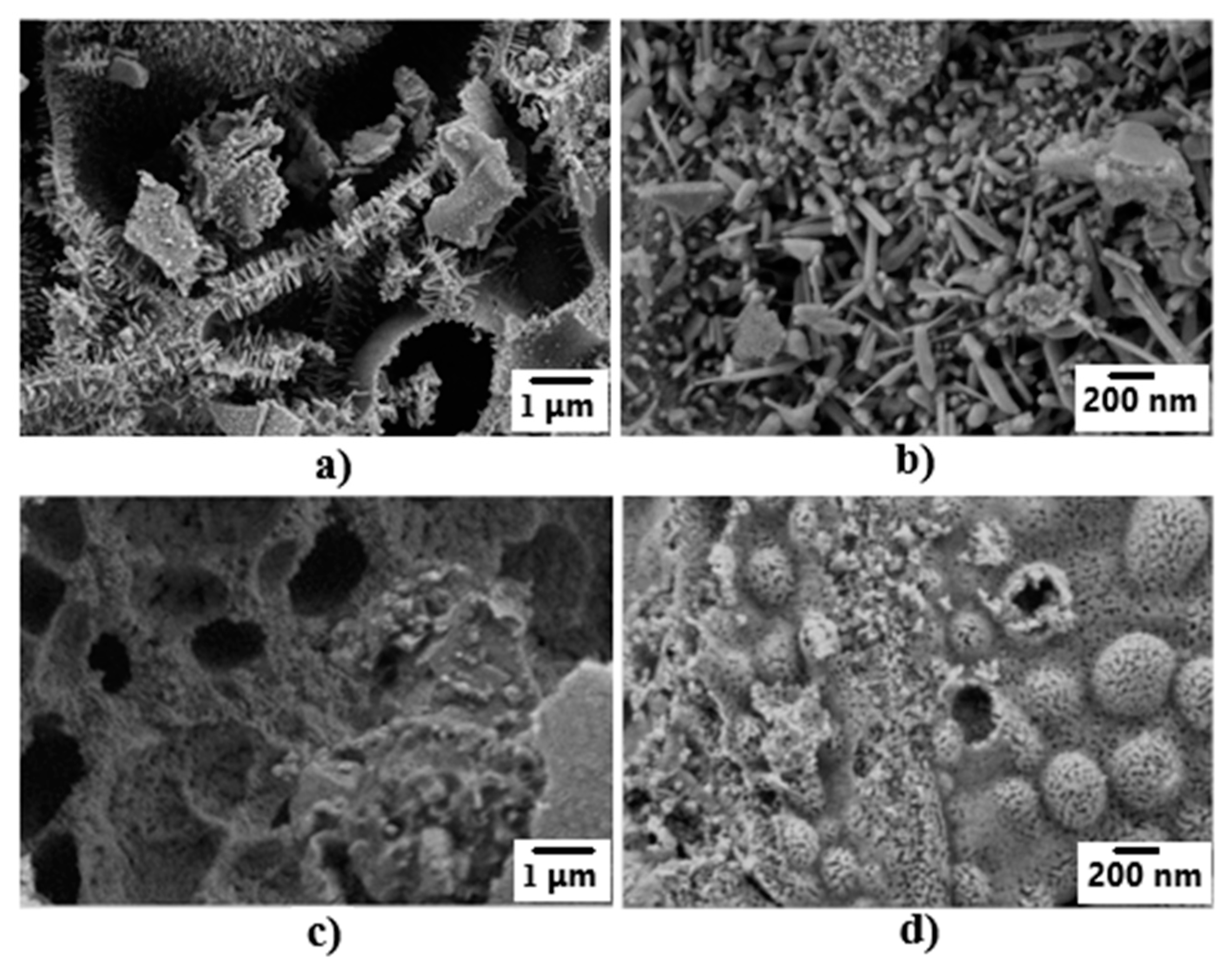
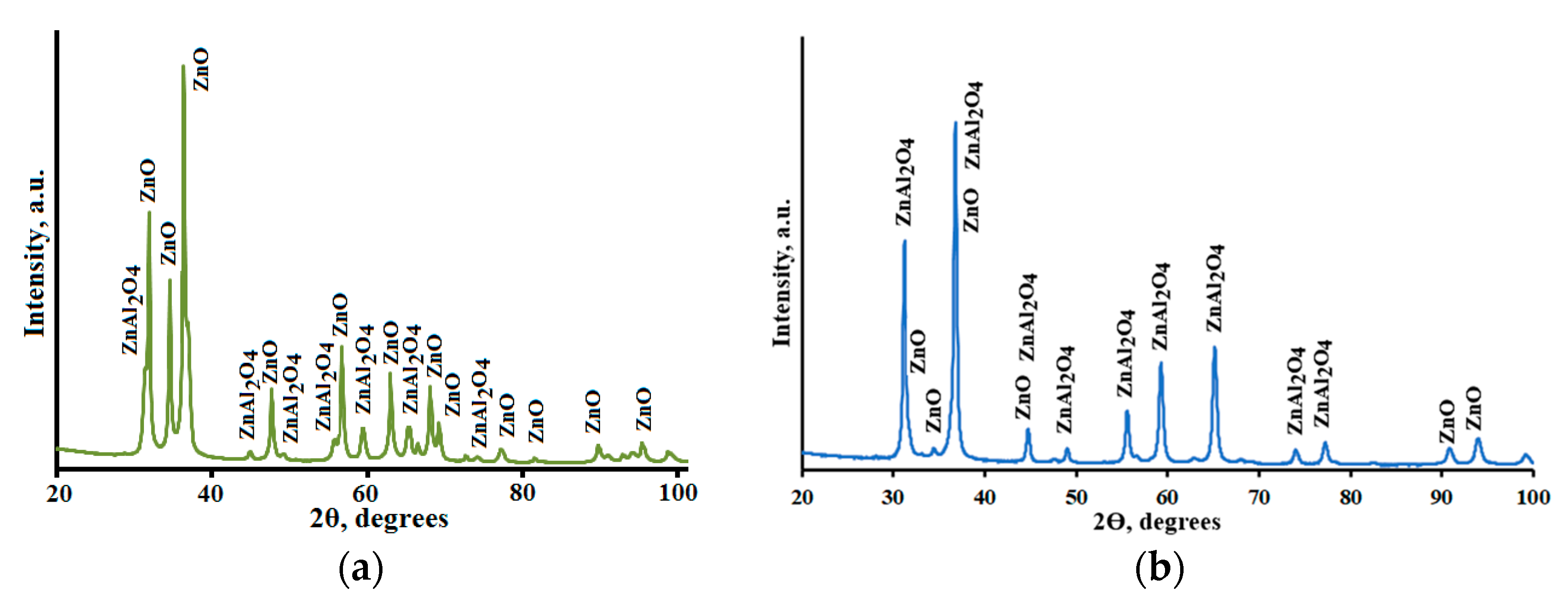
3.1. Crystal Structure and Morphology of ZnO-ZnAl2O4-CuO Composites
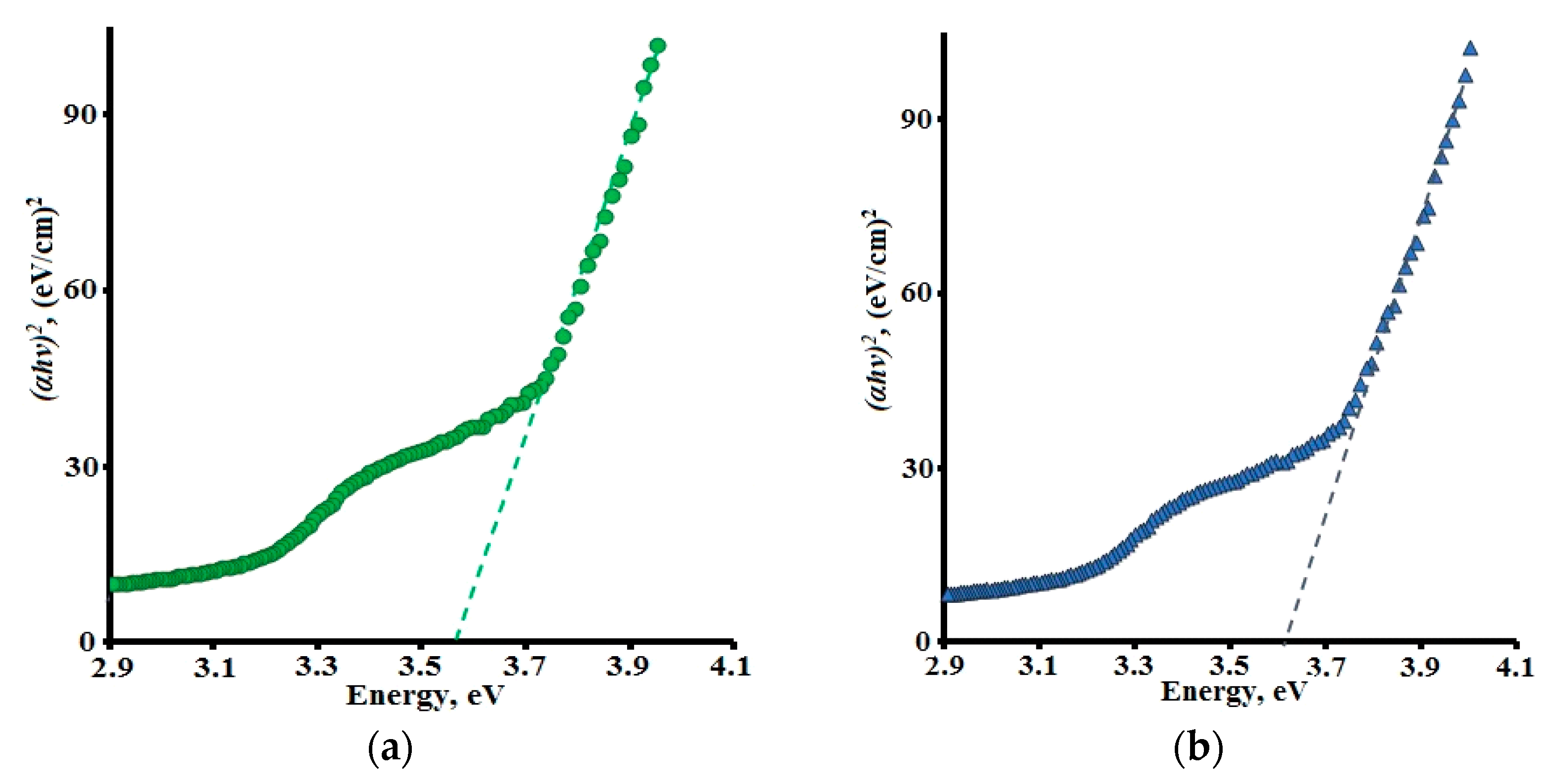
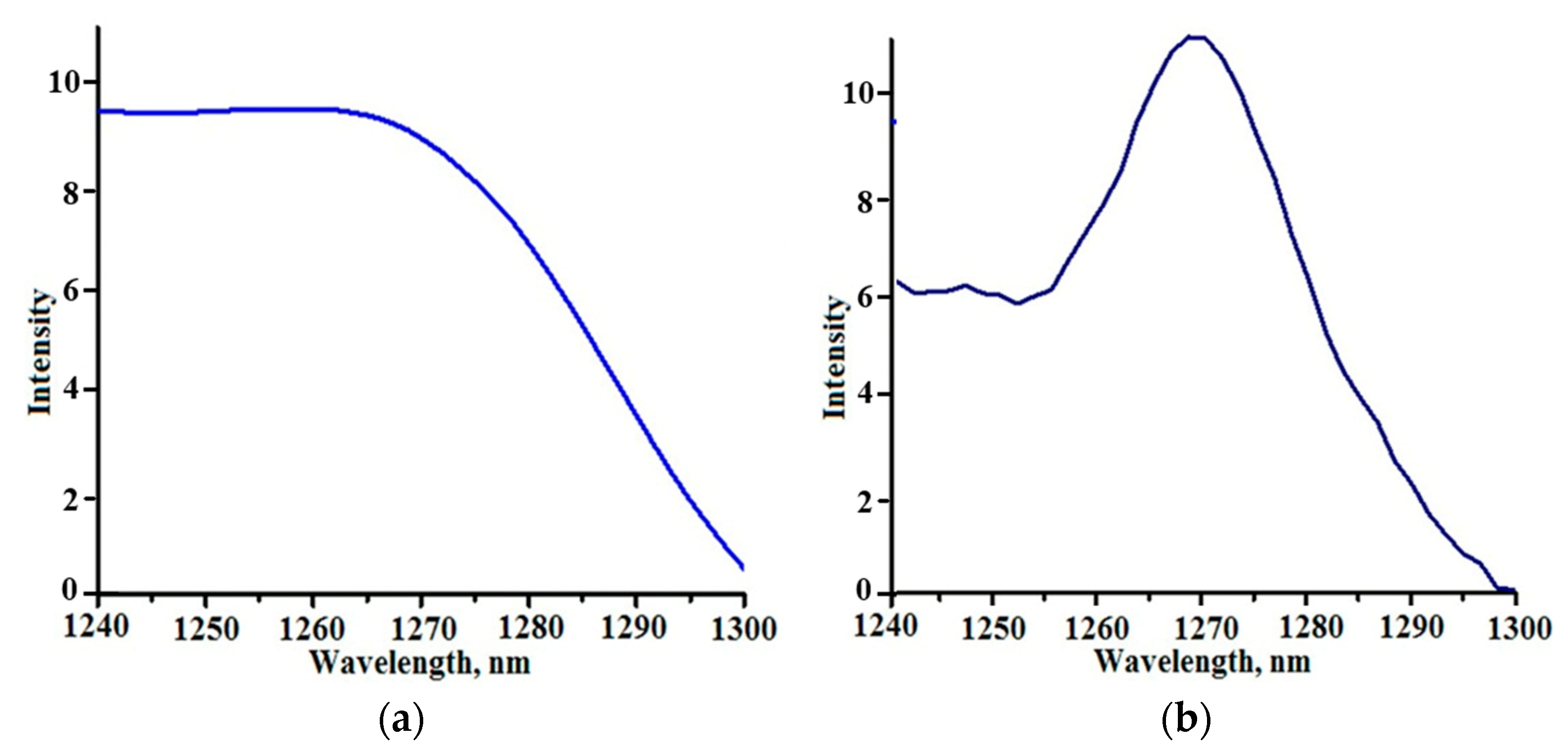
3.2. Band Gap Values
3.3. Photostimulated Discoloration of Dye Solutions
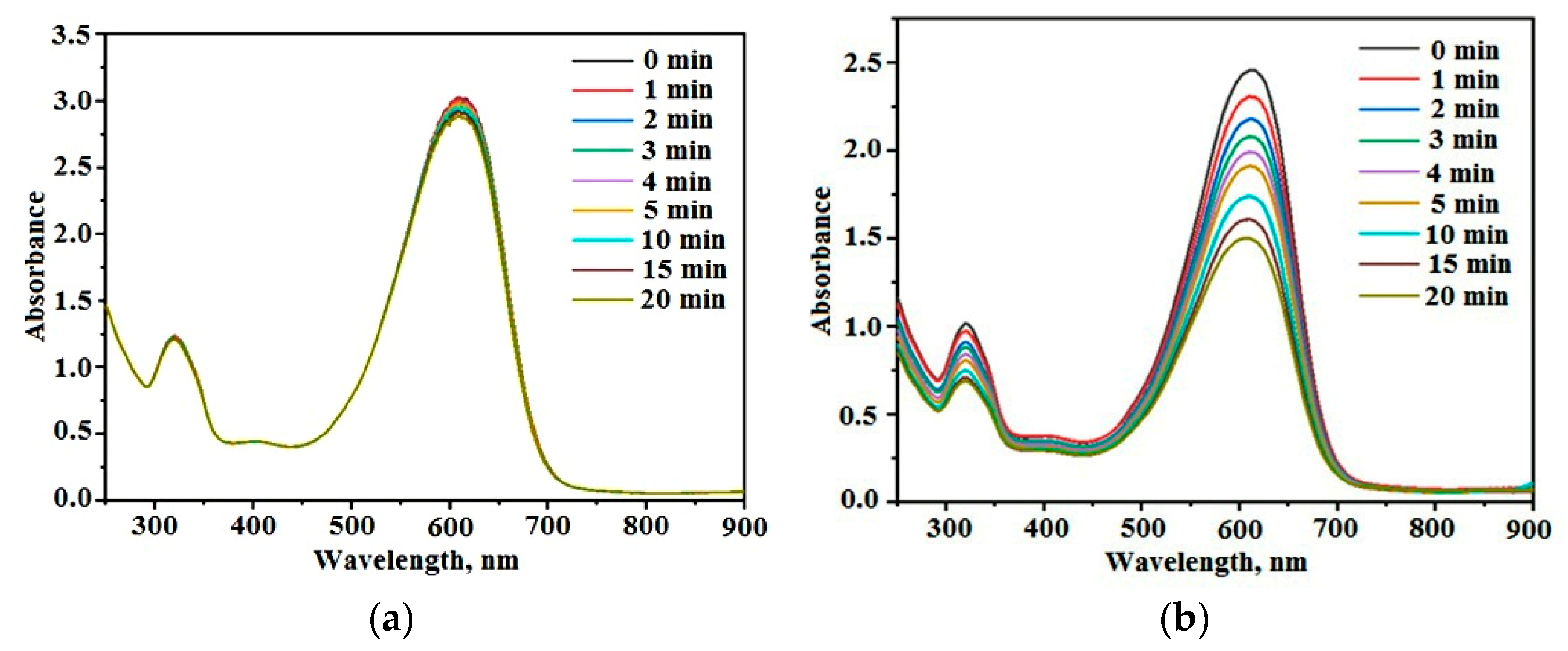
3.3.1. Photolysis of Dye Molecules in Solutions
3.3.2. Photocatalytic Discoloration of Dye Solutions
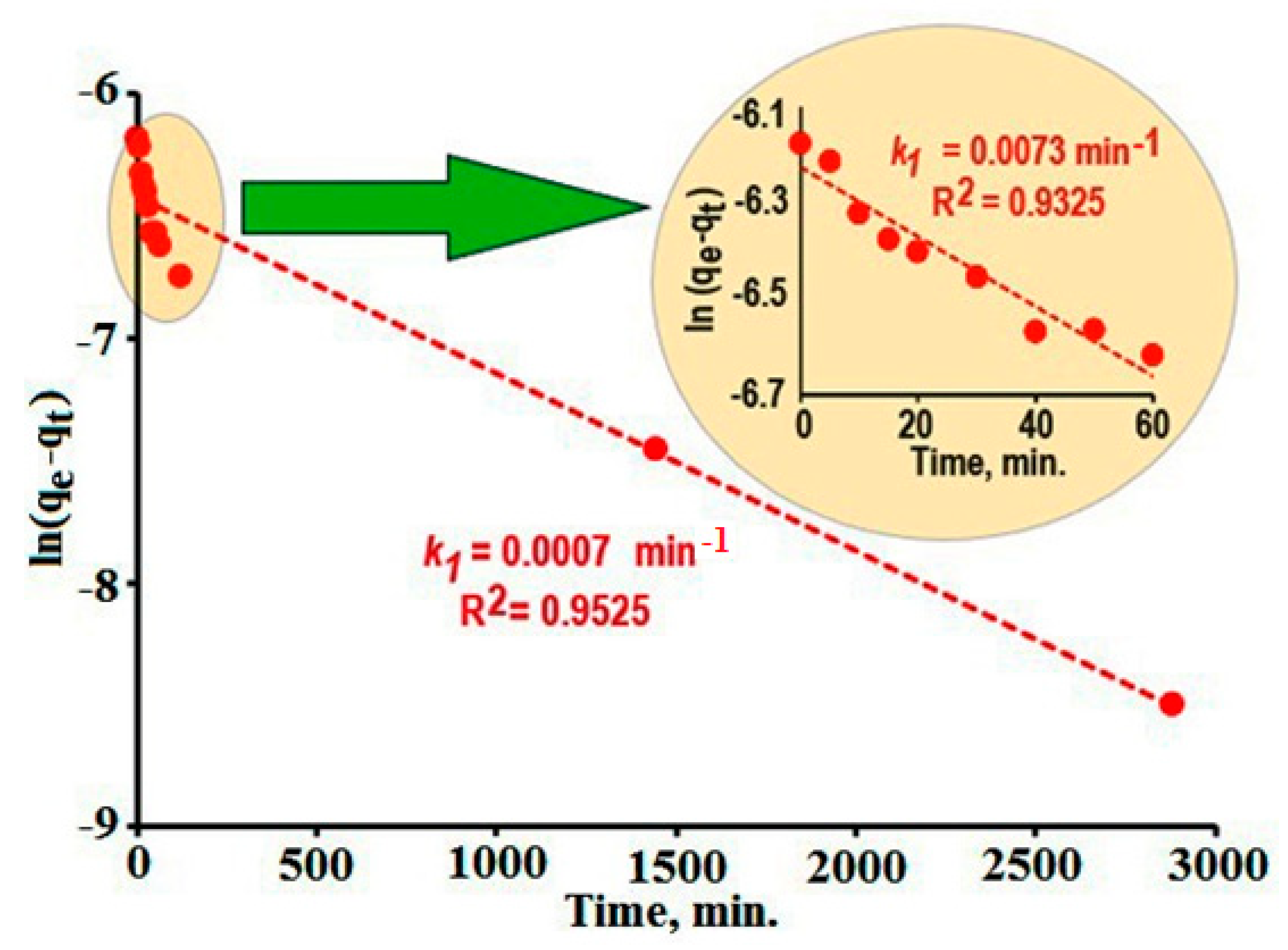
3.3.3. Dye Adsorption from Solutions on the Surfaces of Composites
- The dye photolysis in the liquid phase;
- Its adsorption on the powder surface;
- Photocatalytic dye decomposition.
- Fast adsorption observed at the initial stages of the process (duration of adsorption ~ 120 min);
- Slow adsorption which is proceed at a more long-term process (duration of adsorption > 120 min).
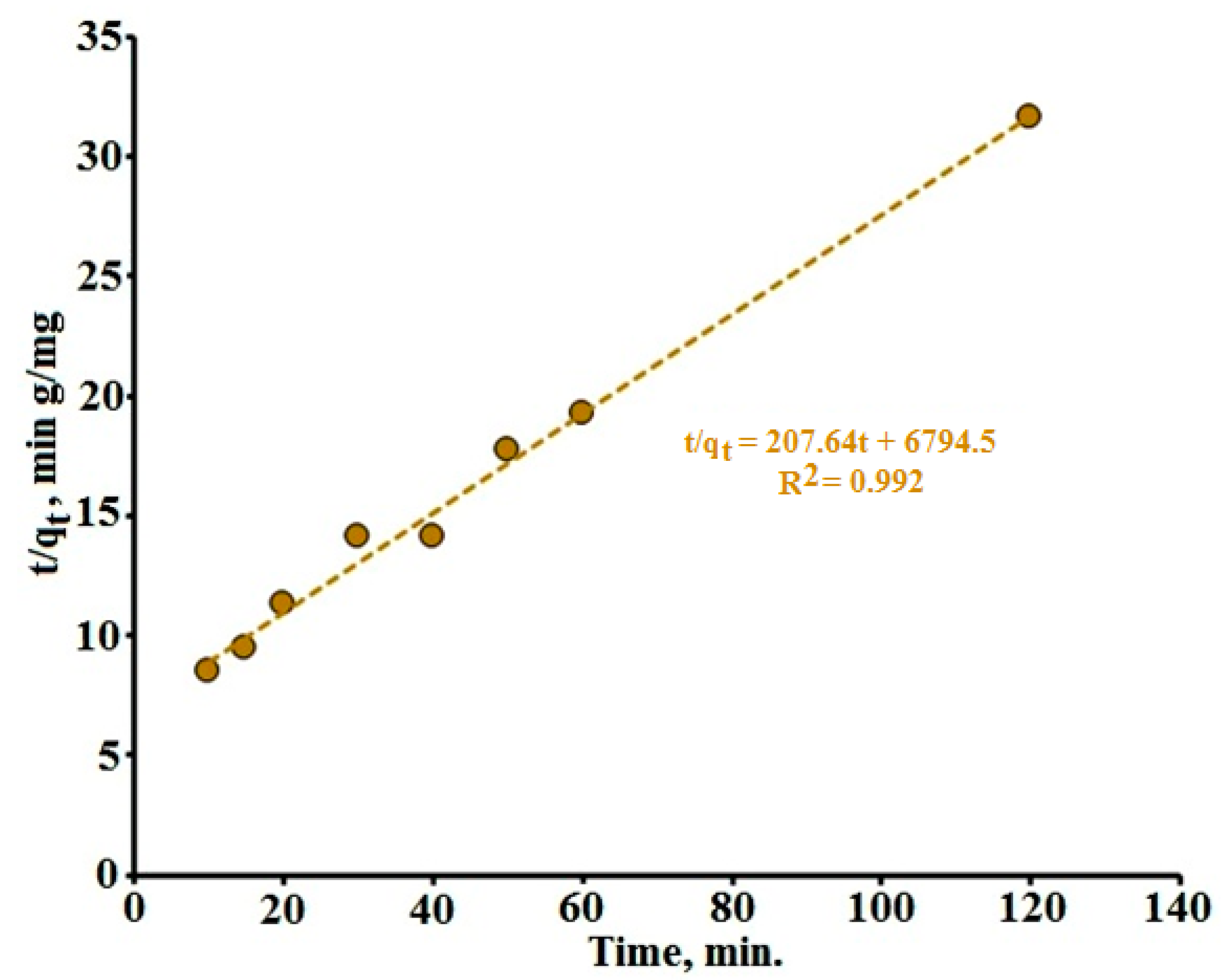
3.4. The Ratio of Adsorption and Photocatalysis Rates
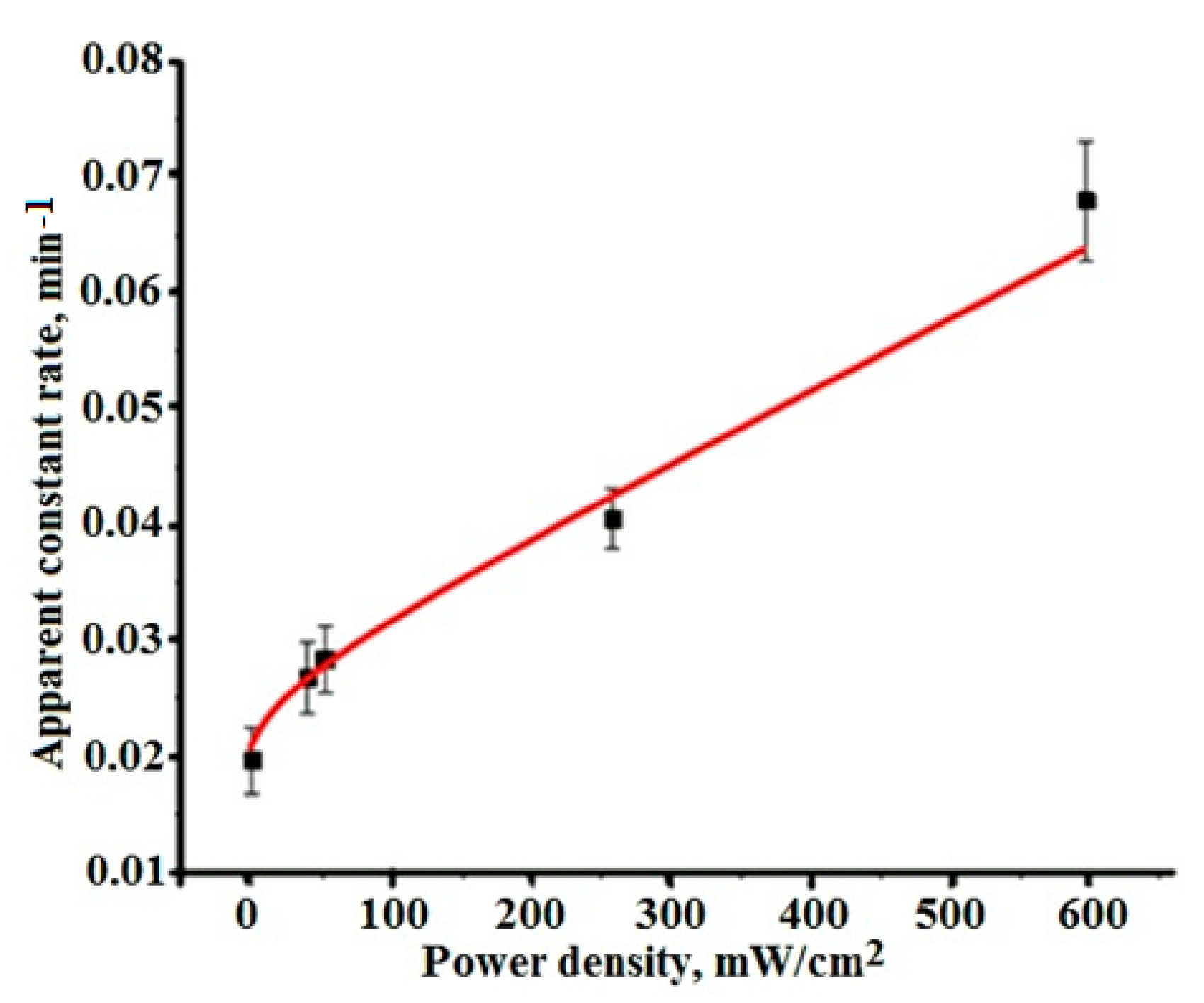
3.5. Influence of the Light Intensity on the Kinetics of Photocatalysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deng, Y. Developing a Langmuir-Type Excitation Equilibrium Equation to Describe the Effect of Light Intensity on the Kinetics of the Photocatalytic Oxidation. Chem. Eng. J. 2018, 337, 220–227. [Google Scholar] [CrossRef]
- Mills, A.; Wang, J.; Ollis, D. Dependence of the Kinetics of Liquid-Phase Photocatalyzed Reactions on Oxygen Concentration and Light Intensity. J. Catal. 2006, 243, 1–6. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z. Study on Light Intensity in the Process of Photocatalytic Degradation of Indoor Gaseous Formaldehyde for Saving Energy. Energy Convers. Manag. 2007, 48, 882–889. [Google Scholar] [CrossRef]
- Bell, S.; Will, G.; Bell, J. Light Intensity Effects on Photocatalytic Water Splitting with a Titania Catalyst. Int. J. Hydrogen Energy 2013, 38, 6938–6947. [Google Scholar] [CrossRef]
- Jadaa, W.; Prakash, A.; Ray, A.K. Photocatalytic Degradation of Diazo Dye over Suspended and Immobilized TiO2 Catalyst in Swirl Flow Reactor: Kinetic Modeling. Processes 2021, 9, 1741. [Google Scholar] [CrossRef]
- Meng, Y.; Huang, X.; Wu, Y.; Wang, X.; Qian, Y. Kinetic Study and Modeling on Photocatalytic Degradation of Para-Chlorobenzoate at Different Light Intensities. Environ. Pollut. 2002, 117, 307–313. [Google Scholar] [CrossRef]
- Xu, Y.; Langford, C.H. Variation of Langmuir Adsorption Constant Determined for TiO2-Photocatalyzed Degradation of Acetophenone under Different Light Intensity. J. Photochem. Photobiol. Chem. 2000, 133, 67–71. [Google Scholar] [CrossRef]
- Li, Y.; Sun, S.; Ma, M.; Ouyang, Y.; Yan, W. Kinetic Study and Model of the Photocatalytic Degradation of Rhodamine B (RhB) by a TiO2-Coated Activated Carbon Catalyst: Effects of Initial RhB Content, Light Intensity and TiO2 Content in the Catalyst. Chem. Eng. J. 2008, 142, 147–155. [Google Scholar] [CrossRef]
- Li Puma, G.; Salvadó-Estivill, I.; Obee, T.N.; Hay, S.O. Kinetics Rate Model of the Photocatalytic Oxidation of Trichloroethylene in Air over TiO2 Thin Films. Sep. Purif. Technol. 2009, 67, 226–232. [Google Scholar] [CrossRef]
- Turchi, C. Photocatalytic Degradation of Organic Water Contaminants: Mechanisms Involving Hydroxyl Radical Attack. J. Catal. 1990, 122, 178–192. [Google Scholar] [CrossRef]
- Wang, X.; Ahmad, M.; Sun, H. Three-Dimensional ZnO Hierarchical Nanostructures: Solution Phase Synthesis and Applications. Materials 2017, 10, 1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peighambardoust, S.J.; Boffito, D.C.; Foroutan, R.; Ramavandi, B. Sono-Photocatalytic Activity of Sea Sediment@400/ZnO Catalyst to Remove Cationic Dyes from Wastewater. J. Mol. Liq. 2022, 367, 120478. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Boffito, D.C.; Ramavandi, B. Sono-Photocatalytic Activity of Cloisite 30B/ZnO/Ag2O Nanocomposite for the Simultaneous Degradation of Crystal Violet and Methylene Blue Dyes in Aqueous Media. Nanomaterials 2022, 12, 3103. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of Photogenerated Reactive Oxygen Species and Correlation with the Antibacterial Properties of Engineered Metal-Oxide Nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Shelemanov, A.A.; Evstropiev, S.K.; Karavaeva, A.V.; Nikonorov, N.V.; Vasilyev, V.N.; Podruhin, Y.F.; Kiselev, V.M. Enhanced Singlet Oxygen Photogeneration by Bactericidal ZnO–MgO–Ag Nanocomposites. Mater. Chem. Phys. 2022, 276, 125204. [Google Scholar] [CrossRef]
- Evstropiev, S.K.; Karavaeva, A.V.; Petrova, M.A.; Nikonorov, N.V.; Vasilyev, V.N.; Lesnykh, L.L.; Dukelskii, K.V. Antibacterial Effect of Nanostructured ZnO-SnO2 Coatings: The Role of Microstructure. Mater. Today Commun. 2019, 21, 100628. [Google Scholar] [CrossRef]
- Hamrouni, A.; Moussa, N.; Parrino, F.; Di Paola, A.; Houas, A.; Palmisano, L. Sol–Gel Synthesis and Photocatalytic Activity of ZnO–SnO2 Nanocomposites. J. Mol. Catal. Chem. 2014, 390, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Manoharan, C.; Pavithra, G.; Dhanapandian, S.; Dhamodharan, P. Effect of In Doping on the Properties and Antibacterial Activity of ZnO Films Prepared by Spray Pyrolysis. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2015, 149, 793–799. [Google Scholar] [CrossRef]
- Tincu, A.; Shelemanov, A.A.; Evstropiev, S.K.; Nikonorov, N.V.; Dukelskii, K.V. Controlled Chemical Transformation and Crystallization Design for the Formation of Multifunctional Cu-Doped ZnO/ZnAl2O4 Composites. J. Inorg. Organomet. Polym. Mater. 2022, 33, 398–406. [Google Scholar] [CrossRef]
- Foletto, E.L.; Battiston, S.; Simões, J.M.; Bassaco, M.M.; Pereira, L.S.F.; de Moraes Flores, É.M.; Müller, E.I. Synthesis of ZnAl2O4 Nanoparticles by Different Routes and the Effect of Its Pore Size on the Photocatalytic Process. Microporous Mesoporous Mater. 2012, 163, 29–33. [Google Scholar] [CrossRef]
- Anchieta, C.G.; Sallet, D.; Foletto, E.L.; da Silva, S.S.; Chiavone-Filho, O.; do Nascimento, C.A.O. Synthesis of Ternary Zinc Spinel Oxides and Their Application in the Photodegradation of Organic Pollutant. Ceram. Int. 2014, 40, 4173–4178. [Google Scholar] [CrossRef]
- Battiston, S.; Rigo, C.; da Severo, E.C.; Mazutti, M.A.; Kuhn, R.C.; Gündel, A.; Foletto, E.L. Synthesis of Zinc Aluminate (ZnAl2O4) Spinel and Its Application as Photocatalyst. Mater. Res. 2014, 17, 734–738. [Google Scholar] [CrossRef]
- Zawadzki, M.; Staszak, W.; López-Suárez, F.E.; Illán-Gómez, M.J.; Bueno-López, A. Preparation, Characterisation and Catalytic Performance for Soot Oxidation of Copper-Containing ZnAl2O4 Spinels. Appl. Catal. Gen. 2009, 371, 92–98. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, L.; Xu, X.; Lei, X.; Xu, S.; Zhang, F. Fabrication and Photocatalytic Properties of Novel ZnO/ZnAl2O4 Nanocomposite with ZnAl2O4 Dispersed inside ZnO Network. AIChE J. 2012, 58, 573–582. [Google Scholar] [CrossRef]
- Shahmirzaee, M.; Shafiee Afarani, M.; Arabi, A.M.; Iran Nejhad, A. In Situ Crystallization of ZnAl2O4/ZnO Nanocomposite on Alumina Granule for Photocatalytic Purification of Wastewater. Res. Chem. Intermed. 2017, 43, 321–340. [Google Scholar] [CrossRef]
- Yuan, X.; Cheng, X.; Jing, Q.; Niu, J.; Peng, D.; Feng, Z.; Wu, X. ZnO/ZnAl2O4 Nanocomposite with 3D Sphere-Like Hierarchical Structure for Photocatalytic Reduction of Aqueous Cr(VI). Materials 2018, 11, 1624. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yan, J.; Zhou, M.; Yang, Y.; Liu, Y.-N. Fabrication and Photocatalytic Properties of Spheres-in-Spheres ZnO/ZnAl2O4 Composite Hollow Microspheres. Appl. Surf. Sci. 2013, 268, 237–245. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, Y.; Jiang, P.; Wang, G.; Zhang, J.; Zhang, C. ZnAl2O4 as a Novel High-Surface-Area Ozonation Catalyst: One-Step Green Synthesis, Catalytic Performance and Mechanism. Chem. Eng. J. 2015, 260, 623–630. [Google Scholar] [CrossRef]
- Akika, F.Z.; Benamira, M.; Lahmar, H.; Trari, M.; Avramova, I.; Suzer, Ş. Structural and Optical Properties of Cu-Doped ZnAl2O4 and Its Application as Photocatalyst for Cr(VI) Reduction under Sunlight. Surf. Interfaces 2020, 18, 100406. [Google Scholar] [CrossRef]
- Saratovskii, A.S.; Bulyga, D.V.; Evstrop’ev, S.K.; Antropova, T.V. Adsorption and Photocatalytic Activity of the Porous Glass–ZnO–Ag Composite and ZnO–Ag Nanopowder. Glass Phys. Chem. 2022, 48, 10–17. [Google Scholar] [CrossRef]
- Vimonses, V.; Chong, M.N.; Jin, B. Evaluation of the Physical Properties and Photodegradation Ability of Titania Nanocrystalline Impregnated onto Modified Kaolin. Microporous Mesoporous Mater. 2010, 132, 201–209. [Google Scholar] [CrossRef]
- Minh, T.T.; Tu, N.T.T.; Van Thi, T.T.; Hoa, L.T.; Long, H.T.; Phong, N.H.; Pham, T.L.M.; Khieu, D.Q. Synthesis of Porous Octahedral ZnO/CuO Composites from Zn/Cu-Based MOF-199 and Their Applications in Visible-Light-Driven Photocatalytic Degradation of Dyes. J. Nanomater. 2019, 2019, 5198045. [Google Scholar] [CrossRef] [Green Version]
- Evstropiev, S.K.; Lesnykh, L.V.; Karavaeva, A.V.; Nikonorov, N.V.; Oreshkina, K.V.; Mironov, L.Y.; Maslennikov, S.Y.; Kolobkova, E.V.; Vasilyev, V.N.; Bagrov, I.V. Intensification of Photodecomposition of Organics Contaminations by Nanostructured ZnO-SnO2 Coatings Prepared by Polymer-Salt Method. Chem. Eng. Process.-Process Intensif. 2019, 142, 107587. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, X.; Zhou, S. Adsorption of Methylene Blue in Water onto Activated Carbon by Surfactant Modification. Water 2020, 12, 587. [Google Scholar] [CrossRef] [Green Version]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous Photocatalytic Degradation of Organic Contaminants over Titanium Dioxide: A Review of Fundamentals, Progress and Problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- El Mouchtari, E.M.; Bahsis, L.; El Mersly, L.; Anane, H.; Lebarillier, S.; Piram, A.; Briche, S.; Wong-Wah-Chung, P.; Rafqah, S. Insights in the Aqueous and Adsorbed Photocatalytic Degradation of Carbamazepine by a Biosourced Composite: Kinetics, Mechanisms and DFT Calculations. Int. J. Environ. Res. 2021, 15, 135–147. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. Classifications, Properties, Recent Synthesis and Applications of Azo Dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; El Harfi, A. Textile Finishing Dyes and Their Impact on Aquatic Environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef]
- Daimon, T.; Nosaka, Y. Formation and Behavior of Singlet Molecular Oxygen in TiO2 Photocatalysis Studied by Detection of Near-Infrared Phosphorescence. J. Phys. Chem. C 2007, 111, 4420–4424. [Google Scholar] [CrossRef]
- Kiselev, V.M.; Kislyakov, I.M.; Burchinov, A.N. Generation of Singlet Oxygen on the Surface of Metal Oxides. Opt. Spectrosc. 2016, 120, 520–528. [Google Scholar] [CrossRef]
- Boltenkov, I.S.; Kolobkova, E.V.; Evstropiev, S.K. Synthesis and Characterization of Transparent Photocatalytic ZnO-Sm2O3 and ZnO-Er2O3 Coatings. J. Photochem. Photobiol. Chem. 2018, 367, 458–464. [Google Scholar] [CrossRef]
- Morkoç, H.; Özgür, Ü. Zinc Oxide: Fundamentals, Materials and Device Technology; Wiley-VCH: Weinheim, Germany, 2009; ISBN 978-3-527-40813-9. [Google Scholar]
- Lim, A.R. Effects of Paramagnetic Interactions by the Partial Replacement of Zn2+ Ions with Cu2+ Ions in Lead-Free Zinc-Based Perovskite (MA)2ZnCl4 Crystal by MAS NMR. AIP Adv. 2019, 9, 105115. [Google Scholar] [CrossRef] [Green Version]
- Evstropiev, S.K.; Soshnikov, I.P.; Kolobkova, E.V.; Evstropyev, K.S.; Nikonorov, N.V.; Khrebtov, A.I.; Dukelskii, K.V.; Kotlyar, K.P.; Oreshkina, K.V.; Nashekin, A.V. Polymer-Salt Synthesis and Characterization of MgO-ZnO Ceramic Coatings with the High Transparency in UV Spectral Range. Opt. Mater. 2018, 82, 81–87. [Google Scholar] [CrossRef]
- Koike, K.; Hama, K.; Nakashima, I.; Takada, G.; Ogata, K.; Sasa, S.; Inoue, M.; Yano, M. Molecular Beam Epitaxial Growth of Wide Bandgap ZnMgO Alloy Films on (111)-Oriented Si Substrate toward UV-Detector Applications. J. Cryst. Growth 2005, 278, 288–292. [Google Scholar] [CrossRef]
- Ohtomo, A.; Kawasaki, M.; Koida, T.; Masubuchi, K.; Koinuma, H.; Sakurai, Y.; Yoshida, Y.; Yasuda, T.; Segawa, Y. MgxZn1−xO as a II–VI Widegap Semiconductor Alloy. Appl. Phys. Lett. 1998, 72, 2466–2468. [Google Scholar] [CrossRef] [Green Version]
- Mang, A.; Reimann, K.; Rübenacke, S. Band Gaps, Crystal-Field Splitting, Spin-Orbit Coupling, and Exciton Binding Energies in ZnO under Hydrostatic Pressure. Solid State Commun. 1995, 94, 251–254. [Google Scholar] [CrossRef]
- Sampath, S.K.; Cordaro, J.F. Optical Properties of Zinc Aluminate, Zinc Gallate, and Zinc Aluminogallate Spinels. J. Am. Ceram. Soc. 2005, 81, 649–654. [Google Scholar] [CrossRef]
- Sawicka-Chudy, P.; Sibiński, M.; Wisz, G.; Rybak-Wilusz, E.; Cholewa, M. Numerical Analysis and Optimization of Cu2O/TiO2, CuO/TiO2, Heterojunction Solar Cells Using SCAPS. J. Phys. Conf. Ser. 2018, 1033, 012002. [Google Scholar] [CrossRef] [Green Version]
- Fox, M.A.; Dulay, M.T. Heterogeneous Photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-Assisted Photocatalytic Degradation of Azo Dyes in Aqueous Solution: Kinetic and Mechanistic Investigations. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Greenway, G.M.; Yue, Y.; Falaras, P.; Tsoukleris, D. The Photocatalytic Activity and Kinetics of the Degradation of an Anionic Azo-Dye in a UV Irradiated Porous Titania Foam. Appl. Catal. B Environ. 2008, 84, 351–355. [Google Scholar] [CrossRef]
- Lagergren, S. About the Theory of So-Called Adsorption of Soluble Substances. Kung Sven Veten Hand. Sven. Vetenskapsakad. Handingarl 1989, 24, 1–39. [Google Scholar]
- Uribe-López, M.C.; Hidalgo-López, M.C.; López-González, R.; Frías-Márquez, D.M.; Núñez-Nogueira, G.; Hernández-Castillo, D.; Alvarez-Lemus, M.A. Photocatalytic Activity of ZnO Nanoparticles and the Role of the Synthesis Method on Their Physical and Chemical Properties. J. Photochem. Photobiol. Chem. 2021, 404, 112866. [Google Scholar] [CrossRef]
- PubChem Release 2021.05.07. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 7 May 2021).
- Abebe, B.; Murthy, H.C.A.; Amare, E. Summary on Adsorption and Photocatalysis for Pollutant Remediation: Mini Review. J. Encapsulation Adsorpt. Sci. 2018, 08, 225–255. [Google Scholar] [CrossRef] [Green Version]


| Sample | Chemical Composition of Solutions, mol.% | Chemical Composition of Powders, mol.% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| H2O | PVP | Propanol-2 | Zn(NO3)2 ∙6H2O | Al(NO3)3 ∙9H2O | CuSO4 ·5H2O | ZnO | ZnAl2O4 | CuO | |
| 1 | 80.533 | 0.002 | 19.010 | 0.320 | 0.133 | 0.002 | 80.16 | 19.83 | 0.04 |
| 2 | 80.561 | 0.002 | 19.010 | 0.162 | 0.258 | 0.002 | 20.81 | 79.18 | 0.01 |
| Photocatalysts | Constant Rate, min−1 | References |
|---|---|---|
| ZnO 100% | 0.022 | [29] |
| ZnO 93.2 mol.% + SnO2 6.8 mol.% | 0.026 | [29] |
| ZnO 95.7 mol.% + Er2O3 4.3 mol.% | 0.012 | [41] |
| ZnO 95.3 mol.% + Er2O3 4.7 mol.% | 0.017 | [41] |
| ZnO 96.2 mol.% + Sm2O3 3.8 mol.% | 0.014 | [41] |
| ZnO 80.16 mol.% + ZnAl2O4 19.83 mol.% + CuO 0.04 mol.% | 0.021 | present work |
| ZnO 20.81 mol.% + ZnAl2O4 79.18 mol.% + CuO 0.01 mol.% | 0.005 | present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shelemanov, A.; Tincu, A.; Evstropiev, S.; Nikonorov, N.; Vasilyev, V. Cu-Doped Porous ZnO-ZnAl2O4 Nanocomposites Synthesized by Polymer-Salt Method for Photocatalytic Water Purification. J. Compos. Sci. 2023, 7, 263. https://doi.org/10.3390/jcs7070263
Shelemanov A, Tincu A, Evstropiev S, Nikonorov N, Vasilyev V. Cu-Doped Porous ZnO-ZnAl2O4 Nanocomposites Synthesized by Polymer-Salt Method for Photocatalytic Water Purification. Journal of Composites Science. 2023; 7(7):263. https://doi.org/10.3390/jcs7070263
Chicago/Turabian StyleShelemanov, Andrey, Artem Tincu, Sergey Evstropiev, Nikolay Nikonorov, and Vladimir Vasilyev. 2023. "Cu-Doped Porous ZnO-ZnAl2O4 Nanocomposites Synthesized by Polymer-Salt Method for Photocatalytic Water Purification" Journal of Composites Science 7, no. 7: 263. https://doi.org/10.3390/jcs7070263







