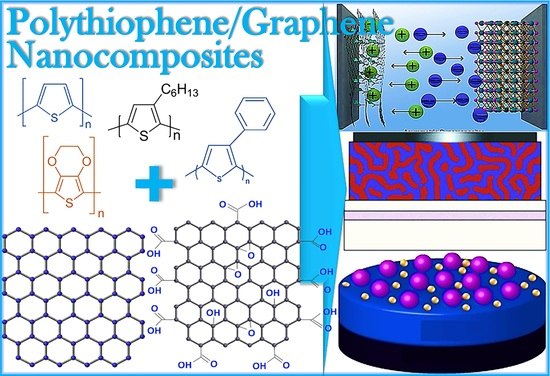
Cutting-Edge Graphene Nanocomposites with Polythiophene—Design, Features and Forefront Potential
Abstract
:1. Introduction
2. Polythiophene and Derived Forms
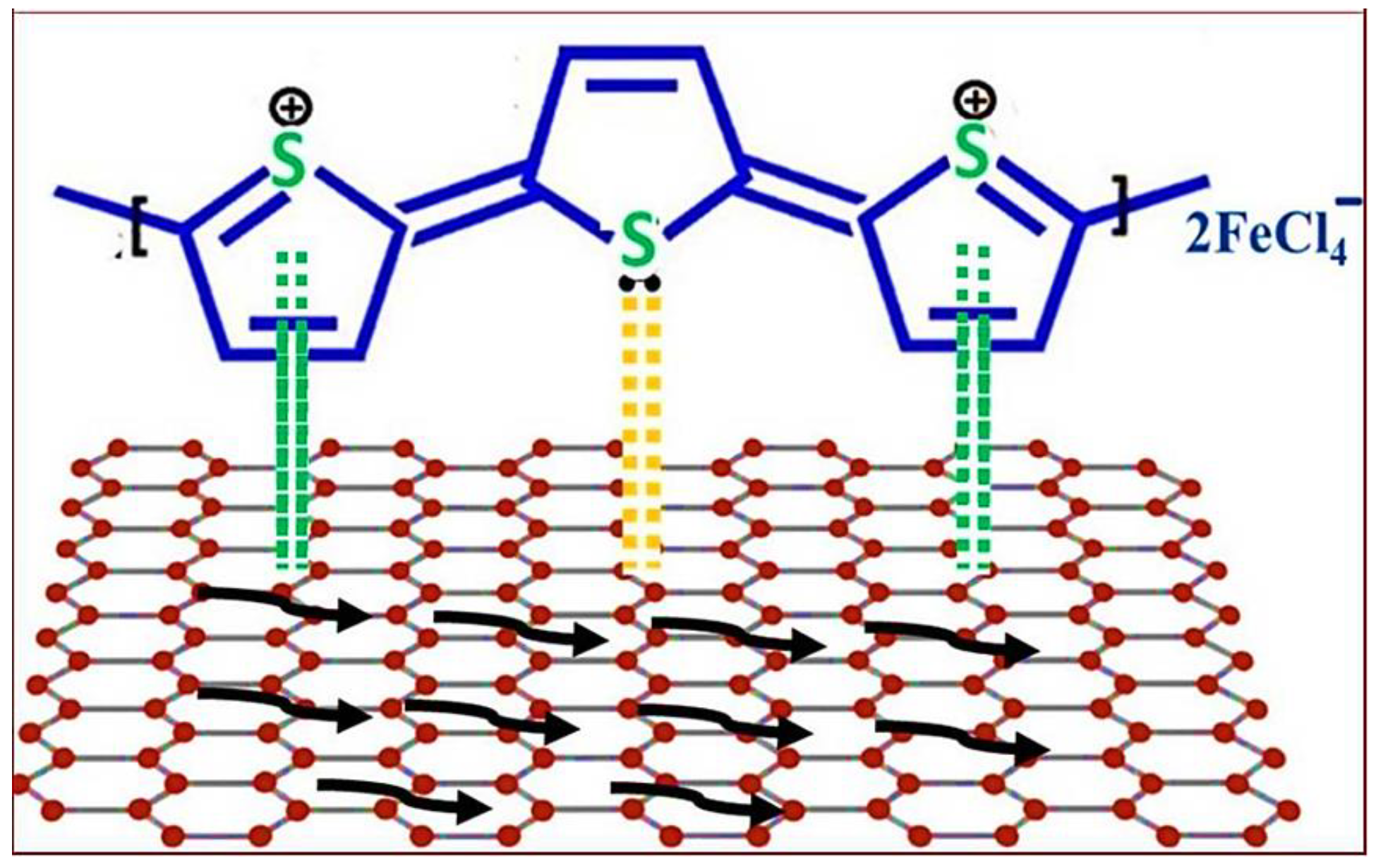
3. Graphene Dispersal Effects in Polythiophene
4. Polythiophene and Graphene-Derived Nanocomposites
5. Technological Potential of Polythiophene and Graphene-Derived Nanocomposites
6. Challenges and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ichakpa, M.; Goodyear, M.; Duthie, J.; Duthie, M.; Wisely, R.; MacPherson, A.; Keyte, J.; Pancholi, K.; Njuguna, J. Investigation on Mechanical and Thermal Properties of 3D-Printed Polyamide 6, Graphene Oxide and Glass-Fibre-Reinforced Composites under Dry, Wet and High Temperature Conditions. J. Compos. Sci. 2023, 7, 227. [Google Scholar] [CrossRef]
- Mohammadsalih, Z.G.; Inkson, B.J.; Chen, B. Structure and properties of polystyrene-co-acrylonitrile/graphene oxide nanocomposites. J. Compos. Sci. 2023, 7, 225. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I.; Zhao, T.; Eisa, M.; Aldaghri, O.; Gupta, M.; Bocchetta, P. Green-Synthesized Graphene for Supercapacitors—Modern Perspectives. J. Compos. Sci. 2023, 7, 108. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I. Conducting Polymer Nanocomposites for Electromagnetic Interference Shielding—Radical Developments. J. Compos. Sci. 2023, 7, 240. [Google Scholar] [CrossRef]
- Mohsenpour, A.; Eisazadeh, H. Preparation and characterization of polythiophene containing Al2O3 nanoparticles using sodium dodecylbenzenesulfonate as a surfactant. Polym. Plast. Technol. Eng. 2015, 54, 57–60. [Google Scholar] [CrossRef]
- Sharif, M.; Heidari, A.; Aghaeinejad Meybodi, A. Polythiophene/Zinc Oxide/Graphene Oxide Ternary Photocatalyst: Synthesis, characterization and application. Polym. Plast. Technol. Mater. 2021, 60, 1450–1460. [Google Scholar] [CrossRef]
- Azimi, M.; Abbaspour, M.; Fazli, A.; Setoodeh, H.; Pourabbas, B. Investigation on electrochemical properties of polythiophene nanocomposite with graphite derivatives as supercapacitor material on breath figure-decorated PMMA electrode. J. Electron. Mater. 2018, 47, 2093–2102. [Google Scholar] [CrossRef]
- Sharif, M.; Pourabas, B. Polythiophene–graphene oxide doped epoxy resin nanocomposites with enhanced electrical, mechanical and thermal properties. RSC Adv. 2016, 6, 93680–93693. [Google Scholar]
- Cao, Y.; Mao, S.; Li, M.; Chen, Y.; Wang, Y. Metal/porous carbon composites for heterogeneous catalysis: Old catalysts with improved performance promoted by N-doping. ACS Catal. 2017, 7, 8090–8112. [Google Scholar] [CrossRef]
- Husain, A.; Ahmad, S.; Mohammad, F. Electrical conductivity and alcohol sensing studies on polythiophene/tin oxide nanocomposites. J. Sci. Adv. Mater. Devices 2020, 5, 84–94. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, J. Scientific Importance of Water-Processable PEDOT–PSS and Preparation, Challenge and New Application in Sensors of Its Film Electrode: A Review. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1121–1150. [Google Scholar] [CrossRef] [Green Version]
- Bakhshi, A.; Ladik, J.; Seel, M. Comparative study of the electronic structure and conduction properties of polypyrrole, polythiophene, and polyfuran and their copolymers. Phys. Rev. B 1987, 35, 704. [Google Scholar] [CrossRef] [PubMed]
- Uflyand, I.E.; Dzhardimalieva, G.I. Thermolysis of Metal Chelates in Polymer Matrices. In Nanomaterials Preparation by Thermolysis of Metal Chelates; Springer: Cham, Switzerland, 2018; pp. 425–458. [Google Scholar]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar]
- Kaloni, T.P.; Schreckenbach, G.; Freund, M.S. Structural and electronic properties of pristine and doped polythiophene: Periodic versus molecular calculations. J. Phys. Chem. C 2015, 119, 3979–3989. [Google Scholar] [CrossRef] [Green Version]
- Kalagi, S.S.; Patil, P.S. Secondary electrochemical doping level effects on polaron and bipolaron bands evolution and interband transition energy from absorbance spectra of PEDOT: PSS thin films. Synth. Met. 2016, 220, 661–666. [Google Scholar] [CrossRef]
- Thanasamy, D.; Jesuraj, D.; Avadhanam, V. A novel route to synthesis polythiophene with great yield and high electrical conductivity without post doping process. Polymer 2019, 175, 32–40. [Google Scholar] [CrossRef]
- Riaz, U.; Farooq, A.; Nabi, N.; Nwanze, F.R.; Yan, F. Synthesis, characterization, and biophysical interaction studies of water-dispersible polypyrrole/polythiophene co-oligomers with bovine serum albumin and human serum albumin: An experimental and theoretical approach. New J. Chem. 2023, 47, 5667–5679. [Google Scholar]
- Deng, S.; Dong, C.; Liu, J.; Meng, B.; Hu, J.; Min, Y.; Tian, H.; Liu, J.; Wang, L. An n-Type Polythiophene Derivative with Excellent Thermoelectric Performance. Angew. Chem. Int. Ed. 2023, 62, e202216049. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mo, D.; Chen, S.; Xu, J.; Lu, B.; Jiang, Q.; Feng, Z.; Xiong, J.; Zhen, S. Poly (thieno [3,4-b]-1,4-oxathiane) and poly (3,4-ethylenedioxythiophene-co-thieno [3,4-b]-1,4-oxathiane)/poly (styrene sulfonic sodium): Preparation, characterization, and optoelectronic performance. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2285–2297. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Lin, Y.-C.; Hung, S.-Y.; Chen, C.-K.; Chiang, Y.-C.; Chueh, C.-C.; Chen, W.-C. Investigation of the mobility–stretchability relationship of ester-substituted polythiophene derivatives. Macromolecules 2020, 53, 4968–4981. [Google Scholar] [CrossRef]
- An, M.; Bai, Q.; Jeong, S.Y.; Ding, J.; Zhao, C.; Liu, B.; Liang, Q.; Wang, Y.; Zhang, G.; Woo, H.Y. Polythiophene Derivatives for Efficient All-Polymer Solar Cells. Adv. Energy Mater. 2023, 2301110. [Google Scholar] [CrossRef]
- Zheng, S. Electrically Conductive Films Formed from Dispersions Comprising Conductive Polymers and Hyperbranched Polymers. European Patent EP2438120B1, 8 May 2013. [Google Scholar]
- Kaloni, T.P.; Giesbrecht, P.K.; Schreckenbach, G.; Freund, M.S. Polythiophene: From fundamental perspectives to applications. Chem. Mater. 2017, 29, 10248–10283. [Google Scholar] [CrossRef]
- Iguchi, H.; Higashi, C.; Funasaki, Y.; Fujita, K.; Mori, A.; Nakasuga, A.; Maruyama, T. Rational and practical exfoliation of graphite using well-defined poly (3-hexylthiophene) for the preparation of conductive polymer/graphene composite. Sci. Rep. 2017, 7, 39937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chunder, A.; Liu, J.; Zhai, L. Reduced Graphene Oxide/Poly (3-hexylthiophene) Supramolecular Composites. Macromol. Rapid Commun. 2010, 31, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, H.; Moss, D.J.; Loh, K.P.; Jia, B. Graphene oxide for photonics, electronics and optoelectronics. Nat. Rev. Chem. 2023, 7, 162–183. [Google Scholar] [CrossRef]
- Nam, Y.T.; Choi, J.; Kang, K.M.; Kim, D.W.; Jung, H.-T. Enhanced stability of laminated graphene oxide membranes for nanofiltration via interstitial amide bonding. ACS Appl. Mater. Interfaces 2016, 8, 27376–27382. [Google Scholar] [CrossRef]
- Chen, J.; Duan, M.; Chen, G. Continuous mechanical exfoliation of graphene sheets via three-roll mill. J. Mater. Chem. 2012, 22, 19625–19628. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Zhou, K.; Gui, Z.; Hu, Y.; Jiang, S.; Tang, G. The influence of cobalt oxide–graphene hybrids on thermal degradation, fire hazards and mechanical properties of thermoplastic polyurethane composites. Compos. Part A Appl. Sci. Manuf. 2016, 88, 10–18. [Google Scholar] [CrossRef]
- Salvatierra, R.V.; Cava, C.E.; Roman, L.S.; Oliveira, M.M.; Zarbin, A.J. The total chemical synthesis of polymer/graphene nanocomposite films. Chem. Commun. 2016, 52, 1629–1632. [Google Scholar] [CrossRef]
- Ciesielski, A.; Samorì, P. Supramolecular Approaches to Graphene: From Self-Assembly to Molecule-Assisted Liquid-Phase Exfoliation. Adv. Mater. 2016, 28, 6030–6051. [Google Scholar] [CrossRef]
- Haar, S.; Ciesielski, A.; Clough, J.; Yang, H.; Mazzaro, R.; Richard, F.; Conti, S.; Merstorf, N.; Cecchini, M.; Morandi, V. A Supramolecular Strategy to Leverage the Liquid-Phase Exfoliation of Graphene in the Presence of Surfactants: Unraveling the Role of the Length of Fatty Acids. Small 2015, 11, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, J.; Xin, Y.; Lubineau, G.; Ma, Q.; Jiang, L. Alcohol recognition by flexible, transparent and highly sensitive graphene-based thin-film sensors. Sci. Rep. 2017, 7, 4317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, J.; Liu, Z.; Yu, J.; Dai, D.; Dai, W.; Du, S.; Li, C.; Jiang, N.; Zhan, Z.; Lin, C.-T. Graphene woven fabric-reinforced polyimide films with enhanced and anisotropic thermal conductivity. Compos. Part A Appl. Sci. Manuf. 2016, 87, 290–296. [Google Scholar] [CrossRef]
- Hu, Z.; Tong, G.; Lin, D.; Chen, C.; Guo, H.; Xu, J.; Zhou, L. Graphene-reinforced metal matrix nanocomposites–a review. Mater. Sci. Technol. 2016, 32, 930–953. [Google Scholar] [CrossRef]
- Munuera, J.; Paredes, J.; Villar-Rodil, S.; Ayán-Varela, M.; Martínez-Alonso, A.; Tascón, J. Electrolytic exfoliation of graphite in water with multifunctional electrolytes: En route towards high quality, oxide-free graphene flakes. Nanoscale 2016, 8, 2982–2998. [Google Scholar] [CrossRef] [Green Version]
- Chethan, B.; Prasad, V.; Sunilkumar, A.; Veena, V.; Thomas, S. Trends in Development of Nanomaterial-Based Sensing Devices. In Recent Developments in Green Electrochemical Sensors: Design, Performance, and Applications; ACS Publications: Washington, DC, USA, 2023; pp. 287–305. [Google Scholar]
- Del Valle, M.; Gacitúa, M.; Hernández, F.; Luengo, M.; Hernández, L. Nanostructured Conducting Polymers and Their Applications in Energy Storage Devices. Polymers 2023, 15, 1450. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal, V.; Colombo, L.; Gellert, P.; Schwab, M.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Idumah, C.I. Design, development, and drug delivery applications of graphene polymeric nanocomposites and bionanocomposites. Emergent Mater. 2023, 6, 777–807. [Google Scholar] [CrossRef]
- Idowu, A.; Thomas, T.; Boesl, B.; Agarwal, A. Cryo-Assisted Extrusion Three-Dimensional Printing of Shape Memory Polymer–Graphene Composites. J. Manuf. Sci. Eng. 2023, 145, 041003. [Google Scholar]
- Pourhashem, S.; Vaezi, M.R.; Rashidi, A.; Bagherzadeh, M.R. Exploring corrosion protection properties of solvent based epoxy-graphene oxide nanocomposite coatings on mild steel. Corros. Sci. 2017, 115, 78–92. [Google Scholar] [CrossRef]
- Jalili, R.; Aboutalebi, S.H.; Esrafilzadeh, D.; Konstantinov, K.; Moulton, S.E.; Razal, J.M.; Wallace, G.G. Organic solvent-based graphene oxide liquid crystals: A facile route toward the next generation of self-assembled layer-by-layer multifunctional 3D architectures. Acs Nano 2013, 7, 3981–3990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, T.S.; Balu, R.; de Campo, L.; Dutta, N.K.; Choudhury, N.R. Sulfonated polythiophene-interfaced graphene for water-redispersible graphene powder with high conductivity and electrocatalytic activity. Energy Adv. 2023, 2, 365–374. [Google Scholar] [CrossRef]
- Greco, G.; Giuri, A.; Bagheri, S.; Seiti, M.; Degryse, O.; Rizzo, A.; Mele, C.; Ferraris, E.; Corcione, C.E. Pedot: PSS/graphene oxide (GO) ternary nanocomposites for electrochemical applications. Molecules 2023, 28, 2963. [Google Scholar] [CrossRef] [PubMed]
- Govindaraj, P.; Sokolova, A.; Salim, N.; Juodkazis, S.; Fuss, F.K.; Fox, B.; Hameed, N. Distribution states of graphene in polymer nanocomposites: A review. Compos. Part B Eng. 2021, 226, 109353. [Google Scholar] [CrossRef]
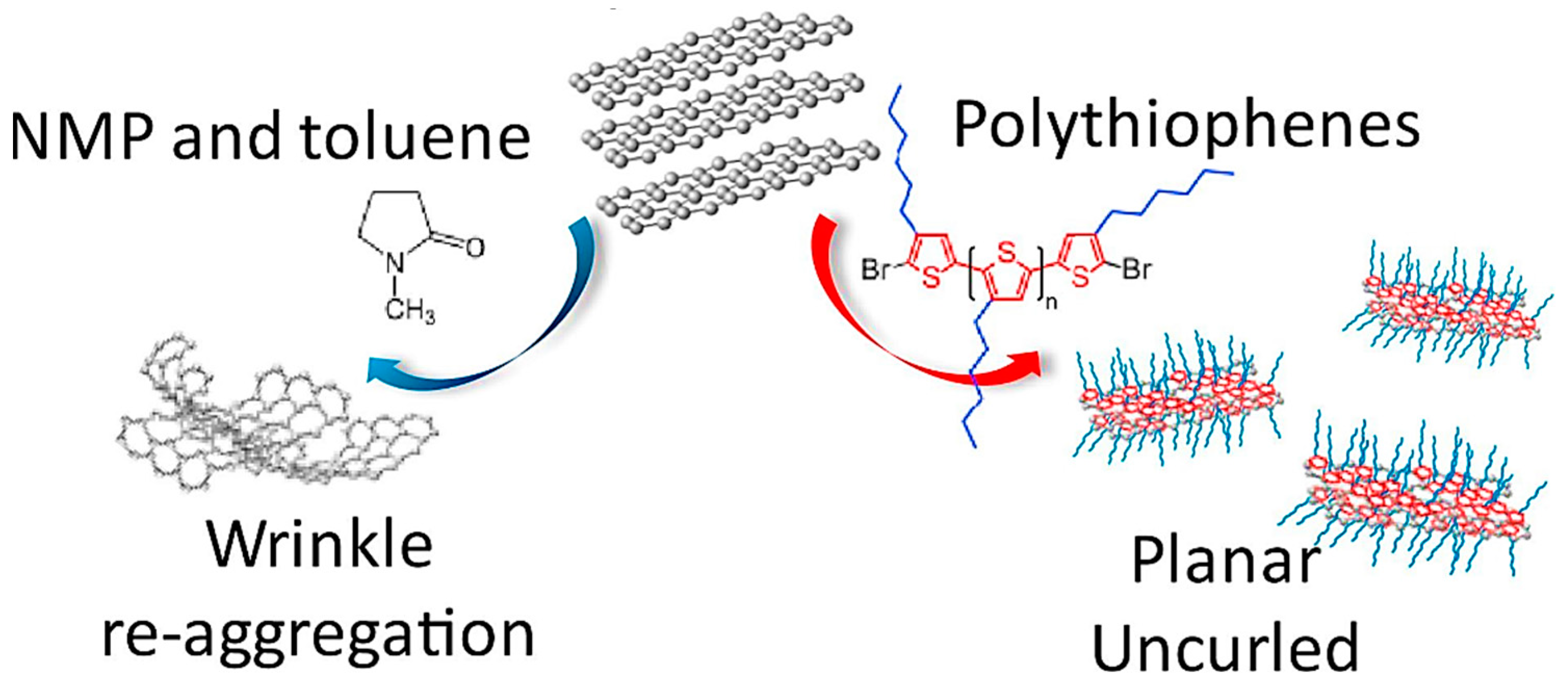
- Iguchi, H.; Miyahara, K.; Higashi, C.; Fujita, K.; Nakagawa, N.; Tamba, S.; Mori, A.; Yoshitani, H.; Nakasuga, A.; Maruyama, T. Preparation of uncurled and planar multilayered graphene using polythiophene derivatives via liquid-phase exfoliation of graphite. FlatChem 2018, 8, 31–39. [Google Scholar] [CrossRef]
- Chen, W.; Gui, X.; Liang, B.; Liu, M.; Lin, Z.; Zhu, Y.; Tang, Z. Controllable fabrication of large-area wrinkled graphene on a solution surface. ACS Appl. Mater. Interfaces 2016, 8, 10977–10984. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Cheong, I.W. Recent studies on dispersion of graphene–polymer composites. Polymers 2021, 13, 2375. [Google Scholar] [CrossRef]
- Husain, A.; Ahmad, S.; Mohammad, F. Synthesis, characterisation and ethanol sensing application of polythiophene/graphene nanocomposite. Mater. Chem. Phys. 2020, 239, 122324. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Kong, Z.; Lv, K.; Teng, C.; Zhu, Y. Conducting-polymer-based materials for electrochemical energy conversion and storage. Adv. Mater. 2017, 29, 1703044. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Liu, J.; Wu, J.; Chen, H.; Bi, H. Design and preparation of a ternary composite of graphene oxide/carbon dots/polypyrrole for supercapacitor application: Importance and unique role of carbon dots. Carbon 2017, 115, 134–146. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J.S.; Jun, J.; Kim, S.G.; Jang, J. Fabrication of water-dispersible and highly conductive PSS-doped PANI/graphene nanocomposites using a high-molecular weight PSS dopant and their application in H2S detection. Nanoscale 2014, 6, 15181–15195. [Google Scholar] [CrossRef] [PubMed]
- Pentzer, E.B.; Bokel, F.A.; Hayward, R.C.; Emrick, T. Nanocomposite “superhighways” by solution assembly of semiconductor nanostructures with ligand-functionalized conjugated polymers. Adv. Mater. 2012, 24, 2254–2258. [Google Scholar] [CrossRef] [PubMed]
- Hajgató, B.; Deleuze, M.S.; Morini, F. Probing Nuclear Dynamics in Momentum Space: A New Interpretation of (e, 2e) Electron Impact Ionization Experiments on Ethanol. J. Phys. Chem. A 2009, 113, 7138–7154. [Google Scholar] [CrossRef]
- Qi, X.; Yao, X.; Deng, S.; Zhou, T.; Fu, Q. Water-induced shape memory effect of graphene oxide reinforced polyvinyl alcohol nanocomposites. J. Mater. Chem. A 2014, 2, 2240–2249. [Google Scholar] [CrossRef]
- Wick, P.; Louw-Gaume, A.E.; Kucki, M.; Krug, H.F.; Kostarelos, K.; Fadeel, B.; Dawson, K.A.; Salvati, A.; Vázquez, E.; Ballerini, L. Classification framework for graphene-based materials. Angew. Chem. Int. Ed. 2014, 53, 7714–7718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laaksonen, P.; Kainlauri, M.; Laaksonen, T.; Shchepetov, A.; Jiang, H.; Ahopelto, J.; Linder, M.B. Interfacial engineering by proteins: Exfoliation and functionalization of graphene by hydrophobins. Angew. Chem. Int. Ed. 2010, 49, 4946–4949. [Google Scholar] [CrossRef]
- Yazid, S.N.A.M.; Adnan, A.A.C.; Isa, I.M.; Saidin, M.I.; Ahmad, M.S.; Fun, C.S. Conducting polymer functionalized graphene-based electrochemical sensors for sensing pollutants in water. J. Electrochem. Sci. Eng. 2023, 13, 251–274. [Google Scholar]
- Kim, N.H.; Kuila, T.; Lee, J.H. Simultaneous reduction, functionalization and stitching of graphene oxide with ethylenediamine for composites application. J. Mater. Chem. A 2013, 1, 1349–1358. [Google Scholar] [CrossRef]
- Kim, D.-H.; Tan, L.-S.; Park, S.-Y. Preparation of water-dispersible graphene using N-methylmorpholine N-oxide monohydrate and its application for the preparation of nanocomposites using PEDOT. J. Mater. Chem. C 2015, 3, 7105–7117. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z.; Zhang, X.; Yang, L.; Zhang, N.; Pan, G.; Yin, S.; Chen, Y.; Wei, J. Polymer photovoltaic cells based on solution-processable graphene and P3HT. Adv. Funct. Mater. 2009, 19, 894–904. [Google Scholar] [CrossRef]
- Cai, X.; Tan, S.; Lin, M.; Xie, A.; Mai, W.; Zhang, X.; Lin, Z.; Wu, T.; Liu, Y. Synergistic antibacterial brilliant blue/reduced graphene oxide/quaternary phosphonium salt composite with excellent water solubility and specific targeting capability. Langmuir 2011, 27, 7828–7835. [Google Scholar] [CrossRef] [PubMed]
- Gopal, J.; Muthu, M.; Sivanesan, I. A Comprehensive Compilation of Graphene/Fullerene Polymer Nanocomposites for Electrochemical Energy Storage. Polymers 2023, 15, 701. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, X.; Yuan, J.; Pu, H.; Liu, Y. Preparation of poly (3-hexylthiophene)/graphene nanocomposite via in situ reduction of modified graphite oxide sheets. Appl. Surf. Sci. 2010, 257, 138–142. [Google Scholar] [CrossRef]
- Ahmed, J.; Faisal, M.; Alsareii, S.; Jalalah, M.; Harraz, F.A. A novel gold-decorated porous silicon-poly (3-hexylthiophene) ternary nanocomposite as a highly sensitive and selective non-enzymatic dopamine electrochemical sensor. J. Alloys Compd. 2023, 931, 167403. [Google Scholar] [CrossRef]
- Tienne, L.G.P.; Paula, T.P.; Marques, M.d.F.V. Preparation and optical-electronic properties of thin films comprising P3HT and few-layer graphene nanosheets. Mater. Sci. Eng. B 2023, 293, 116505. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Chen, P.; Xia, R.; Wu, B.; Qian, J. Interfacial Modulation of Graphene by Polythiophene with Controlled Molecular Weight to Enhance Thermal Conductivity. Membranes 2021, 11, 895. [Google Scholar] [CrossRef]
- Shah, R.; Kausar, A.; Muhammad, B. Exploration of polythiophene/graphene, poly (methyl methacrylate)/graphene and polythiophene-co-poly (methyl methacrylate)/graphene nanocomposite obtained via in-situ technique. J. Plast. Film Sheeting 2015, 31, 144–157. [Google Scholar] [CrossRef]
- An, Y.-C.; Gao, X.-X.; Jiang, W.-L.; Han, J.-L.; Ye, Y.; Chen, T.-M.; Ren, R.-Y.; Zhang, J.-H.; Liang, B.; Li, Z.-L. A critical review on graphene oxide membrane for industrial wastewater treatment. Environ. Res. 2023, 223, 115409. [Google Scholar] [CrossRef]
- Zhou, A.; Yu, T.; Liang, X.; Yin, S. H2O2-free strategy derived from Hummers method for preparing graphene oxide with high oxidation degree. FlatChem 2023, 38, 100487. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, H.; Shrama, R.; Kumari, R. Synthesis, Processing, and Applications of 2D (nano) materials: A sustainable approach. Surf. Interfaces 2023, 39, 102925. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Zhou, N.; Tan, J.; Ashley, J.; Wang, W.; Wu, F.; Shen, J.; Zhang, M. Study on a Novel Poly (vinyl alcohol)/Graphene Oxide-Citicoline Sodium-Lanthanum Wound Dressing: Biocompatibility, Bioactivity, Antimicrobial Activity, and Wound Healing Effect. Chem. Eng. J. 2020, 395, 125059. [Google Scholar] [CrossRef]
- Demirel, E.; Karaca, E.; Durmaz, Y.Y. Effective PEGylation Method to Improve Biocompatibility of Graphene Derivatives. Eur. Polym. J. 2020, 124, 109504. [Google Scholar] [CrossRef]
- Bu, Y.; Kim, B.S. Eco-friendly production of functionalized few-layer graphene using coffee waste extract and in-situ growth of copper oxide nanoparticles. J. Environ. Chem. Eng. 2023, 11, 109350. [Google Scholar] [CrossRef]
- Abdelkarim, R.B.; Yahiaoui, A.; Belbachir, M.; Khelil, A. Synthesis and Properties of Polythiophène Benzylidene and Their Photovoltaic Applications. Mater. Sci. Appl. 2011, 2, 1014. [Google Scholar]
- Wang, S.; Liu, M.; Araby, S.; Wang, X.; Abdelsalam, A.A.; Xue, H.; Meng, Q. Reinforcing interlaminar interface of carbon fiber reinforced metal laminates by graphene. Compos. Struct. 2023, 311, 116814. [Google Scholar] [CrossRef]
- Yang, L.; Tan, X.; Wang, Z.; Zhang, X. Supramolecular polymers: Historical development, preparation, characterization, and functions. Chem. Rev. 2015, 115, 7196–7239. [Google Scholar] [CrossRef]
- Nayebi, P.; Zaminpayma, E. A molecular dynamic simulation study of mechanical properties of graphene–polythiophene composite with Reax force field. Phys. Lett. A 2016, 380, 628–633. [Google Scholar] [CrossRef]
- Hsieh, G.-W.; Lin, Z.-R.; Hung, C.-Y.; Lin, S.-Y.; Yang, C.-R. Graphene-induced enhancement of charge carrier mobility and air stability in organic polythiophene field effect transistors. Org. Electron. 2018, 54, 27–33. [Google Scholar] [CrossRef]
- Pandey, R.K.; Sahu, P.K.; Singh, M.K.; Prakash, R. Fast grown self-assembled polythiophene/graphene oxide nanocomposite thin films at air–liquid interface with high mobility used in polymer thin film transistors. J. Mater. Chem. C 2018, 6, 9981–9989. [Google Scholar]
- Liscio, A.; Veronese, G.P.; Treossi, E.; Suriano, F.; Rossella, F.; Bellani, V.; Rizzoli, R.; Samorì, P.; Palermo, V. Charge transport in graphene–polythiophene blends as studied by Kelvin Probe Force Microscopy and transistor characterization. J. Mater. Chem. 2011, 21, 2924–2931. [Google Scholar] [CrossRef]
- El Gemayel, M.; Narita, A.; Dössel, L.F.; Sundaram, R.S.; Kiersnowski, A.; Pisula, W.; Hansen, M.R.; Ferrari, A.C.; Orgiu, E.; Feng, X. Graphene nanoribbon blends with P3HT for organic electronics. Nanoscale 2014, 6, 6301–6314. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, K.; Botiz, I.; Stingelin, N.; Kayunkid, N.; Sommer, M.; Koch, F.P.V.; Nguyen, H.; Coulembier, O.; Dubois, P.; Brinkmann, M. Controllable processes for generating large single crystals of poly (3-hexylthiophene). Angew. Chem. Int. Ed. 2012, 51, 11131–11135. [Google Scholar] [CrossRef] [PubMed]
- Alvi, F.; Ram, M.K.; Basnayaka, P.; Stefanakos, E.; Goswami, Y.; Hoff, A.; Kumar, A. Electrochemical supercapacitors based on graphene-conducting polythiophenes nanocomposite. ECS Trans. 2011, 35, 167. [Google Scholar] [CrossRef]
- Tian, F.; Li, H.; Li, M.; Li, C.; Lei, Y.; Yang, B. Synthesis of one-dimensional poly (3, 4-ethylenedioxythiophene)-graphene composites for the simultaneous detection of hydroquinone, catechol, resorcinol, and nitrite. Synth. Met. 2017, 226, 148–156. [Google Scholar] [CrossRef]
- Gökce, H.; Alpaslan, Y.B.; Zeyrek, C.T.; Ağar, E.; Güder, A.; Özdemir, N.; Alpaslan, G. Structural, spectroscopic, radical scavenging activity, molecular docking and DFT studies of a synthesized Schiff base compound. J. Mol. Struct. 2019, 1179, 205–215. [Google Scholar] [CrossRef]
- Alabadi, A.; Razzaque, S.; Dong, Z.; Wang, W.; Tan, B. Graphene oxide-polythiophene derivative hybrid nanosheet for enhancing performance of supercapacitor. J. Power Sources 2016, 306, 241–247. [Google Scholar] [CrossRef]
- Jing, H.; Min, M.; Seo, S.; Lu, B.; Yoon, Y.; Lee, S.M.; Hwang, E.; Lee, H. Non-metal catalytic synthesis of graphene from a polythiophene monolayer on silicon dioxide. Carbon 2015, 86, 272–278. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, J.; Bao, F.; Jiang, S.; Zhang, X.; Ma, R. Covalent functionalization of graphene with polythiophene through a Suzuki coupling reaction. RSC Adv. 2015, 5, 42754–42761. [Google Scholar] [CrossRef]
- Forcherio, G.T.; Benamara, M.; Roper, D.K. Electron energy loss spectroscopy of hot electron transport between gold nanoantennas and molybdenum disulfide by plasmon excitation. Adv. Opt. Mater. 2017, 5, 1600572. [Google Scholar] [CrossRef]
- Shamsayei, M.; Yamini, Y.; Asiabi, H. Polythiophene/graphene oxide nanostructured electrodeposited coating for on-line electrochemically controlled in-tube solid-phase microextraction. J. Chromatogr. A 2016, 1475, 8–17. [Google Scholar] [CrossRef]
- Molaei, K.; Bagheri, H.; Asgharinezhad, A.A.; Ebrahimzadeh, H.; Shamsipur, M. SiO2-coated magnetic graphene oxide modified with polypyrrole–polythiophene: A novel and efficient nanocomposite for solid phase extraction of trace amounts of heavy metals. Talanta 2017, 167, 607–616. [Google Scholar] [CrossRef]
- Wang, Z.; Li, P.; Chen, Y.; He, J.; Zhang, W.; Schmidt, O.G.; Li, Y. Pure thiophene–sulfur doped reduced graphene oxide: Synthesis, structure, and electrical properties. Nanoscale 2014, 6, 7281–7287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, Z.; Xiao, Q.; Wang, D.; Yi, G.; Xu, Z.; Li, B.; Zhang, C. Improved electrochemical performance of biomass-derived nanoporous carbon/sulfur composites cathode for lithium-sulfur batteries by nitrogen doping. Electrochim. Acta 2016, 202, 131–139. [Google Scholar] [CrossRef]
- Bora, C.; Pegu, R.; Saikia, B.J.; Dolui, S.K. Synthesis of polythiophene/graphene oxide composites by interfacial polymerization and evaluation of their electrical and electrochemical properties. Polym. Int. 2014, 63, 2061–2067. [Google Scholar] [CrossRef]
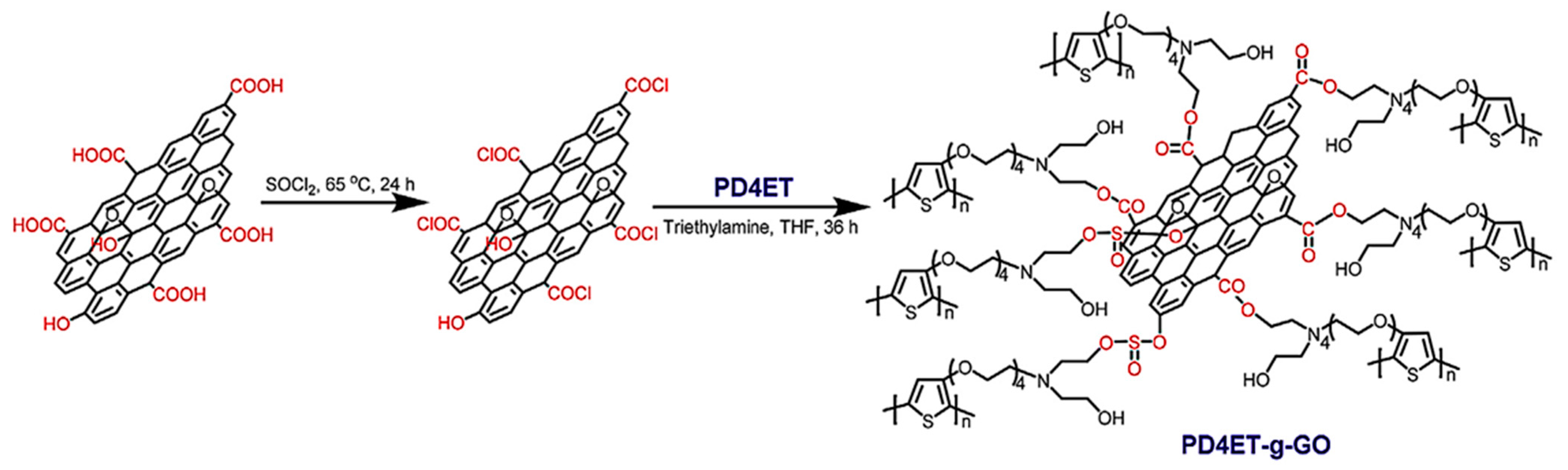
- Li, Y.; Zhou, M.; Wang, Y.; Pan, Q.; Gong, Q.; Xia, Z.; Li, Y. Remarkably enhanced performances of novel polythiophene-grafting-graphene oxide composite via long alkoxy linkage for supercapacitor application. Carbon 2019, 147, 519–531. [Google Scholar]
- Jalilian, N.; Ebrahimzadeh, H.; Asgharinezhad, A.A.; Molaei, K. Extraction and determination of trace amounts of gold (III), palladium (II), platinum (II) and silver (I) with the aid of a magnetic nanosorbent made from Fe3O4-decorated and silica-coated graphene oxide modified with a polypyrrole-polythiophene copolymer. Microchim. Acta 2017, 184, 2191–2200. [Google Scholar] [CrossRef]
- Chang, Y.-P.; Ren, C.-L.; Qu, J.-C.; Chen, X.-G. Preparation and characterization of Fe3O4/graphene nanocomposite and investigation of its adsorption performance for aniline and p-chloroaniline. Appl. Surf. Sci. 2012, 261, 504–509. [Google Scholar] [CrossRef]
- Mehdinia, A.; Khodaee, N.; Jabbari, A. Fabrication of graphene/Fe3O4@ polythiophene nanocomposite and its application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Anal. Chim. Acta 2015, 868, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yang, L.; Liu, N. Recent Progress on Poly (3, 4-Ethylenedioxythiophene): Poly (Styrenesulfonate) Bioelectrodes. Small Sci. 2023, 3, 2300008. [Google Scholar] [CrossRef]
- Sriprachuabwong, C.; Karuwan, C.; Wisitsorrat, A.; Phokharatkul, D.; Lomas, T.; Sritongkham, P.; Tuantranont, A. Inkjet-printed graphene-PEDOT: PSS modified screen printed carbon electrode for biochemical sensing. J. Mater. Chem. 2012, 22, 5478–5485. [Google Scholar] [CrossRef]
- Tung, T.T.; Castro, M.; Kim, T.Y.; Suh, K.S.; Feller, J.-F. Graphene quantum resistive sensing skin for the detection of alteration biomarkers. J. Mater. Chem. 2012, 22, 21754–21766. [Google Scholar] [CrossRef]
- Choi, K.S.; Liu, F.; Choi, J.S.; Seo, T.S. Fabrication of free-standing multilayered graphene and poly (3,4-ethylenedioxythiophene) composite films with enhanced conductive and mechanical properties. Langmuir 2010, 26, 12902–12908. [Google Scholar] [CrossRef]
- Tung, T.T.; Kim, T.Y.; Shim, J.P.; Yang, W.S.; Kim, H.; Suh, K.S. Poly (ionic liquid)-stabilized graphene sheets and their hybrid with poly (3,4-ethylenedioxythiophene). Org. Electron. 2011, 12, 2215–2224. [Google Scholar] [CrossRef]
- Tung, T.T.; Castro, M.; Feller, J.-F.; Kim, T.Y.; Suh, K.S. Hybrid film of chemically modified graphene and vapor-phase-polymerized PEDOT for electronic nose applications. Org. Electron. 2013, 14, 2789–2794. [Google Scholar] [CrossRef]
- Tung, T.T.; Chen, S.J.; Fumeaux, C.; Kim, T.; Losic, D. N-doped reduced graphene oxide-PEDOT nanocomposites for implementation of a flexible wideband antenna for wearable wireless communication applications. Nanotechnology 2021, 32, 245711. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Miao, X.; Hua, S.; An, L.; Gao, X.; Jiang, W.; Qu, D.; Zhou, Z.; Liu, X.; Sun, Z. Structure defects assisted photocatalytic H2 production for polythiophene nanofibers. Appl. Catal. B Environ. 2017, 211, 98–105. [Google Scholar] [CrossRef]
- Labastide, J.A.; Baghgar, M.; McKenna, A.; Barnes, M.D. Time-and polarization-resolved photoluminescence decay from isolated polythiophene (P3HT) nanofibers. J. Phys. Chem. C 2012, 116, 23803–23811. [Google Scholar] [CrossRef]
- Barnes, M.D.; Baghar, M. Optical probes of chain packing structure and exciton dynamics in polythiophene films, composites, and nanostructures. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1121–1129. [Google Scholar] [CrossRef]
- Tran, T.S.; Balu, R.; Nguyen, C.K.; Mata, J.; Truong, V.K.; Dutta, N.K.; Choudhury, N.R. Graphene Nanosheets Stabilized by P3HT Nanoparticles for Printable Metal-Free Electrocatalysts for Oxygen Reduction. ACS Appl. Nano Mater. 2023, 6, 908–917. [Google Scholar] [CrossRef]
- Tran, T.S.; Balu, R.; Mata, J.; Dutta, N.K.; Choudhury, N.R. 3D printed graphene aerogels using conductive nanofibrillar network formulation. Nano Trends 2023, 2, 100011. [Google Scholar] [CrossRef]
- Maity, N.; Ghosh, R.; Nandi, A.K. Optoelectronic properties of self-assembled nanostructures of polymer functionalized polythiophene and graphene. Langmuir 2018, 34, 7585–7597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Y.B.; Lee, J.-M. Graphene for supercapacitor applications. J. Mater. Chem. A 2013, 1, 14814–14843. [Google Scholar] [CrossRef]
- Smaisim, G.F.; Abed, A.M.; Al-Madhhachi, H.; Hadrawi, S.K.; Al-Khateeb, H.M.M.; Kianfar, E. Graphene-based important carbon structures and nanomaterials for energy storage applications as chemical capacitors and supercapacitor electrodes: A review. BioNanoScience 2023, 13, 219–248. [Google Scholar] [CrossRef]
- Sahoo, B.; Pandey, V.; Dogonchi, A.; Thatoi, D.; Nayak, N.; Nayak, M. Synthesis, characterization and electrochemical aspects of graphene based advanced supercapacitor electrodes. Fuel 2023, 345, 128174. [Google Scholar] [CrossRef]
- Chen, W.; Xue, G. Low potential electrochemical syntheses of heteroaromatic conducting polymers in a novel solvent system based on trifluroborate–ethyl ether. Prog. Polym. Sci. 2005, 30, 783–811. [Google Scholar] [CrossRef]
- Heeney, M.; Bailey, C.; Genevicius, K.; Shkunov, M.; Sparrowe, D.; Tierney, S.; McCulloch, I. Stable polythiophene semiconductors incorporating thieno [2, 3-b] thiophene. J. Am. Chem. Soc. 2005, 127, 1078–1079. [Google Scholar] [CrossRef]
- Shah, R.; Kausar, A.; Muhammad, B.; Khan, M. Investigation on thermal conductivity and physical properties of polythiophene/p-phenylenediamine-graphene oxide and polythiophene-co-poly (methyl methacrylate)/p-phenylenediamine graphene oxide composites. Compos. Interfaces 2016, 23, 887–899. [Google Scholar] [CrossRef]
- Kim, T.-H.; Choi, K.-I.; Kim, H.; Oh, S.H.; Koo, J.; Nah, Y.-C. Long-term cyclability of electrochromic poly (3-hexyl thiophene) films modified by surfactant-assisted graphene oxide layers. ACS Appl. Mater. Interfaces 2017, 9, 20223–20230. [Google Scholar] [CrossRef]
- Male, U.; Modigunta, J.K.R. Design and synthesis of polyaniline-grafted reduced graphene oxide via azobenzene pendants for high-performance supercapacitors. Polymer 2017, 110, 242–249. [Google Scholar] [CrossRef]
- Benson, E.E.; Zhang, H.; Schuman, S.A.; Nanayakkara, S.U.; Bronstein, N.D.; Ferrere, S.; Blackburn, J.L.; Miller, E.M. Balancing the hydrogen evolution reaction, surface energetics, and stability of metallic MoS2 nanosheets via covalent functionalization. J. Am. Chem. Soc. 2018, 140, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jamal, R.; Wang, Y.; Yang, L.; Liu, F.; Abdiryim, T. Functionalization of graphene oxide and its composite with poly (3, 4-ethylenedioxythiophene) as electrode material for supercapacitors. Nanoscale Res. Lett. 2015, 10, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Kumar, A.; Khan, R. A highly sensitive amperometric immunosensor probe based on gold nanoparticle functionalized poly (3,4-ethylenedioxythiophene) doped with graphene oxide for efficient detection of aflatoxin B1. Synth. Met. 2018, 235, 136–144. [Google Scholar] [CrossRef]
- Yin, Z.; Zhu, J.; He, Q.; Cao, X.; Tan, C.; Chen, H.; Yan, Q.; Zhang, H. Graphene-based materials for solar cell applications. Adv. Energy Mater. 2014, 4, 1300574. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hammed, W.A.; Yahya, R.B.; Mahmud, H.N.M.E. Prospects of conducting polymer and graphene as counter electrodes in dye-sensitized solar cells. J. Polym. Res. 2016, 23, 192. [Google Scholar] [CrossRef]
- Vovchenko, L.L.; Matzui, L.Y.; Perets, Y.S.; Milovanov, Y.S. Dielectric Properties and AC Conductivity of Epoxy/Hybrid Nanocarbon Filler Composites. In Nanochemistry, Biotechnology, Nanomaterials, and Their Applications: Selected Proceedings of the 5th International Conference Nanotechnology and Nanomaterials (NANO2017), Chernivtsi, Ukraine, 23–26 August 2017; Springer International Publishing: Berlin/Heidelberg, Germany; pp. 377–393.
- Lee, J.K.Y.; Chen, N.; Peng, S.; Li, L.; Tian, L.; Thakor, N.; Ramakrishna, S. Polymer-based composites by electrospinning: Preparation & functionalization with nanocarbons. Prog. Polym. Sci. 2018, 86, 40–84. [Google Scholar]
- Hill, C.M.; Zhu, Y.; Pan, S. Fluorescence and electroluminescence quenching evidence of interfacial charge transfer in poly (3-hexylthiophene): Graphene oxide bulk heterojunction photovoltaic devices. Acs Nano 2011, 5, 942–951. [Google Scholar] [CrossRef]
- Niu, Z.; Liu, L.; Zhang, L.; Zhou, W.; Chen, X.; Xie, S. Programmable nanocarbon-based architectures for flexible supercapacitors. Adv. Energy Mater. 2015, 5, 1500677. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611. [Google Scholar] [CrossRef] [Green Version]
- Antoniadou, M.; Stathatos, E.; Boukos, N.; Stefopoulos, A.; Kallitsis, J.; Krebs, F.C.; Lianos, P. Study of hybrid solar cells made of multilayer nanocrystalline titania and poly (3-octylthiophene) or poly-(3-(2-methylhex-2-yl)-oxy-carbonyldithiophene). Nanotechnology 2009, 20, 495201. [Google Scholar] [CrossRef]
- Parlak, E.A. The blend ratio effect on the photovoltaic performance and stability of poly (3-hexylthiophene):[6,6]-phenyl-C61 butyric acid methyl ester (PCBM) and poly (3-octylthiophene): PCBM solar cells. Sol. Energy Mater. Sol. Cells 2012, 100, 174–184. [Google Scholar]
- Stylianakis, M.M.; Stratakis, E.; Koudoumas, E.; Kymakis, E.; Anastasiadis, S.H. Organic bulk heterojunction photovoltaic devices based on polythiophene–graphene composites. ACS Appl. Mater. Interfaces 2012, 4, 4864–4870. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, S.; Yang, W.; Yuan, W.; Xu, J.; Jiang, Y. In situ polymerization deposition of porous conducting polymer on reduced graphene oxide for gas sensor. ACS Appl. Mater. Interfaces 2014, 6, 13807–13814. [Google Scholar] [CrossRef] [PubMed]
- Nag, A.; Mitra, A.; Mukhopadhyay, S.C. Graphene and its sensor-based applications: A review. Sens. Actuators A Phys. 2018, 270, 177–194. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.; Blake, P.; Katsnelson, M.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- De Castro, E.S.; Genders, J.D.; Weinberg, N.L. Solid State Gas Sensor and Filter Assembly. WO Patents WO1997009613A1, 13 March 1998. [Google Scholar]
- Varela-Rizo, H.; Martín-Gullón, I.; Terrones, M. Hybrid films with graphene oxide and metal nanoparticles could now replace indium tin oxide. ACS Nano 2012, 6, 4565–4572. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Ahn, J.; Lee, K.B.; Kim, D.; Kim, J. Graphene-based flexible NO2 chemical sensors. Thin Solid Film. 2012, 520, 5459–5462. [Google Scholar] [CrossRef]
- Hangarter, C.M.; Chartuprayoon, N.; Hernández, S.C.; Choa, Y.; Myung, N.V. Hybridized conducting polymer chemiresistive nano-sensors. Nano Today 2013, 8, 39–55. [Google Scholar] [CrossRef]
- Bai, S.; Guo, J.; Sun, J.; Tang, P.; Chen, A.; Luo, R.; Li, D. Enhancement of NO2-sensing performance at room temperature by graphene-modified polythiophene. Ind. Eng. Chem. Res. 2016, 55, 5788–5794. [Google Scholar] [CrossRef]
- Peng, D.; Wang, S.; Tian, Z.; Wu, X.; Wu, Y.; Wu, H.; Xin, Q.; Chen, J.; Cao, X.; Jiang, Z. Facilitated transport membranes by incorporating graphene nanosheets with high zinc ion loading for enhanced CO2 separation. J. Membr. Sci. 2017, 522, 351–362. [Google Scholar] [CrossRef]
- Noreen, H.; Iqbal, J.; Hassan, W.; Rahman, G.; Yaseen, M.; Rahman, A.U. Synthesis of graphene nanoplatelets/polythiophene nanocomposites with enhanced photocatalytic degradation of bromophenol blue and antibacterial properties. Mater. Res. Bull. 2021, 142, 111435. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Bahrani, S.; Yousefi, K.; Behbudi, G.; Babapoor, A.; Omidifar, N.; Lai, C.W.; Gholami, A.; Chiang, W.-H. Recent advancements in polythiophene-based materials and their biomedical, geno sensor and DNA detection. Int. J. Mol. Sci. 2021, 22, 6850. [Google Scholar] [CrossRef] [PubMed]

| Derivative | Structure |
|---|---|
| Poly(3-hexylthiophene) |  |
| Poly(3-octylthiophene) |  |
| Poly(3-dodecylthiophene) |  |
| Poly(isothianaphthene) |  |
| Poly(l,4-di-(2-thienyl)benzene) |  |
| Poly(thieno [3,2-b]thiophene) |  |
| Poly(dithieno [3,2-b:2′,3′-d]thiophene) | 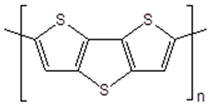 |
| Sample | Resistivity (Ω cm) | Conductivity (S cm−1) |
|---|---|---|
| Polythiophene | 4.90 | 5.8 × 10−5 |
| Polythiophene with graphene oxide 1 wt.% | 2.80 | 0.8 × 10−4 |
| Polythiophene with graphene oxide 2 wt.% | 2.36 | 1.2 × 10−4 |
| Polythiophene with graphene oxide 3 wt.% | 1.97 | 2.7 × 10−4 |
| GO-PITC (wt.%) | Jsc (mA cm−2) | Voc (mV) | Ff | η (%) |
|---|---|---|---|---|
| 0 | 0.04 | 0.40 | 0.28 | 0.004 |
| 10 | 3.96 | 0.57 | 0.39 | 0.88 |
| 20 | 4.34 | 0.51 | 0.46 | 1.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kausar, A.; Ahmad, I.; Zhao, T.; Aldaghri, O.; Ibnaouf, K.H.; Eisa, M.H. Cutting-Edge Graphene Nanocomposites with Polythiophene—Design, Features and Forefront Potential. J. Compos. Sci. 2023, 7, 319. https://doi.org/10.3390/jcs7080319
Kausar A, Ahmad I, Zhao T, Aldaghri O, Ibnaouf KH, Eisa MH. Cutting-Edge Graphene Nanocomposites with Polythiophene—Design, Features and Forefront Potential. Journal of Composites Science. 2023; 7(8):319. https://doi.org/10.3390/jcs7080319
Chicago/Turabian StyleKausar, Ayesha, Ishaq Ahmad, Tingkai Zhao, Osamah Aldaghri, Khalid H. Ibnaouf, and M. H. Eisa. 2023. "Cutting-Edge Graphene Nanocomposites with Polythiophene—Design, Features and Forefront Potential" Journal of Composites Science 7, no. 8: 319. https://doi.org/10.3390/jcs7080319