CoP/EEBP/N-FLGS Nanocomposite as an Efficient Electrocatalyst of Hydrogen Evolution Reaction in Alkaline Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Electrochemically Exfoliated Black Phosphorus (EEBP)
2.3. Preparation of CoP/EEBP
2.4. Preparation of CoP/EEBP/N-FLGS
2.5. Characterization
2.6. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huangfu, Z.; Hu, H.; Xie, N.; Zhu, Y.-Q.; Chen, H.; Wang, Y. The Heterogeneous Influence of Economic Growth on Environmental Pollution: Evidence from Municipal Data of China. Pet. Sci. 2020, 17, 1180–1193. [Google Scholar] [CrossRef]
- Tian, L.; Li, Z.; Wang, P.; Zhai, X.; Wang, X.; Li, T. Carbon Quantum Dots for Advanced Electrocatalysis. J. Energy Chem. 2021, 55, 279–294. [Google Scholar] [CrossRef]
- Do, M.N.; Berezina, N.M.; Bazanov, M.I.; Gyseinov, S.S.; Berezin, M.M.; Koifman, O.I. Electrochemical Behavior of a Number of Bispyridyl-Substituted Porphyrins and Their Electrocatalytic Activity in Molecular Oxygen Reduction Reaction. J. Porphyrins Phthalocyanines 2016, 20, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Sazali, N. Emerging Technologies by Hydrogen: A Review. Int. J. Hydrogen Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, B. Recent Advances in Transition Metal Phosphide Nanomaterials: Synthesis and Applications in Hydrogen Evolution Reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, Y. Noble Metal-Free Hydrogen Evolution Catalysts for Water Splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Popczun, E.J.; McKone, J.R.; Read, C.G.; Biacchi, A.J.; Wiltrout, A.M.; Lewis, N.S.; Schaak, R.E. Nanostructured Nickel Phosphide as an Electrocatalyst for the Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 9267–9270. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Qin, Y.; Zhang, W.; Wang, L.; Luo, M.; Yang, H.; Guo, S. Recent Advances on Water-Splitting Electrocatalysis Mediated by Noble-Metal-Based Nanostructured Materials. Adv. Energy Mater. 2020, 10, 1903120. [Google Scholar] [CrossRef]
- Ruqia, B.; Choi, S. Catalytic Surface Specificity on Pt and Pt–Ni(OH)2 Electrodes for the Hydrogen Evolution Reaction in Alkaline Electrolytes and Their Nanoscaled Electrocatalysts. ChemSusChem 2018, 11, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.; Jiang, B.; Shen, W.; Chen, Y.; Li, Y.; Shen, Y.; Yin, K.; Shao, M. Ir-Au Bimetallic Nanoparticle Modified Silicon Nanowires with Ultralow Content of Ir for Hydrogen Evolution Reaction. ChemCatChem 2019, 11, 2126–2130. [Google Scholar] [CrossRef]
- Ma, F.; Xu, C.; Lyu, F.; Song, B.; Sun, S.; Li, Y.Y.; Lu, J.; Zhen, L. Construction of FeP Hollow Nanoparticles Densely Encapsulated in Carbon Nanosheet Frameworks for Efficient and Durable Electrocatalytic Hydrogen Production. Adv. Sci. 2019, 6, 1801490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakati, N.; Maiti, J.; Lee, S.H.; Jee, S.H.; Viswanathan, B.; Yoon, Y.S. Anode Catalysts for Direct Methanol Fuel Cells in Acidic Media: Do We Have Any Alternative for Pt or Pt–Ru? Chem. Rev. 2014, 114, 12397–12429. [Google Scholar] [CrossRef]
- Antolini, E. Palladium in Fuel Cell Catalysis. Energy Environ. Sci. 2009, 2, 915. [Google Scholar] [CrossRef]
- Faber, M.S.; Jin, S. Earth-Abundant Inorganic Electrocatalysts and Their Nanostructures for Energy Conversion Applications. Energy Environ. Sci. 2014, 7, 3519–3542. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y. Recent Advances in Heterogeneous Electrocatalysts for the Hydrogen Evolution Reaction. J. Mater. Chem. A 2015, 3, 14942–14962. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.; Cui, W.; Jiang, P.; Cheng, N.; Asiri, A.M.; Sun, X. Carbon Nanotubes Decorated with CoP Nanocrystals: A Highly Active Non-Noble-Metal Nanohybrid Electrocatalyst for Hydrogen Evolution. Angew. Chem. Int. Ed. 2014, 53, 6710–6714. [Google Scholar] [CrossRef]
- Yu, S.H.; Chua, D.H.C. Toward High-Performance and Low-Cost Hydrogen Evolution Reaction Electrocatalysts: Nanostructuring Cobalt Phosphide (CoP) Particles on Carbon Fiber Paper. ACS Appl. Mater. Interfaces 2018, 10, 14777–14785. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Cao, L.; Dai, P.; Gu, X.; Liu, D.; Li, L.; Wang, Y.; Zhao, X. Metal-Organic Frameworks Derived Nanotube of Nickel-Cobalt Bimetal Phosphides as Highly Efficient Electrocatalysts for Overall Water Splitting. Adv. Funct. Mater. 2017, 27, 1703455. [Google Scholar] [CrossRef]
- Yu, D.; Ilango, P.R.; Han, S.; Ye, M.; Hu, Y.; Li, L.; Peng, S. Metal-Organic Framework Derived Co@NC/CNT Hybrid as a Multifunctional Electrocatalyst for Hydrogen and Oxygen Evolution Reaction and Oxygen Reduction Reaction. Int. J. Hydrogen Energy 2019, 44, 32054–32065. [Google Scholar] [CrossRef]
- Lu, M.; Li, L.; Chen, D.; Li, J.; Klyui, N.I.; Han, W. MOF-Derived Nitrogen-Doped CoO@CoP Arrays as Bifunctional Electrocatalysts for Efficient Overall Water Splitting. Electrochim. Acta 2020, 330, 135210. [Google Scholar] [CrossRef]
- Sun, T.; Dong, J.; Huang, Y.; Ran, W.; Chen, J.; Xu, L. Highly Active and Stable Electrocatalyst of Ni 2 P Nanoparticles Supported on 3D Ordered Macro-/Mesoporous Co–N-Doped Carbon for Acidic Hydrogen Evolution Reaction. J. Mater. Chem. A 2018, 6, 12751–12758. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, K.; Ricciardulli, A.G.; Zhang, P.; Liao, Z.; Lohe, M.R.; Zschech, E.; Blom, P.W.M.; Pisula, W.; Müllen, K.; et al. A Rational Delamination Strategy towards Defect-Free, High- Mobility, Few-Layered Black Phosphorus Flakes. Angew. Chem. 2018, 130, 4767–4771. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Rui, K.; Hng, H.H.; Hippalgaonkar, K.; Xu, J.; Sun, W.; Zhu, J.; Yan, Q.; Huang, W. 2D Black Phosphorus for Energy Storage and Thermoelectric Applications. Small 2017, 13, 1700661. [Google Scholar] [CrossRef]
- Mei, J.; Liao, T.; Sun, Z. Opportunities and Challenges of Black Phosphorus for Electrocatalysis and Rechargeable Batteries. Adv. Sustain. Syst. 2022, 6, 2200301. [Google Scholar] [CrossRef]
- Baboukani, A.R.; Khakpour, I.; Drozd, V.; Allagui, A.; Wang, C. Single-step exfoliation of black phosphorus and deposition of phosphorene via bipolar electrochemistry for capacitive energy storage application. J. Mater. Chem. A 2019, 7, 25548–25556. [Google Scholar] [CrossRef]
- Cheng, J.; Gao, L.; Li, T.; Mei, S.; Wang, C.; Wen, B.; Huang, W.; Li, C.; Zheng, G.; Wang, H.; et al. Two-dimensional black phosphorus nanomaterials: Emerging advances in electrochemical energy storage science. Nano-Micro Lett. 2020, 12, 1–34. [Google Scholar] [CrossRef]
- Baboukani, A.R.; Khakpour, I.; Drozd, V.; Wang, C. Liquid-Based Exfoliation of Black Phosphorus into Phosphorene and Its Application for Energy Storage Devices. Small Struct. 2021, 2, 2000148. [Google Scholar] [CrossRef]
- Liu, H.; Hu, K.; Yan, D.; Chen, R.; Zou, Y.; Liu, H.; Wang, S. Recent advances on black phosphorus for energy storage, catalysis, and sensor applications. Adv. Mater. 2018, 30, 1800295. [Google Scholar] [CrossRef]
- Gao, W.; Zhou, Y.; Wu, X.; Shen, Q.; Ye, J.; Zou, Z. State-of-the-Art Progress in Diverse Black Phosphorus-Based Structures: Basic Properties, Synthesis, Stability, Photo- and Electrocatalysis-Driven Energy Conversion. Adv. Funct. Mater. 2021, 31, 2005197. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, J.; Yang, M.; Fang, Z.; Jian, J.; Yu, D.; Chen, X.; Dai, L. Ultrathin Black Phosphorus-on-Nitrogen Doped Graphene for Efficient Overall Water Splitting: Dual Modulation Roles of Directional Interfacial Charge Transfer. J. Am. Chem. Soc. 2019, 141, 4972–4979. [Google Scholar] [CrossRef] [PubMed]
- Kochergin, V.K.; Komarova, N.S.; Kotkin, A.S.; Manzhos, R.A.; Vasiliev, V.P.; Krivenko, A.G. Plasma Electrochemical Synthesis of Graphene-Phosphorene Composite and Its Catalytic Activity towards Hydrogen Evolution Reaction. C 2022, 8, 79. [Google Scholar] [CrossRef]
- Krivenko, A.G.; Manzhos, R.A.; Kotkin, A.S.; Kochergin, V.K.; Piven, N.P.; Manzhos, A.P. Production of Few-Layer Graphene Structures in Different Modes of Electrochemical Exfoliation of Graphite by Voltage Pulses. Instrum. Sci. Technol. 2019, 47, 535–544. [Google Scholar] [CrossRef]
- Belkin, P.N.; Yerokhin, A.; Kusmanov, S.A. Plasma Electrolytic Saturation of Steels with Nitrogen and Carbon. Surf. Coat. Technol. 2016, 307, 1194–1218. [Google Scholar] [CrossRef]
- Kochergin, V.K.; Manzhos, R.A.; Khodos, I.I.; Krivenko, A.G. One-Step Synthesis of Nitrogen-Doped Few-Layer Graphene Structures Decorated with Mn1.5Co1.5O4 Nanoparticles for Highly Efficient Electrocatalysis of Oxygen Reduction Reaction. Mendeleev Commun. 2022, 32, 492–494. [Google Scholar] [CrossRef]
- Callejas, J.F.; Read, C.G.; Popczun, E.J.; McEnaney, J.M.; Schaak, R.E. Nanostructured Co2P Electrocatalyst for the Hydrogen Evolution Reaction and Direct Comparison with Morphologically Equivalent CoP. Chem. Mater. 2015, 27, 3769–3774. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Huang, H.; Yang, N.; Yu, B.; Wen, M.; Wang, X.; Chu, P.K.; Yu, X.-F. In-Plane Black Phosphorus/Dicobalt Phosphide Heterostructure for Efficient Electrocatalysis. Angew. Chem. 2018, 130, 2630–2634. [Google Scholar] [CrossRef]
- Ha, D.-H.; Moreau, L.M.; Bealing, C.R.; Zhang, H.; Hennig, R.G.; Robinson, R.D. The Structural Evolution and Diffusion during the Chemical Transformation from Cobalt to Cobalt Phosphide Nanoparticles. J. Mater. Chem. 2011, 21, 11498. [Google Scholar] [CrossRef] [Green Version]
- Kotkin, A.S.; Kochergin, V.K.; Kabachkov, E.N.; Shulga, Y.M.; Lobach, A.S.; Manzhos, R.A.; Krivenko, A.G. One-Step Plasma Electrochemical Synthesis and Oxygen Electrocatalysis of Nanocomposite of Few-Layer Graphene Structures with Cobalt Oxides. Mater. Today Energy 2020, 17, 100459. [Google Scholar] [CrossRef]
- Manivasakan, P.; Ramasamy, P.; Kim, J. Use of Urchin-like NixCo3−xO4 Hierarchical Nanostructures Based on Non-Precious Metals as Bifunctional Electrocatalysts for Anion-Exchange Membrane Alkaline Alcohol Fuel Cells. Nanoscale 2014, 6, 9665–9672. [Google Scholar] [CrossRef]
- Liu, T.; Yan, X.; Xi, P.; Chen, J.; Qin, D.; Shan, D.; Devaramani, S.; Lu, X. Nickel–Cobalt Phosphide Nanowires Supported on Ni Foam as a Highly Efficient Catalyst for Electrochemical Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2017, 42, 14124–14132. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zhao, J.; Yang, K.; Liang, J.; Liu, D.; Hu, W.; Liu, D.; Liu, Y.; Liu, C. Monodispersed Nickel Phosphide Nanocrystals with Different Phases: Synthesis, Characterization and Electrocatalytic Properties for Hydrogen Evolution. J. Mater. Chem. A 2015, 3, 1656–1665. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, Z.; Chen, Z.; Lv, C.; Meng, H.; Zhang, C. Ni12P5 Nanoparticles as an Efficient Catalyst for Hydrogen Generation via Electrolysis and Photoelectrolysis. ACS Nano 2014, 8, 8121–8129. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Wik, S.D.; Cavell, R.G.; Mar, A. Examination of the Bonding in Binary Transition-Metal Monophosphides MP (M = Cr, Mn, Fe, Co) by X-Ray Photoelectron Spectroscopy. Inorg. Chem. 2005, 44, 8988–8998. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Luo, J.; Cheng, S.; Gong, H.; Liu, J.; Xu, C.; Zhao, Z.; Sun, Y.; Song, W.; et al. Porous N, P Co-Doped Carbon-Coated Ultrafine Co2P Nanoparticles Derived from DNA: An Electrocatalyst for Highly Efficient Hydrogen Evolution Reaction. Electrochim. Acta 2021, 393, 139051. [Google Scholar] [CrossRef]
- Briggs, D.; Seah, M.P. Practical Surface Analysis: By Auger and X-Ray Photoelectron Spectroscopy, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 1990; p. 674. [Google Scholar]
- Duan, D.; Feng, J.; Liu, S.; Wang, Y.; Zhou, X. MOF-Derived Cobalt Phosphide as Highly Efficient Electrocatalysts for Hydrogen Evolution Reaction. J. Electroanal. Chem. 2021, 892, 115300. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Asiri, A.M.; Sun, X. Self-Supported Nanoporous Cobalt Phosphide Nanowire Arrays: An Efficient 3D Hydrogen-Evolving Cathode over the Wide Range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590. [Google Scholar] [CrossRef]
- Lv, X.; Ren, J.-T.; Wang, Y.; Liu, Y.-P.; Yuan, Z.-Y. Well-Defined Phase-Controlled Cobalt Phosphide Nanoparticles Encapsulated in Nitrogen-Doped Graphitized Carbon Shell with Enhanced Electrocatalytic Activity for Hydrogen Evolution Reaction in all-pH. ACS Sustain. Chem. Eng. 2019, 7, 8993–9001. [Google Scholar] [CrossRef]
- Song, J.; Zhu, C.; Xu, B.Z.; Fu, S.; Engelhard, M.H.; Ye, R.; Du, D.; Beckman, S.P.; Lin, Y. Bimetallic Cobalt-Based Phosphide Zeolitic Imidazolate Framework: CoPx Phase-Dependent Electrical Conductivity and Hydrogen Atom Adsorption Energy for Efficient Overall Water Splitting. Adv. Energy Mater. 2017, 7, 1601555. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, X.-Y.; Paik, U. Nickel Cobalt Phosphides Quasi-Hollow Nanocubes as an Efficient Electrocatalyst for Hydrogen Evolution in Alkaline Solution. Chem. Commun. 2016, 52, 1633–1636. [Google Scholar] [CrossRef]
- Yang, M.; Feng, F.; Wang, K.; Li, S.; Huang, X.; Gong, L.; Ma, L.; Li, R. Synthesis of Metal Phosphide Nanoparticles Supported on Porous N-Doped Carbon Derived from Spirulina for Universal-pH Hydrogen Evolution. ChemSusChem 2020, 13, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Guo, B.-Y.; Chen, Q.-W.; Dong, B.; Zhang, J.-Q.; Qin, J.-F.; Xie, J.-Y.; Yang, M.; Wang, L.; Chai, Y.-M.; et al. Ultrafine and Highly-Dispersed Bimetal Ni2P/Co2P Encapsulated by Hollow N-Doped Carbon Nanospheres for Efficient Hydrogen Evolution. Int. J. Hydrogen Energy 2019, 44, 14908–14917. [Google Scholar] [CrossRef]
- Chen, A.; Fu, L.; Xiang, W.; Wei, W.; Liu, D.; Liu, C. Facile Synthesis of Ni5P4 Nanosheets/Nanoparticles for Highly Active and Durable Hydrogen Evolution. Int. J. Hydrogen Energy 2021, 46, 11701–11710. [Google Scholar] [CrossRef]
- Yang, S.; Chen, L.; Wei, W.; Lv, X.; Xie, J. CoP Nanoparticles Encapsulated in Three-Dimensional N-Doped Porous Carbon for Efficient Hydrogen Evolution Reaction in a Broad pH Range. Appl. Surf. Sci. 2019, 476, 749–756. [Google Scholar] [CrossRef]
- Tabassum, H.; Guo, W.; Meng, W.; Mahmood, A.; Zhao, R.; Wang, Q.; Zou, R. Metal-Organic Frameworks Derived Cobalt Phosphide Architecture Encapsulated into B/N Co-Doped Graphene Nanotubes for All pH Value Electrochemical Hydrogen Evolution. Adv. Energy Mater. 2017, 7, 1601671. [Google Scholar] [CrossRef]
- Danilovic, N.; Subbaraman, R.; Strmcnik, D.; Stamenkovic, V.; Markovic, N. Electrocatalysis of the HER in Acid and Alkaline Media. J. Serb. Chem. Soc. 2013, 78, 2007–2015. [Google Scholar] [CrossRef]
- Sheng, W.; Gasteiger, H.A.; Shao-Horn, Y. Hydrogen Oxidation and Evolution Reaction Kinetics on Platinum: Acid vs Alkaline Electrolytes. J. Electrochem. Soc. 2010, 157, B1529. [Google Scholar] [CrossRef]
- Li, X.; Hao, X.; Abudula, A.; Guan, G. Nanostructured Catalysts for Electrochemical Water Splitting: Current State and Prospects. J. Mater. Chem. A 2016, 4, 11973–12000. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, L.; Liu, J. Recent Advances in Cobalt-Based Electrocatalysts for Hydrogen and Oxygen Evolution Reactions. J. Alloys Compd. 2020, 821, 153542. [Google Scholar] [CrossRef]
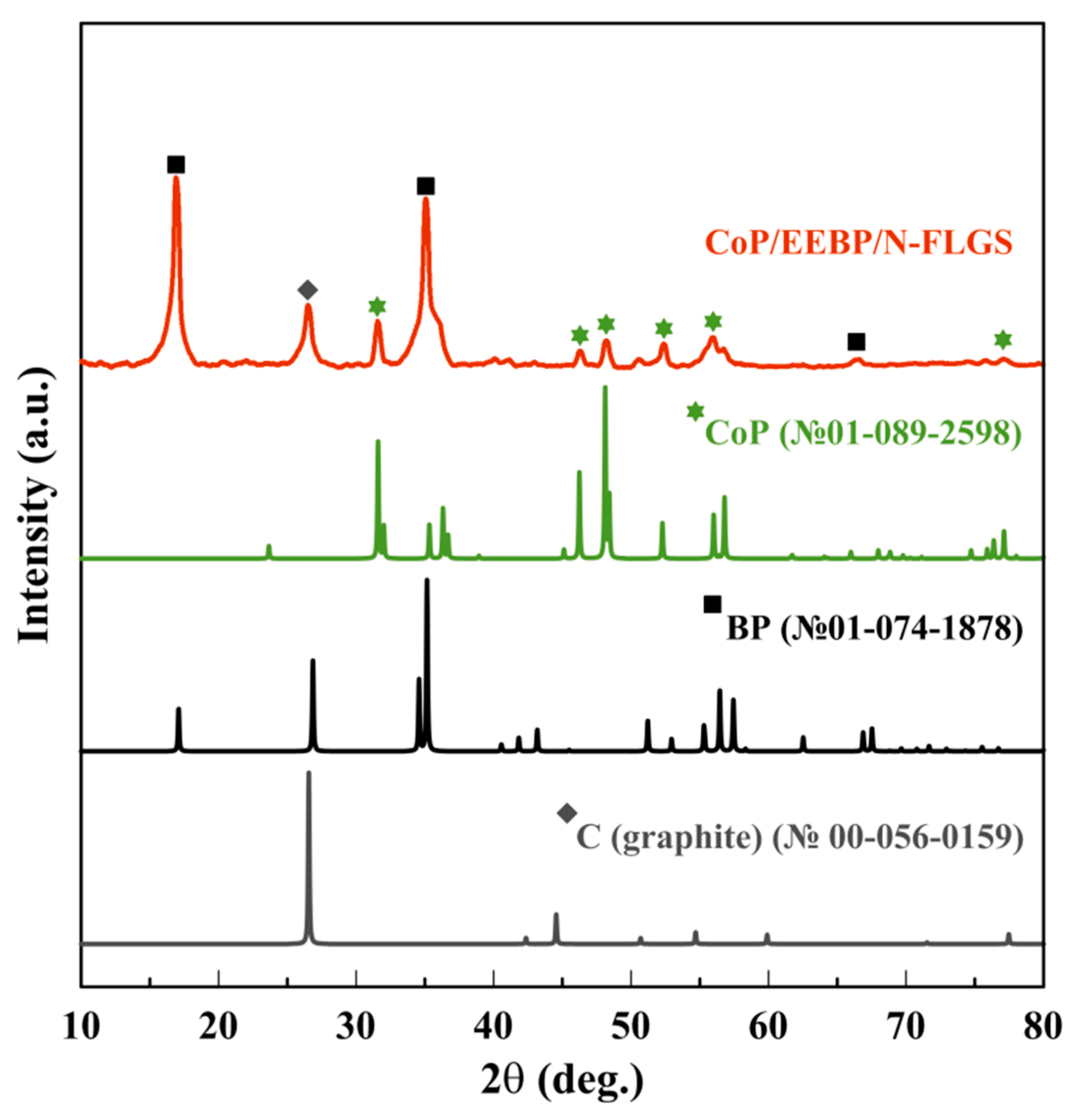


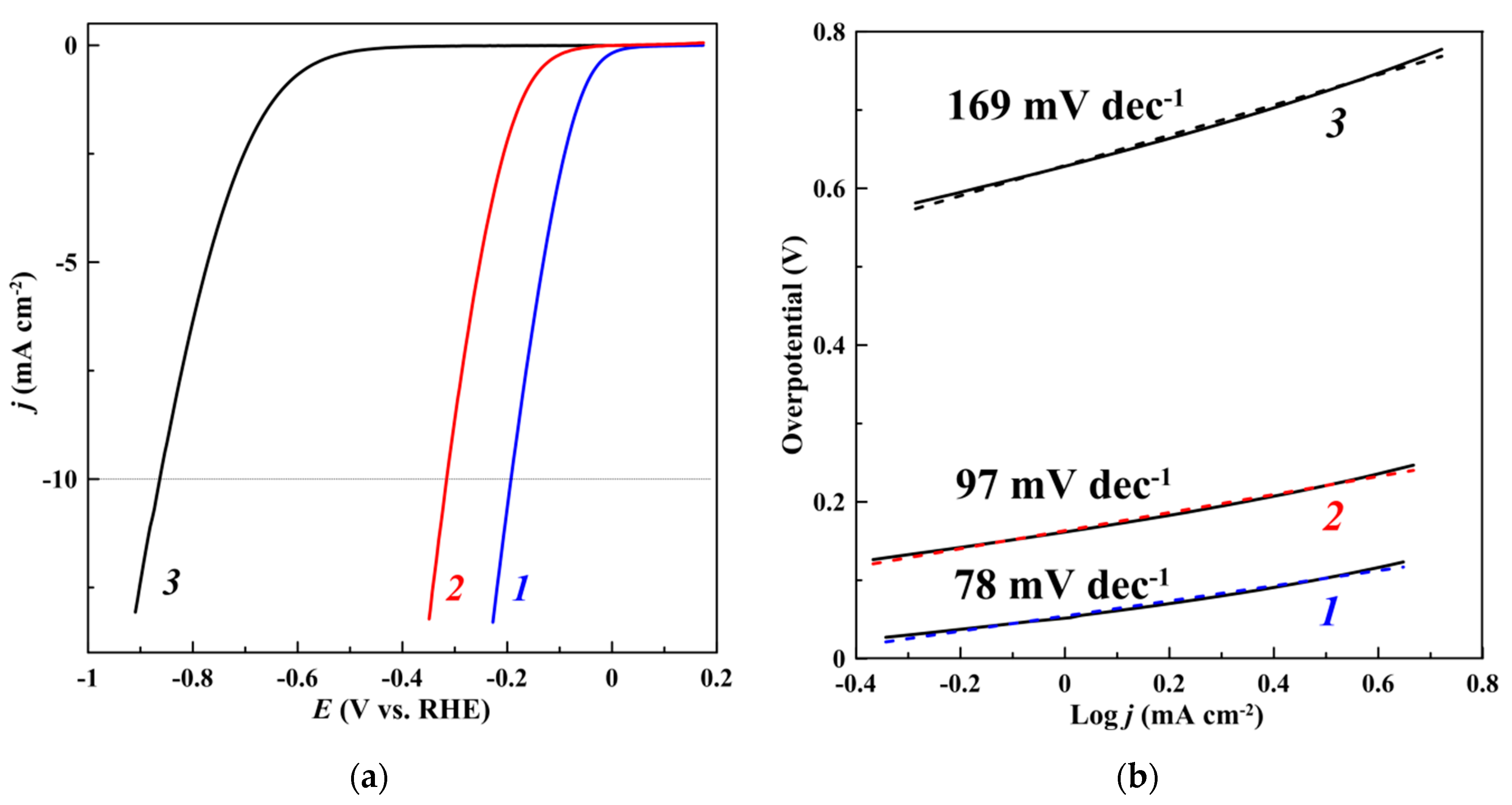
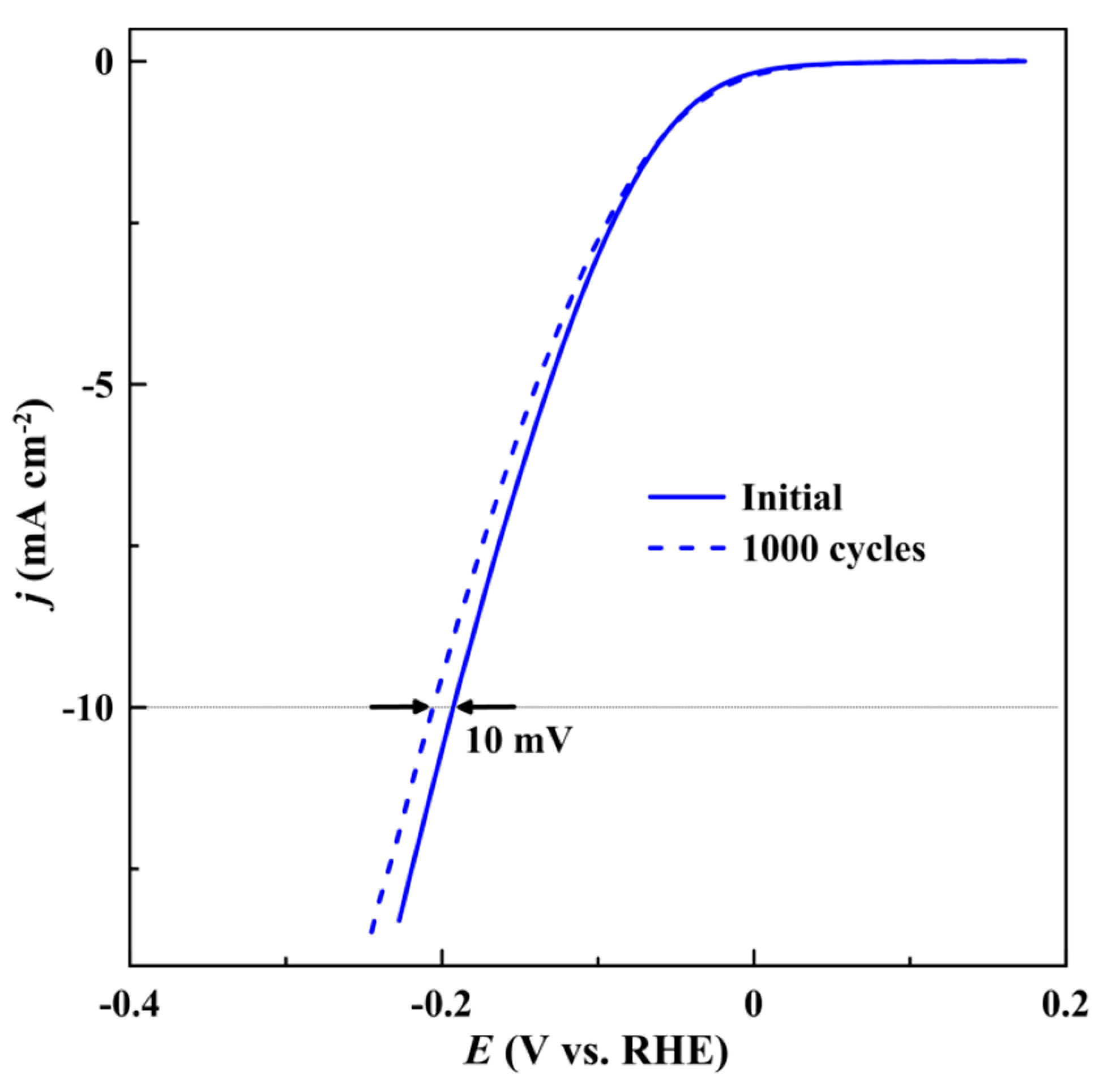
| Catalyst | η10 1, mV | Tafel slope, mV dec−1 |
|---|---|---|
| EEBP | 865 | 169 |
| CoP/EEBP | 315 | 97 |
| CoP/EEBP/N-FLGS | 190 | 78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochergin, V.K.; Kotkin, A.S.; Manzhos, R.A.; Krivenko, A.G.; Khodos, I.I.; Kabachkov, E.N. CoP/EEBP/N-FLGS Nanocomposite as an Efficient Electrocatalyst of Hydrogen Evolution Reaction in Alkaline Media. J. Compos. Sci. 2023, 7, 328. https://doi.org/10.3390/jcs7080328
Kochergin VK, Kotkin AS, Manzhos RA, Krivenko AG, Khodos II, Kabachkov EN. CoP/EEBP/N-FLGS Nanocomposite as an Efficient Electrocatalyst of Hydrogen Evolution Reaction in Alkaline Media. Journal of Composites Science. 2023; 7(8):328. https://doi.org/10.3390/jcs7080328
Chicago/Turabian StyleKochergin, Valerii K., Alexander S. Kotkin, Roman A. Manzhos, Alexander G. Krivenko, Igor I. Khodos, and Eugene N. Kabachkov. 2023. "CoP/EEBP/N-FLGS Nanocomposite as an Efficient Electrocatalyst of Hydrogen Evolution Reaction in Alkaline Media" Journal of Composites Science 7, no. 8: 328. https://doi.org/10.3390/jcs7080328







