A Review of Biomass Wood Ash in Alkali-Activated Materials: Treatment, Application, and Outlook
Abstract
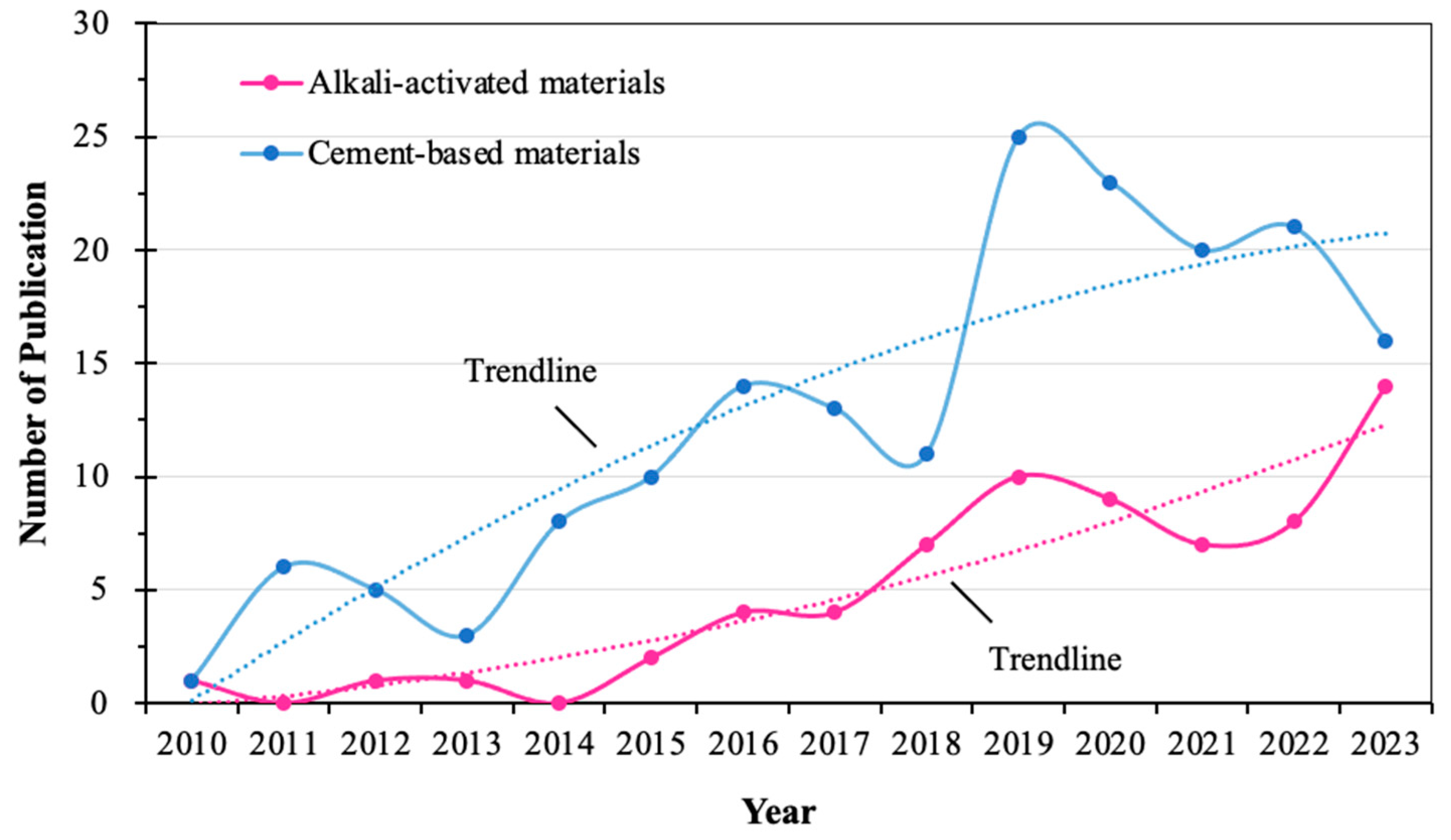
:1. Introduction
2. Characterisation of Biomass Wood Ash
3. Preparation of Alkali-Activated Composites
| Category | Precursor | Alkali Activator | Testing Methods | Temperature Treatment | Curing Humidity | Major Outcomes | Reference |
|---|---|---|---|---|---|---|---|
| Paste | BWA, BSS | KOH, K2SiO3 | Compressive strength, flexural strength, FTIR, bulk density, XRD, thermal conductivity, SEM-EDS | 60 or 20 °C for 24 h | 90% | Thermal curing at 60 °C increased mechanical properties at early curing ages. The addition of up to 50 wt% of BSS contributed to increased compressive and flexural strengths. | [58] |
| Paste | BWA, FA, GGBS | NaOH, Na2SiO3 | Compressive strength, workability, ultrasonic pulse velocity (UPV), XRD, TG, FTIR, SEM-EDS | Room temperature | 65% | Replacement of 30 wt% BWA significantly improved compressive strength and microstructure compared to the reference mixture. | [70] |
| Paste | MK, BWA | NaOH, Na2SiO3 | Rheological tests, isothermal calorimetry, FTIR, greenhouse gas emissions | - | - | The specific surface area of BWA greatly influenced the rheological parameters and the hydration kinetics of the geopolymers, and BWA had 65% less CO2 emissions than MK. | [27] |
| Mortar | FA, BWA | KOH, Na2SiO3 | MIP, compressive strength, flexural strength, XRD, TG, SEM | 70 °C for 24 h | - | The inclusion of treated BWA enhanced compressive and flexural strength. The control mix and treated BWA specimens displayed better porosity. | [71] |
| Paste, concrete | BWA, PG | NaOH, Na2SiO3 | Compressive strength, flexural strength, SEM, XRD, total porosity | 60 °C for 24 h | - | 15–20% PG replacement led to improved compressive strength. NaOH/Na2SiO3 as activator solution resulted in reduced open porosity and improved samples’ resistance to freezing and thawing. | [72] |
| Paste | BWA, zeolitic waste | NaOH | Compressive strength, XRD, FTIR, SEM | 60 °C for 24 h | - | 3% zeolitic waste replacement. | [73] |
| Paste, mortar | BWA, silica fume | NaOH | XRD, SEM-EDS, FTIR, compressive strength, total porosity, Methylene blue concentration reduction | 24 °C | 50–60% | The alkaline activation of pastes and mortars with 5 M solution exhibited the best levels of compressive strength. Alkaline-activated mortars presented lower water absorption than mortars without activation. | [65] |
| Paste | BWA, glass powder (GP) | NaOH | Compressive strength, flexural strength, XRD, SEM-EDS, | Room temperature | - | With the increase in NaOH content and reduction in GP, both compressive and flexural strength grew. | [75] |
| Paste | BWA, PG | Na2CO3, Na2SiO3 | Setting time, density, UPV, compressive strength, water absorption, TG, XRD, SEM | 20 and 40 °C | - | The mechanical properties increased with the increase in temperature and Na2CO3/Na2SiO3 ratio. | [61] |
| Paste, mortar | BWA, MK | NaOH, Na2SiO3 | XRD, SEM-EDS, workability, bulk density, water absorption, compressive strength | Room temperature | 65% for 24 h | Binder/aggregate ratio influenced the properties of the mortar. With an increasing number of aggregates, the compressive strength decreased. | [64] |
| Mortar | BWA, FA | NaOH, Na2SiO3 | Compressive strength, flexural strength, water absorption, total porosity, XRD, SEM | 70 °C for 24 h | - | Incorporation of BWA up to 20% increased the early strength due to the formation of CSH gel. Only the mortar with 10% BWA replacement showed positive 28-day results. | [77] |
| Paste | BWA, MK | NaOH, Na2SiO3 | XRD, TG, SEM-EDS, compressive strength, bulk density, water absorption | 40, 20 °C and room temperature | 65%, submerged in water and open condition | Samples obtained with hydrothermal conditions and SS:SH = 1.0 can be used for pollutant adsorption and those produced at room condition with SS:SH = 1.5 can be employed as dense mortars. | [79] |
| Aggregate | BWA, GGBS | Na2SiO3 | Bulk density, water absorption, crushing strength, pore distribution, XRD, EDS | 30 °C | 65% | By increasing the content of GGBS from 0% to 30%, the crushing strength improved from 0.84 MPa to 2.25 MPa, and the water absorption decreased from 24.0% to 12.5%. | [76] |
| Paste | BWA, MK, calcined clay | NaOH, Na2SiO3 | Bulk density, apparent porosity, water absorption, compressive strength, thermal conductivity, XRD, FTIR, SEM-EDS | 60 °C for 24 h | - | BWA and calcined clays improved the mechanical properties of geopolymer. | [80] |
| Mortar | BWA, GGBFS | NaOH, Na2SiO3, Na2CO3 | Workability, compressive strength, flexural strength, SEM, total porosity, EDS, XRD, pH value, isothermal calorimetry | Room temperature | - | Grinding wood ash can improve the mechanical properties of alkali-activated systems compared to untreated wood ash, and the incorporation of wood ash increased the porosity of the binder matrix. | [5] |
| Paste | BWA, silica gel by-product | NaOH | Compressive strength, XRD, FTIR, SEM | 60 °C for 24 h | - | The compressive strength of AAB specimens relied on the amounts of silica gel by-product and alkali activator. The highest strength (21.6 MPa) was observed in specimens with 35% of silica gel by-product. | [74] |
| Paste | BWA, MK | NaOH, Na2SiO3 | XRD, FTIR, SEM-EDS, bulk density, apparent porosity, water absorption, compressive strength | 60 °C for 24 h | - | Concentration of at least 8 M NaOH is required to obtain optimum mechanical properties and up to 50 wt% addition of BWA increased compressive strength. | [81] |
| Mortar | FA, BWA | NaOH, Na2SiO3 | SEM-EDS, FTIR, catalytic tests, metal sorption | Room temperature | - | Geopolymers possess optical and photocatalytic properties. Geopolymers based on BWA had better sorption properties. | [83] |
| Paste, mortar | BWA, MK | NaOH, Na2SiO3 | Workability, compressive strength, XRD, TG, SEM-EDS | 60 °C for 48 h then at 60 °C | RH 95% | Incorporation of MK improved mechanical properties. The highest compressive strength was obtained for 40% MK incorporated mortars for around 38 MPa. | [110] |
| Paste, mortar, concrete | FA, BWA | NaOH, Na2SiO3 | Setting time, workability, bulk density, flexural strength, sulphate resistance, freeze-thaw resistance | Room temperature | - | The mixtures with 20% BWA showed better sulphate resistance than those with only FA. | [82] |
4. Properties of Alkali-Activated Composites Containing Biomass Wood Ash
4.1. Workability and Setting Time
4.2. Mechanical Properties
4.3. Durability
4.4. Bulk Density
4.5. Phase Identification
4.6. Microstructure and Porosity
5. Lifecycle Assessment and Analysis of Eco-Efficiency
6. Discussion on the State-of-the-Art Research
7. Conclusions and Future Research Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huntzinger, D.N.; Eatmon, T.D. A Life-Cycle Assessment of Portland Cement Manufacturing: Comparing the Traditional Process with Alternative Technologies. J. Clean. Prod. 2009, 17, 668–675. [Google Scholar] [CrossRef]
- Frías, M.; Rodríguez, O.; de Rojas, M.I.S.; Villar-Cociña, E.; Rodrigues, M.S.; Junior, H.S. Advances on the Development of Ternary Cements Elaborated with Biomass Ashes Coming from Different Activation Process. Constr. Build. Mater. 2017, 136, 73–80. [Google Scholar] [CrossRef]
- Pierrehumbert, R. There Is No Plan B for Dealing with the Climate Crisis. Bull. At. Sci. 2019, 75, 215–221. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, P.; Zhang, Z. Application of Silica-Rich Biomass Ash Solid Waste in Geopolymer Preparation: A Review. Constr. Build. Mater. 2022, 356, 129142. [Google Scholar] [CrossRef]
- Ercan, E.E.T.; Cwirzen, A.; Habermehl-Cwirzen, K. The Effects of Partial Replacement of Ground Granulated Blast Furnace Slag by Ground Wood Ash on Alkali-Activated Binder Systems. Materials 2023, 16, 5347. [Google Scholar] [CrossRef] [PubMed]
- Pasupathy, K.; Sanjayan, J.; Rajeev, P. Evaluation of Alkalinity Changes and Carbonation of Geopolymer Concrete Exposed to Wetting and Drying. J. Build. Eng. 2021, 35, 102029. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Yang, T.; Li, L.; Zhu, H.; Wang, H. Conversion of Local Industrial Wastes into Greener Cement through Geopolymer Technology: A Case Study of High-Magnesium Nickel Slag. J. Clean. Prod. 2017, 141, 463–471. [Google Scholar] [CrossRef]
- Awoyera, P.O.; Adesina, A.; Sivakrishna, A.; Gobinath, R.; Kumar, K.R.; Srinivas, A. Alkali Activated Binders: Challenges and Opportunities. Mater. Today Proc. 2020, 27, 40–43. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Fořt, J.; Klapiszewska, I.; Thomas, M.; Klapiszewski, Ł.; Černý, R. A Literature Review of the Latest Trends and Perspectives Regarding Alkali-Activated Materials in Terms of Sustainable Development. J. Mater. Res. Technol. 2023, 25, 5394–5425. [Google Scholar] [CrossRef]
- Patil, P.P.; Katare, V.D. Development of Sustainable Alkali-Activated Binder for Building Products Using Sugarcane Bagasse Ash: A Review. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Mendes, B.C.; Pedroti, L.G.; Vieira, C.M.F.; Marvila, M.; Azevedo, A.R.G.; de Carvalho, J.M.F.; Ribeiro, J.C.L. Application of Eco-Friendly Alternative Activators in Alkali-Activated Materials: A Review. J. Build. Eng. 2021, 35, 102010. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and Other Alkali Activated Materials: Why, How, and What? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Akturk, B.; Kizilkanat, A.B.; Kabay, N. Effect of Calcium Hydroxide on Fresh State Behavior of Sodium Carbonate Activated Blast Furnace Slag Pastes. Constr. Build. Mater. 2019, 212, 388–399. [Google Scholar] [CrossRef]
- Yuan, B.; Yu, Q.L.; Brouwers, H.J.H. Assessing the Chemical Involvement of Limestone Powder in Sodium Carbonate Activated Slag. Mater. Struct. 2017, 50, 136. [Google Scholar] [CrossRef]
- Ibrahim, M.; Maslehuddin, M. An Overview of Factors Influencing the Properties of Alkali-Activated Binders. J. Clean. Prod. 2021, 286, 124972. [Google Scholar] [CrossRef]
- Elahi, M.M.A.; Hossain, M.M.; Karim, M.R.; Zain, M.F.M.; Shearer, C. A Review on Alkali-Activated Binders: Materials Composition and Fresh Properties of Concrete. Constr. Build. Mater. 2020, 260, 119788. [Google Scholar] [CrossRef]
- Çelikten, S.; Sarıdemir, M.; Deneme, İ.Ö. Mechanical and Microstructural Properties of Alkali-Activated Slag and Slag + fly Ash Mortars Exposed to High Temperature. Constr. Build. Mater. 2019, 217, 50–61. [Google Scholar] [CrossRef]
- Gijbels, K.; Iacobescu, R.I.; Pontikes, Y.; Schreurs, S.; Schroeyers, W. Alkali-Activated Binders Based on Ground Granulated Blast Furnace Slag and Phosphogypsum. Constr. Build. Mater. 2019, 215, 371–380. [Google Scholar] [CrossRef]
- Wu, M.; Shen, W.; Xiong, X.; Zhao, L.; Yu, Z.; Sun, H.; Xu, G.; Zhao, Q.; Wang, G.; Zhang, W. Effects of the Phosphogypsum on the Hydration and Microstructure of Alkali Activated Slag Pastes. Constr. Build. Mater. 2023, 368, 130391. [Google Scholar] [CrossRef]
- Kim, T. Characteristics of Alkali-Activated Slag Cement-Based Ultra-Lightweight Concrete with High-Volume Cenosphere. Constr. Build. Mater. 2021, 302, 124165. [Google Scholar] [CrossRef]
- Zheng, Y.; Xuan, D.; Shen, B.; Ma, K. Shrinkage Mitigation of Alkali-Activated Fly Ash/Slag Mortar by Using Phosphogypsum Waste. Constr. Build. Mater. 2023, 375, 130978. [Google Scholar] [CrossRef]
- Pan, Z.; Tao, Z.; Cao, Y.F.; Wuhrer, R.; Murphy, T. Compressive Strength and Microstructure of Alkali-Activated Fly Ash/Slag Binders at High Temperature. Cem. Concr. Compos. 2018, 86, 9–18. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R.; Roviello, G.; Capasso, I.; Caputo, D.; Aprea, P.; Liguori, B.; Ferone, C. Thermal Cycling Stability of Fly Ash Based Geopolymer Mortars. Compos. B Eng. 2017, 129, 11–17. [Google Scholar] [CrossRef]
- Huseien, G.F.; Sam, A.R.M.; Shah, K.W.; Mirza, J.; Tahir, M.M. Evaluation of Alkali-Activated Mortars Containing High Volume Waste Ceramic Powder and Fly Ash Replacing GBFS. Constr. Build. Mater. 2019, 210, 78–92. [Google Scholar] [CrossRef]
- Olatoyan, O.J.; Kareem, M.A.; Adebanjo, A.U.; Olawale, S.O.A.; Alao, K.T. Potential Use of Biomass Ash as a Sustainable Alternative for Fly Ash in Concrete Production: A Review. Hybrid Adv. 2023, 4, 100076. [Google Scholar] [CrossRef]
- Ilari, A.; Duca, D.; Boakye-Yiadom, K.A.; Gasperini, T.; Toscano, G. Carbon Footprint and Feedstock Quality of a Real Biomass Power Plant Fed with Forestry and Agricultural Residues. Resources 2022, 11, 7. [Google Scholar] [CrossRef]
- Silvestro, L.; Scolaro, T.P.; Ruviaro, A.S.; Lima, G.T.d.S.; Gleize, P.J.P.; Pelisser, F. Use of Biomass Wood Ash to Produce Sustainable Geopolymeric Pastes. Constr. Build. Mater. 2023, 370, 130641. [Google Scholar] [CrossRef]
- Pels, J.R.; Nie, D.S. De Utilization of Ashes from Biomass Combustion and Gasification. In Proceedings of the 14th European Biomass Conference & Exhibition, Paris, France, 17–21 October 2005. [Google Scholar]
- Van Eijk, R.J. Options for Increased Utilization of Ash from Biomass Combustion and Co-Firing. In IEA Bioenergy Task 32; Kema: Arnhem, The Netherlands, 2012. [Google Scholar]
- Santhosh, K.G.; Subhani, S.M.; Bahurudeen, A. Sustainable Reuse of Palm Oil Fuel Ash in Concrete, Alkali-Activated Binders, Soil Stabilisation, Bricks and Adsorbent: A Waste to Wealth Approach. Ind. Crop. Prod. 2022, 183, 114954. [Google Scholar] [CrossRef]
- Santhosh, K.G.; Subhani, S.M.; Bahurudeen, A. Recycling of Palm Oil Fuel Ash and Rice Husk Ash in the Cleaner Production of Concrete. J. Clean. Prod. 2022, 354, 131736. [Google Scholar] [CrossRef]
- Murugesan, T.; Vidjeapriya, R.; Bahurudeen, A. Development of Sustainable Alkali Activated Binder for Construction Using Sugarcane Bagasse Ash and Marble Waste. Sugar Tech 2020, 22, 885–895. [Google Scholar] [CrossRef]
- Gedela Santhosh, K.; Subhani, S.M.; Bahurudeen, A. An Investigation on the Effectiveness of Quarry Dust and Sugarcane Bagasse Ash in Na-Based Alkali-Activated Binder. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Chougan, M.; Ghaffar, S.H.; Sikora, P.; Mijowska, E.; Kukułka, W.; Stephan, D. Boosting Portland Cement-Free Composite Performance via Alkali-Activation and Reinforcement with Pre-Treated Functionalised Wheat Straw. Ind. Crop. Prod. 2022, 178, 114648. [Google Scholar] [CrossRef]
- Das, D.; Laskar, S.M.; Hussain, B. Study on Slag-Rice Husk Ash Based Alkali Activated Concrete. ASPS Conf. Proc. 2022, 1, 147–151. [Google Scholar] [CrossRef]
- Dhasindrakrishna, K.; Ramakrishnan, S.; Pasupathy, K.; Sanjayan, J. Synthesis and Performance of Intumescent Alkali-Activated Rice Husk Ash for Fire-Resistant Applications. J. Build. Eng. 2022, 51, 104281. [Google Scholar] [CrossRef]
- Matalkah, F.; Soroushian, P.; Abideen, S.U.; Peyvandi, A. Use of Non-Wood Biomass Combustion Ash in Development of Alkali-Activated Concrete. Constr. Build. Mater. 2016, 121, 491–500. [Google Scholar] [CrossRef]
- Zhu, C.J.; Pundienė, I.; Pranckevičienė, J.; Kligys, M.; Korjakins, A.; Vitola, L. Influence of Alkaline Activator Solution Ratio on the Properties of Biomass Fly Ash-Based Alkali-Activated Materials. J. Phys. Conf. Ser. 2023, 2423, 012033. [Google Scholar] [CrossRef]
- Athira, V.S.; Charitha, V.; Athira, G.; Bahurudeen, A. Agro-Waste Ash Based Alkali-Activated Binder: Cleaner Production of Zero Cement Concrete for Construction. J. Clean. Prod. 2021, 286, 125429. [Google Scholar] [CrossRef]
- Prabagar, S.; Thuraisingam, S.; Prabagar, J. The Influence of Eco-Friendly Ash on Sustainable Construction Material: A Review. Appl. Sci. Innov. Res. 2022, 6, 39. [Google Scholar] [CrossRef]
- Das, S.K.; Adediran, A.; Kaze, C.R.; Mustakim, S.M.; Leklou, N. Production, Characteristics, and Utilization of Rice Husk Ash in Alkali Activated Materials: An Overview of Fresh and Hardened State Properties. Constr. Build. Mater. 2022, 345, 128341. [Google Scholar]
- Ercan, E.E.T.; Andreas, L.; Cwirzen, A.; Habermehl-Cwirzen, K. Wood Ash as Sustainable Alternative Raw Material for the Production of Concrete—A Review. Materials 2023, 16, 2557. [Google Scholar] [CrossRef]
- Wang, R.; Haller, P. Applications of Wood Ash as a Construction Material in Civil Engineering: A Review. Biomass Conv. Biorefin. 2022, 1–21. [Google Scholar] [CrossRef]
- Martínez-García, R.; Jagadesh, P.; Zaid, O.; Șerbănoiu, A.A.; Fraile-Fernández, F.J.; de Prado-Gil, J.; Qaidi, S.M.A.; Grădinaru, C.M. The Present State of the Use of Waste Wood Ash as an Eco-Efficient Construction Material: A Review. Materials 2022, 15, 5349. [Google Scholar] [CrossRef] [PubMed]
- Isa, M.N.; Awang, H.; Muthusamy, K. A Potential Environmental Sustainability of Wood Ash in Normal and Geopolymer Concrete—A Review. Adv. Sci. Technol. Res. J. 2023, 17, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.; Díazlópez, J.L.; Agrela, F.; Rosales, J. Eco-efficient Cement-based Materials Using Biomass Bottom Ash: A Review. Appl. Sci. 2020, 10, 8026. [Google Scholar] [CrossRef]
- AL-Kharabsheh, B.N.; Arbili, M.M.; Majdi, A.; Ahmad, J.; Deifalla, A.F.; Hakamy, A. A Review on Strength and Durability Properties of Wooden Ash Based Concrete. Materials 2022, 15, 7282. [Google Scholar] [CrossRef] [PubMed]
- Ayobami, A.B. Performance of Wood Bottom Ash in Cement-Based Applications and Comparison with Other Selected Ashes: Overview. Resour. Conserv. Recycl. 2021, 166, 105351. [Google Scholar] [CrossRef]
- Nascimento, L.C.; Junior, G.B.; Xavier, G.d.C.; Monteiro, S.N.; Vieira, C.M.F.; de Azevedo, A.R.G.; Alexandre, J. Use of Wood Bottom Ash in Cementitious Materials: A Review. J. Mater. Res. Technol. 2023, 23, 4226–4243. [Google Scholar] [CrossRef]
- Tamanna, K.; Raman, S.N.; Jamil, M.; Hamid, R. Utilization of Wood Waste Ash in Construction Technology: A Review. Constr. Build. Mater. 2020, 237, 117654. [Google Scholar] [CrossRef]
- Cheah, C.B.; Ramli, M. The Implementation of Wood Waste Ash as a Partial Cement Replacement Material in the Production of Structural Grade Concrete and Mortar: An Overview. Resour. Conserv. Recycl. 2011, 55, 669–685. [Google Scholar] [CrossRef]
- Rajamma, R.; Senff, L.; Ribeiro, M.J.; Labrincha, J.A.; Ball, R.J.; Allen, G.C.; Ferreira, V.M. Biomass Fly Ash Effect on Fresh and Hardened State Properties of Cement Based Materials. Compos. B Eng. 2015, 77, 1–9. [Google Scholar] [CrossRef]
- Berra, M.; Mangialardi, T.; Paolini, A.E. Reuse of Woody Biomass Fly Ash in Cement-Based Materials. Constr. Build. Mater. 2015, 76, 286–296. [Google Scholar] [CrossRef]
- Barbosa, R.; Lapa, N.; Dias, D.; Mendes, B. Concretes Containing Biomass Ashes: Mechanical, Chemical, and Ecotoxic Performances. Constr. Build. Mater. 2013, 48, 457–463. [Google Scholar] [CrossRef]
- Rissanen, J.; Ohenoja, K.; Kinnunen, P.; Romagnoli, M.; Illikainen, M. Milling of Peat-Wood Fly Ash: Effect on Water Demand of Mortar and Rheology of Cement Paste. Constr. Build. Mater. 2018, 180, 143–153. [Google Scholar] [CrossRef]
- Nagrockienė, D.; Daugėla, A. Investigation into the Properties of Concrete Modified with Biomass Combustion Fly Ash. Constr. Build. Mater. 2018, 174, 369–375. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An Overview of the Composition and Application of Biomass Ash. Part 1. Phase-Mineral and Chemical Composition and Classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Gómez-Casero, M.A.; Pérez-Villarejo, L.; Castro, E.; Eliche-Quesada, D. Effect of Steel Slag and Curing Temperature on the Improvement in Technological Properties of Biomass Bottom Ash Based Alkali-Activated Materials. Constr. Build. Mater. 2021, 302, 124205. [Google Scholar] [CrossRef]
- Maschowski, C.; Kruspan, P.; Garra, P.; Arif, A.T.; Trouvé, G.; Gieré, R. Physicochemical and Mineralogical Characterization of Biomass Ash from Different Power Plants in the Upper Rhine Region. Fuel 2019, 258, 116020. [Google Scholar] [CrossRef] [PubMed]
- Sathonsaowaphak, A.; Chindaprasirt, P.; Pimraksa, K. Workability and Strength of Lignite Bottom Ash Geopolymer Mortar. J. Hazard. Mater. 2009, 168, 44–50. [Google Scholar] [CrossRef]
- Zhu, C.; Pundienė, I.; Pranckevičienė, J.; Kligys, M. Effects of Na2CO3/Na2SiO3 Ratio and Curing Temperature on the Structure Formation of Alkali-Activated High-Carbon Biomass Fly Ash Pastes. Materials 2022, 15, 8354. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Characterisation of Fly Ashes. Potential Reactivity as Alkaline Cements. Fuel 2003, 82, 2259–2265. [Google Scholar] [CrossRef]
- Antunes Boca Santa, R.A.; Soares, C.; Riella, H.G. Geopolymers Obtained from Bottom Ash as Source of Aluminosilicate Cured at Room Temperature. Constr. Build. Mater. 2017, 157, 459–466. [Google Scholar] [CrossRef]
- Saeli, M.; Senff, L.; Tobaldi, D.M.; Seabra, M.P.; Labrincha, J.A. Novel Biomass Fly Ash-Based Geopolymeric Mortars Using Lime Slaker Grits as Aggregate for Applications in Construction: Influence of Granulometry and Binder/Aggregate Ratio. Constr. Build. Mater. 2019, 227, 116643. [Google Scholar] [CrossRef]
- Silva, T.H.; Lara, L.F.S.; Silva, G.J.B.; Provis, J.L.; Bezerra, A.C.S. Alkali-Activated Materials Produced Using High-Calcium, High-Carbon Biomass Ash. Cem. Concr. Compos. 2022, 132, 104646. [Google Scholar] [CrossRef]
- Carević, I.; Baričević, A.; Štirmer, N.; Šantek Bajto, J. Correlation between Physical and Chemical Properties of Wood Biomass Ash and Cement Composites Performances. Constr. Build. Mater. 2020, 256, 119450. [Google Scholar] [CrossRef]
- Rajamma, R.; Ball, R.J.; Tarelho, L.A.C.; Allen, G.C.; Labrincha, J.A.; Ferreira, V.M. Characterisation and Use of Biomass Fly Ash in Cement-Based Materials. J. Hazard. Mater. 2009, 172, 1049–1060. [Google Scholar] [CrossRef]
- Ngueyep, M.; Leroy, L. Valorization of Wood Ashes as Partial Replacement of Portland Cement: Mechanical Performance and Durability. Eur. J. Sci. Res. 2019, 151, 468–478. [Google Scholar]
- Carević, I.; Serdar, M.; Štirmer, N.; Ukrainczyk, N. Preliminary Screening of Wood Biomass Ashes for Partial Resources Replacements in Cementitious Materials. J. Clean. Prod. 2019, 229, 1045–1064. [Google Scholar] [CrossRef]
- Thi, K.D.T.; Liao, M.C.; Vo, D.H. The Characteristics of Alkali-Activated Slag-Fly Ash Incorporating the High Volume Wood Bottom Ash: Mechanical Properties and Microstructures. Constr. Build. Mater. 2023, 394, 132240. [Google Scholar] [CrossRef]
- Ates, F.; Park, K.T.; Kim, K.W.; Woo, B.H.; Kim, H.G. Effects of Treated Biomass Wood Fly Ash as a Partial Substitute for Fly Ash in a Geopolymer Mortar System. Constr. Build. Mater. 2023, 376, 131063. [Google Scholar] [CrossRef]
- Vaičiukyniene, D.; Nizevičiene, D.; Kantautas, A.; Bocullo, V.; Kiele, A. Alkali Activated Paste and Concrete Based on of Biomass Bottom Ash with Phosphogypsum. Appl. Sci. 2020, 10, 5190. [Google Scholar] [CrossRef]
- Vaiciukyniene, D.; Nizeviciene, D.; Mikelioniene, A.; Radzevicius, A. Utilization of Zeolitic Waste in Alkali-Activated Biomass Bottom Ash Blends. Molecules 2020, 25, 3053. [Google Scholar] [CrossRef]
- Vaičiukynienė, D.; Nizevičienė, D.; Kantautas, A.; Kielė, A.; Bocullo, V. Alkali Activated Binders Based on Biomass Bottom Ash and Silica By-Product Blends. Waste Biomass Valorization 2021, 12, 1095–1105. [Google Scholar] [CrossRef]
- Silva, G.J.B.; Santana, V.P.; Wójcik, M. Investigation on Mechanical and Microstructural Properties of Alkali-Activated Materials Made of Wood Biomass Ash and Glass Powder. Powder Technol. 2021, 377, 900–912. [Google Scholar] [CrossRef]
- Lin, J.; Tan, T.H.; Yeo, J.S.; Goh, Y.; Ling, T.C.; Mo, K.H. Municipal Woody Biomass Waste Ash-Based Cold-Bonded Artificial Lightweight Aggregate Produced by One-Part Alkali-Activation Method. Constr. Build. Mater. 2023, 394, 131619. [Google Scholar] [CrossRef]
- Abdulkareem, O.A.; Ramli, M.; Matthews, J.C. Production of Geopolymer Mortar System Containing High Calcium Biomass Wood Ash as a Partial Substitution to Fly Ash: An Early Age Evaluation. Compos. B Eng. 2019, 174, 106941. [Google Scholar] [CrossRef]
- Hassan, H.S.; Abdel-Gawwad, H.A.; Vásquez-García, S.R.; Israde-Alcántara, I.; Flores-Ramirez, N.; Rico, J.L.; Mohammed, M.S. Cleaner Production of One-Part White Geopolymer Cement Using Pre-Treated Wood Biomass Ash and Diatomite. J. Clean. Prod. 2019, 209, 1420–1428. [Google Scholar] [CrossRef]
- De Rossi, A.; Simão, L.; Ribeiro, M.J.; Hotza, D.; Moreira, R.F.P.M. Study of Cure Conditions Effect on the Properties of Wood Biomass Fly Ash Geopolymers. J. Mater. Res. Technol. 2020, 9, 7518–7528. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Calero-Rodríguez, A.; Bonet-Martínez, E.; Pérez-Villarejo, L.; Sánchez-Soto, P.J. Geopolymers Made from Metakaolin Sources, Partially Replaced by Spanish Clays and Biomass Bottom Ash. J. Build. Eng. 2021, 40, 102761. [Google Scholar] [CrossRef]
- Jurado-Contreras, S.; Bonet-Martínez, E.; Sánchez-Soto, P.J.; Gencel, O.; Eliche-Quesada, D. Synthesis and Characterization of Alkali-Activated Materials Containing Biomass Fly Ash and Metakaolin: Effect of the Soluble Salt Content of the Residue. Arch. Civ. Mech. Eng. 2022, 22, 121. [Google Scholar] [CrossRef]
- Bijeljić, J.; Ristić, N.; Grdić, D.; Pavlović, M. Possibilities of Biomass Wood Ash Usage in Geopolymer Mixtures. Teh. Vjesn. 2023, 30, 52–60. [Google Scholar] [CrossRef]
- Sitarz-Palczak, E.; Kalembkiewicz, J.; Galas, D. Comparative Study on the Characteristics of Coal Fly Ash and Biomass Ash Geopolymers. Arch. Environ. Prot. 2019, 45, 126–135. [Google Scholar] [CrossRef]
- De, S.; Mishra, S.; Poonguzhali, E.; Rajesh, M.; Tamilarasan, K. Fractionation and Characterization of Lignin from Waste Rice Straw: Biomass Surface Chemical Composition Analysis. Int. J. Biol. Macromol. 2020, 145, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Etiégni, L.; Campbell, A.G. Physical and Chemical Characteristics of Wood Ash. Bioresour. Technol. 1991, 37, 173–178. [Google Scholar] [CrossRef]
- Karrech, A.; Dong, M.; Elchalakani, M.; Shahin, M.A. Sustainable Geopolymer Using Lithium Concentrate Residues. Constr. Build. Mater. 2019, 228, 116740. [Google Scholar] [CrossRef]
- Shi, C.; Krivenko, P.V.; Roy, D. Alkali-Activated Cements and Concretes; CRC Press: London, UK, 2006. [Google Scholar]
- Li, C.; Sun, H.; Li, L. A Review: The Comparison between Alkali-Activated Slag (Si + Ca) and Metakaolin (Si + Al) Cements. Cem. Concr. Res. 2010, 40, 1341–1349. [Google Scholar] [CrossRef]
- Mohammed, B.S.; Haruna, S.; Wahab, M.M.A.; Liew, M.S.; Haruna, A. Mechanical and Microstructural Properties of High Calcium Fly Ash One-Part Geopolymer Cement Made with Granular Activator. Heliyon 2019, 5, e022552019. [Google Scholar] [CrossRef]
- Lee, W.K.W.; Van Deventer, J.S.J. The Effect of Ionic Contaminants on the Early-Age Properties of Alkali-Activated Fly Ash-Based Cements. Cem. Concr. Res. 2002, 32, 577–584. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Role of Slag in Microstructural Development and Hardening of Fly Ash-Slag Geopolymer. Cem. Concr. Res. 2013, 43, 70–80. [Google Scholar] [CrossRef]
- Kim, E.H. Understanding Effects of Silicon/Aluminum Ratio and Calcium Hydroxide on Chemical Composition, Nanostructure and Compressive Strength for Metakaolin Geopolymers. Master’s Thesis, University of Illinois at Urbana-Champaign, Urbana, IL, USA, 2012. [Google Scholar]
- de la Grée, G.C.H.D.; Florea, M.V.A.; Keulen, A.; Brouwers, H.J.H. Contaminated Biomass Fly Ashes—Characterization and Treatment Optimization for Reuse as Building Materials. Waste Manag. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Peng, Y.-X.; Ni, X.; Zhu, Z.-C.; Yu, Z.-F.; Yin, Z.-X.; Li, T.-Q.; Liu, S.-Y.; Zhao, L.-L.; Xu, J. Friction and Wear of Liner and Grinding Ball in Iron Ore Ball Mill. Tribol. Int. 2017, 115, 506–517. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R. Mechanical Activation of Fly Ash: Effect on Reaction, Structure and Properties of Resulting Geopolymer. Ceram. Int. 2011, 37, 533–541. [Google Scholar] [CrossRef]
- Hamzaoui, R.; Bouchenafa, O.; Guessasma, S.; Leklou, N.; Bouaziz, A. The Sequel of Modified Fly Ashes Using High Energy Ball Milling on Mechanical Performance of Substituted Past Cement. Mater. Des. 2016, 90, 29–37. [Google Scholar] [CrossRef]
- Amaral, R.C.; Rohden, A.B.; Garcez, M.R.; Andrade, J.J.d.O. Reuse of Wood Ash from Biomass Combustion in Non-Structural Concrete: Mechanical Properties, Durability, and Eco-Efficiency. J. Mater. Cycles Waste Manag. 2022, 24, 2439–2454. [Google Scholar] [CrossRef]
- Komljenović, M.; Baščarević, Z.; Bradić, V. Mechanical and Microstructural Properties of Alkali-Activated Fly Ash Geopolymers. J. Hazard. Mater. 2010, 181, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Tchakouté, H.K.; Rüscher, C.H.; Kong, S.; Kamseu, E.; Leonelli, C. Geopolymer Binders from Metakaolin Using Sodium Waterglass from Waste Glass and Rice Husk Ash as Alternative Activators: A Comparative Study. Constr. Build. Mater. 2016, 114, 276–289. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon Dioxide Equivalent (CO2-e) Emissions: A Comparison between Geopolymer and OPC Cement Concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Jeon, D.; Jun, Y.; Jeong, Y.; Oh, J.E. Microstructural and Strength Improvements through the Use of Na2CO3 in a Cementless Ca(OH)2-Activated Class F Fly Ash System. Cem. Concr. Res. 2015, 67, 215–225. [Google Scholar] [CrossRef]
- Khale, D.; Chaudhary, R. Mechanism of Geopolymerization and Factors Influencing Its Development: A Review. J. Mater. Sci. 2007, 42, 729–746. [Google Scholar] [CrossRef]
- Phair, J.W.; Van Deventer, J.S.J. Effect of Silicate Activator PH on the Leaching and Material Characteristics of Waste-Based Inorganic Polymers. Miner. Eng. 2001, 14, 289–304. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The Geopolymerisation of Alumino-Silicate Minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- PQ Europe Sodium and Potassium Silicates. Soidium and Potasium Silicates: Versatile Compounds for Your Applications. 2004, 2. Available online: https://www.scribd.com/document/382255676/sodium-and-potassium-silicates-brochure-eng-oct-2004-pdf (accessed on 1 April 2024).
- Singh, B.; Rahman, M.R.; Paswan, R.; Bhattacharyya, S.K. Effect of Activator Concentration on the Strength, ITZ and Drying Shrinkage of Fly Ash/Slag Geopolymer Concrete. Constr. Build. Mater. 2016, 118, 171–179. [Google Scholar] [CrossRef]
- Ishwarya, G.; Singh, B.; Deshwal, S.; Bhattacharyya, S.K. Effect of Sodium Carbonate/Sodium Silicate Activator on the Rheology, Geopolymerization and Strength of Fly Ash/Slag Geopolymer Pastes. Cem. Concr. Compos. 2019, 97, 226–238. [Google Scholar] [CrossRef]
- Chithiraputhiran, S.; Neithalath, N. Isothermal Reaction Kinetics and Temperature Dependence of Alkali Activation of Slag, Fly Ash and Their Blends. Constr. Build. Mater. 2013, 45, 233–242. [Google Scholar] [CrossRef]
- Xu, H.; Gong, W.; Syltebo, L.; Izzo, K.; Lutze, W.; Pegg, I.L. Effect of Blast Furnace Slag Grades on Fly Ash Based Geopolymer Waste Forms. Fuel 2014, 133, 332–340. [Google Scholar] [CrossRef]
- Rajamma, R.; Labrincha, J.A.; Ferreira, V.M. Alkali Activation of Biomass Fly Ash-Metakaolin Blends. Fuel 2012, 98, 265–271. [Google Scholar] [CrossRef]
- Rodrigue Kaze, C.; Ninla Lemougna, P.; Alomayri, T.; Assaedi, H.; Adesina, A.; Kumar Das, S.; Lecomte-Nana, G.L.; Kamseu, E.; Chinje Melo, U.; Leonelli, C. Characterization and Performance Evaluation of Laterite Based Geopolymer Binder Cured at Different Temperatures. Constr. Build. Mater. 2021, 270, 121443. [Google Scholar] [CrossRef]
- Zribi, M.; Samet, B.; Baklouti, S. Effect of Curing Temperature on the Synthesis, Structure and Mechanical Properties of Phosphate-Based Geopolymers. J. Non Cryst. Solids 2019, 511, 62–67. [Google Scholar] [CrossRef]
- Mustafa Al Bakri, A.M.; Kamarudin, H.; Bnhussain, M.; Nizar, K.; Rafiza, A.R.; Zarina, Y. The Processing, Characterization, and Properties of Fly Ash Based Geopolymer Concrete. Rev. Adv. Mater. Sci. 2012, 30, 90–97. [Google Scholar]
- Ozer, I.; Soyer-Uzun, S. Relations between the Structural Characteristics and Compressive Strength in Metakaolin Based Geopolymers with Different Molar Si/Al Ratios. Ceram. Int. 2015, 41, 10192–10198. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Reid, A.; Aravinthan, T. Effects of Fly Ash Source and Curing Procedure on Strength Development of Geopolymers. In Proceedings of the Incorporating Sustainable Practice in Mechanics of Structures and Materials—Proceedings of the 21st Australian Conference on the Mechanics of Structures and Materials, Melbourne, Australia, 7–10 December 2010; Scimago Journal & Country Rank: Granada, Spain, 2011. [Google Scholar]
- Cheah, C.B.; Part, W.K.; Ramli, M. The Hybridizations of Coal Fly Ash and Wood Ash for the Fabrication of Low Alkalinity Geopolymer Load Bearing Block Cured at Ambient Temperature. Constr. Build. Mater. 2015, 88, 41–55. [Google Scholar] [CrossRef]
- Sun, B.; Sun, Y.; Ye, G.; De Schutter, G. A Mix Design Methodology of Slag and Fly Ash-Based Alkali-Activated Paste. Cem. Concr. Compos. 2022, 126, 104368. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Myers, R.J.; San Nicolas, R.; van Deventer, J.S.J. Role of Carbonates in the Chemical Evolution of Sodium Carbonate-Activated Slag Binders. Mater. Struct. 2014, 48, 517–529. [Google Scholar] [CrossRef]
- Cheah, C.B.; Ramli, M. The Engineering Properties of High Performance Concrete with HCWA-DSF Supplementary Binder. Constr. Build. Mater. 2013, 40, 93–103. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; Van Deventer, J.S.J. The Coexistence of Geopolymeric Gel and Calcium Silicate Hydrate at the Early Stage of Alkaline Activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Gallé, C. Effect of Drying on Cement-Based Materials Pore Structure as Identified by Mercury Intrusion Porosimetry—A Comparative Study between Oven-, Vacuum-, and Freeze-Drying. Cem. Concr. Res. 2001, 31, 1467–1477. [Google Scholar] [CrossRef]
- Nemaleu, J.G.D.; Kaze, R.C.; Tome, S.; Alomayri, T.; Assaedi, H.; Kamseu, E.; Melo, U.C.; Sglavo, V.M. Powdered Banana Peel in Calcined Halloysite Replacement on the Setting Times and Engineering Properties on the Geopolymer Binders. Constr. Build. Mater. 2021, 279, 122480. [Google Scholar] [CrossRef]
- Rovnaník, P. Effect of Curing Temperature on the Development of Hard Structure of Metakaolin-Based Geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Composition and Microstructure of Alkali Activated Fly Ash Binder: Effect of the Activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Košnář, Z.; Mercl, F.; Perná, I.; Tlustoš, P. Investigation of Polycyclic Aromatic Hydrocarbon Content in Fly Ash and Bottom Ash of Biomass Incineration Plants in Relation to the Operating Temperature and Unburned Carbon Content. Sci. Total Environ. 2016, 563–564, 53–61. [Google Scholar] [CrossRef]
- Perná, I.; Šupová, M.; Hanzlíček, T.; Špaldoňová, A. The Synthesis and Characterization of Geopolymers Based on Metakaolin and High LOI Straw Ash. Constr. Build. Mater. 2019, 228, 116765. [Google Scholar] [CrossRef]
- Yuan, J.; He, P.; Jia, D.; Yang, C.; Zhang, Y.; Yan, S.; Yang, Z.; Duan, X.; Wang, S.; Zhou, Y. Effect of Curing Temperature and SiO2/K2O Molar Ratio on the Performance of Metakaolin-Based Geopolymers. Ceram. Int. 2016, 42, 16184–16190. [Google Scholar] [CrossRef]
- Le, T.; Wang, Q.; Ravindra, A.V.; Li, X.; Ju, S. Microwave Intensified Synthesis of Zeolite-Y from Spent FCC Catalyst after Acid Activation. J. Alloys Compd. 2019, 776, 437–446. [Google Scholar] [CrossRef]
- Huseien, G.F.; Ismail, M.; Khalid, N.H.A.; Hussin, M.W.; Mirza, J. Compressive Strength and Microstructure of Assorted Wastes Incorporated Geopolymer Mortars: Effect of Solution Molarity. Alex. Eng. J. 2018, 57, 3375–3386. [Google Scholar] [CrossRef]
- Oh, J.E.; Monteiro, P.J.M.; Jun, S.S.; Choi, S.; Clark, S.M. The Evolution of Strength and Crystalline Phases for Alkali-Activated Ground Blast Furnace Slag and Fly Ash-Based Geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]
- Timakul, P.; Rattanaprasit, W.; Aungkavattana, P. Improving Compressive Strength of Fly Ash-Based Geopolymer Composites by Basalt Fibers Addition. Ceram. Int. 2016, 42, 6288–6295. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Cristelo, N.; Miranda, T.; Palomo, Á. Sustainable Alkali Activated Materials: Precursor and Activator Derived from Industrial Wastes. J. Clean. Prod. 2017, 162, 1200–1209. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, H.; El-Korchi, T.; Zhang, G.; Tao, M. Experimental Feasibility Study of Geopolymer as the Next-Generation Soil Stabilizer. Constr. Build. Mater. 2013, 47, 1468–1478. [Google Scholar] [CrossRef]
- Sore, S.O.; Messan, A.; Prud’homme, E.; Escadeillas, G.; Tsobnang, F. Synthesis and Characterization of Geopolymer Binders Based on Local Materials from Burkina Faso—Metakaolin and Rice Husk Ash. Constr. Build. Mater. 2016, 124, 301–311. [Google Scholar] [CrossRef]
- De Rossi, A.; Simão, L.; Ribeiro, M.J.; Novais, R.M.; Labrincha, J.A.; Hotza, D.; Moreira, R.F.P.M. In-Situ Synthesis of Zeolites by Geopolymerization of Biomass Fly Ash and Metakaolin. Mater. Lett. 2019, 236, 644–648. [Google Scholar] [CrossRef]
- de Moraes Pinheiro, S.M.; Font, A.; Soriano, L.; Tashima, M.M.; Monzó, J.; Borrachero, M.V.; Payá, J. Olive-Stone Biomass Ash (OBA): An Alternative Alkaline Source for the Blast Furnace Slag Activation. Constr. Build. Mater. 2018, 178, 327–338. [Google Scholar] [CrossRef]
- Firdous, R.; Hirsch, T.; Klimm, D.; Lothenbach, B.; Stephan, D. Reaction of Calcium Carbonate Minerals in Sodium Silicate Solution and Its Role in Alkali-Activated Systems. Miner. Eng. 2021, 165, 106849. [Google Scholar] [CrossRef]
- Sun, R.; Fang, C.; Zhang, H.; Ling, Y.; Feng, J.; Qi, H.; Ge, Z. Chemo-Mechanical Properties of Alkali-Activated Slag/Fly Ash Paste Incorporating White Mud. Constr. Build. Mater. 2021, 291, 123312. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; Van Deventer, J.S.J.; Lukey, G.C. The Effect of Composition and Temperature on the Properties of Fly Ash- and Kaolinite-Based Geopolymers. Chem. Eng. J. 2002, 89, 63–73. [Google Scholar] [CrossRef]
- Le Troëdec, M.; Dalmay, P.; Patapy, C.; Peyratout, C.; Smith, A.; Chotard, T. Mechanical Properties of Hemp-Lime Reinforced Mortars: Influence of the Chemical Treatment of Fibers. J. Compos. Mater. 2011, 45, 2347–2357. [Google Scholar] [CrossRef]
- Tchakoute, H.K.; Elimbi, A.; Yanne, E.; Djangang, C.N. Utilization of Volcanic Ashes for the Production of Geopolymers Cured at Ambient Temperature. Cem. Concr. Compos. 2013, 38, 75–81. [Google Scholar] [CrossRef]
- Bayiha, B.N.; Billong, N.; Yamb, E.; Kaze, R.C.; Nzengwa, R. Effect of Limestone Dosages on Some Properties of Geopolymer from Thermally Activated Halloysite. Constr. Build. Mater. 2019, 217, 28–35. [Google Scholar] [CrossRef]
- Nana, A.; Alomayri, T.S.; Venyite, P.; Kaze, R.C.; Assaedi, H.S.; Nobouassia, C.B.; Sontia, J.V.M.; Ngouné, J.; Kamseu, E.; Leonelli, C. Mechanical Properties and Microstructure of a Metakaolin-Based Inorganic Polymer Mortar Reinforced with Quartz Sand. Silicon 2022, 14, 263–274. [Google Scholar] [CrossRef]
- Cheah, C.B.; Part, W.K.; Ramli, M. The Long Term Engineering Properties of Cementless Building Block Work Containing Large Volume of Wood Ash and Coal Fly Ash. Constr. Build. Mater. 2017, 143, 522–536. [Google Scholar] [CrossRef]
- Mathivet, V.; Jouin, J.; Gharzouni, A.; Sobrados, I.; Celerier, H.; Rossignol, S.; Parlier, M. Acid-Based Geopolymers: Understanding of the Structural Evolutions during Consolidation and after Thermal Treatments. J. Non Cryst. Solids 2019, 512, 90–97. [Google Scholar] [CrossRef]
- Ma, Y.; Ye, G.; Hu, J. Micro-Mechanical Properties of Alkali-Activated Fly Ash Evaluated by Nanoindentation. Constr. Build. Mater. 2017, 147, 407–416. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Rüscher, C.H. Mechanical and Microstructural Properties of Metakaolin-Based Geopolymer Cements from Sodium Waterglass and Phosphoric Acid Solution as Hardeners: A Comparative Study. Appl. Clay Sci. 2017, 140, 81–87. [Google Scholar] [CrossRef]
- Huo, Z.; Xu, X.; Lü, Z.; Song, J.; He, M.; Li, Z.; Wang, Q.; Yan, L. Synthesis of Zeolite NaP with Controllable Morphologies. Microporous Mesoporous Mater. 2012, 158, 137–140. [Google Scholar] [CrossRef]
- Moudio, A.M.N.; Tchakouté, H.K.; Ngnintedem, D.L.V.; Andreola, F.; Kamseu, E.; Nanseu-Njiki, C.P.; Leonelli, C.; Rüscher, C.H. Influence of the Synthetic Calcium Aluminate Hydrate and the Mixture of Calcium Aluminate and Silicate Hydrates on the Compressive Strengths and the Microstructure of Metakaolin-Based Geopolymer Cements. Mater. Chem. Phys. 2021, 264, 124459. [Google Scholar] [CrossRef]
- Hu, X.; Shi, C.; Shi, Z.; Zhang, L. Compressive Strength, Pore Structure and Chloride Transport Properties of Alkali-Activated Slag/Fly Ash Mortars. Cem. Concr. Compos. 2019, 104, 103392. [Google Scholar] [CrossRef]
- Sun, B.; Ye, G.; de Schutter, G. A Review: Reaction Mechanism and Strength of Slag and Fly Ash-Based Alkali-Activated Materials. Constr. Build. Mater. 2022, 326, 126843. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Reaction Kinetics, Gel Character and Strength of Ambient Temperature Cured Alkali Activated Slag-Fly Ash Blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef]
- Cheah, C.B.; Samsudin, M.H.; Ramli, M.; Part, W.K.; Tan, L.E. The Use of High Calcium Wood Ash in the Preparation of Ground Granulated Blast Furnace Slag and Pulverized Fly Ash Geopolymers: A Complete Microstructural and Mechanical Characterization. J. Clean. Prod. 2017, 156, 114–123. [Google Scholar] [CrossRef]
- Puertas, F.; González-Fonteboa, B.; González-Taboada, I.; Alonso, M.M.; Torres-Carrasco, M.; Rojo, G.; Martínez-Abella, F. Alkali-Activated Slag Concrete: Fresh and Hardened Behaviour. Cem. Concr. Compos. 2018, 85, 22–31. [Google Scholar] [CrossRef]
- Fu, Q.; Bu, M.; Zhang, Z.; Xu, W.; Yuan, Q.; Niu, D. Hydration Characteristics and Microstructure of Alkali-Activated Slag Concrete: A Review. Engineering 2023, 20, 162–179. [Google Scholar] [CrossRef]
- Caldas, L.R.; Saraiva, A.B.; Lucena, A.F.P.; Da Gloria, M.Y.; Santos, A.S.; Filho, R.D.T. Building Materials in a Circular Economy: The Case of Wood Waste as CO2-Sink in Bio Concrete. Resour. Conserv. Recycl. 2021, 166, 105346. [Google Scholar] [CrossRef]
- Ohenoja, K.; Rissanen, J.; Kinnunen, P.; Illikainen, M. Direct Carbonation of Peat-Wood Fly Ash for Carbon Capture and Utilization in Construction Application. J. CO2 Util. 2020, 40, 101203. [Google Scholar] [CrossRef]
- Kannan, V.; Raja Priya, P. Evaluation of the Permeability of High Strength Concrete Using Metakaolin and Wood Ash as Partial Replacement for Cement. SN Appl. Sci. 2021, 3, 90. [Google Scholar] [CrossRef]
- Gaudreault, C.; Lama, I.; Sain, D. Is the Beneficial Use of Wood Ash Environmentally Beneficial? A Screening-Level Life Cycle Assessment and Uncertainty Analysis. J. Ind. Ecol. 2020, 24, 1300–1309. [Google Scholar] [CrossRef]
- Celik, K.; Meral, C.; Petek Gursel, A.; Mehta, P.K.; Horvath, A.; Monteiro, P.J.M. Mechanical Properties, Durability, and Life-Cycle Assessment of Self-Consolidating Concrete Mixtures Made with Blended Portland Cements Containing Fly Ash and Limestone Powder. Cem. Concr. Compos. 2015, 56, 59–72. [Google Scholar] [CrossRef]
- Šķēls, P.; Bondars, K.; Plonis, R.; Haritonovs, V.; Paeglītis, A. Usage of Wood Fly Ash in Stabilization of Unbound Pavement Layers and Soils. In Proceedings of the 13th Baltic Sea Geotechnical Conference “Historocal Experiences and Challenges of Geotechnical Problems in Baltic Sea Region”, Vilnius, Lithuania, 15–17 September 2016. [Google Scholar]
- da Costa, T.P.; Quinteiro, P.; Arroja, L.; Dias, A.C. Environmental Performance of Different End-of-Life Alternatives of Wood Fly Ash by a Consequential Perspective. Sustain. Mater. Technol. 2022, 32, e004112022. [Google Scholar] [CrossRef]
- da Costa, T.P.; Quinteiro, P.; Tarelho, L.A.C.; Arroja, L.; Dias, A.C. Environmental Assessment of Valorisation Alternatives for Woody Biomass Ash in Construction Materials. Resour. Conserv. Recycl. 2019, 148, 67–79. [Google Scholar] [CrossRef]
- Amiandamhen, S.O.; Adamopoulos, S.; Adl-Zarrabi, B.; Yin, H.; Norén, J. Recycling Sawmilling Wood Chips, Biomass Combustion Residues, and Tyre Fibres into Cement-Bonded Composites: Properties of Composites and Life Cycle Analysis. Constr. Build. Mater. 2021, 297, 123781. [Google Scholar] [CrossRef]
- Gabrijel, I.; Rukavina, M.J.; Štirmer, N. Influence of Wood Fly Ash on Concrete Properties through Filling Effect Mechanism. Materials 2021, 14, 7164. [Google Scholar] [CrossRef]
- Kim, K.W.; Park, K.T.; Ates, F.; Kim, H.G.; Woo, B.H. Effect of Pretreated Biomass Fly Ash on the Mechanical Properties and Durability of Cement Mortar. Case Stud. Constr. Mater. 2023, 18, e017542023. [Google Scholar] [CrossRef]
- Vilutiene, T.; Kalibatiene, D.; Hosseini, M.R.; Pellicer, E.; Zavadskas, E.K. Building Information Modeling (BIM) for Structural Engineering: A Bibliometric Analysis of the Literature. Adv. Civ. Eng. 2019, 2019, 5290690. [Google Scholar] [CrossRef]
- Waltman, L.; Van Eck, N.J. A Smart Local Moving Algorithm for Large-Scale Modularity-Based Community Detection. Eur. Phys. J. B 2013, 86, 471. [Google Scholar] [CrossRef]
- Hosseini, M.R.; Maghrebi, M.; Akbarnezhad, A.; Martek, I.; Arashpour, M. Analysis of Citation Networks in Building Information Modeling Research. J. Constr. Eng. Manag. 2018, 144, 04018064. [Google Scholar] [CrossRef]
- Turk, J.; Cotič, Z.; Mladenovič, A.; Šajna, A. Environmental Evaluation of Green Concretes versus Conventional Concrete by Means of LCA. Waste Manag. 2015, 45, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Ghufran, M.; Khan, K.I.A.; Ullah, F.; Nasir, A.R.; Al Alahmadi, A.A.; Alzaed, A.N.; Alwetaishi, M. Circular Economy in the Construction Industry: A Step towards Sustainable Development. Buildings 2022, 12, 1004. [Google Scholar] [CrossRef]
- Norouzi, M.; Chàfer, M.; Cabeza, L.F.; Jiménez, L.; Boer, D. Circular Economy in the Building and Construction Sector: A Scientific Evolution Analysis. J. Build. Eng. 2021, 44, 102704. [Google Scholar] [CrossRef]
- Usta, M.C.; Yörük, C.R.; Uibu, M.; Hain, T.; Gregor, A.; Trikkel, A. CO2 Curing of Ca-Rich Fly Ashes to Produce Cement-Free Building Materials. Minerals 2022, 12, 513. [Google Scholar] [CrossRef]

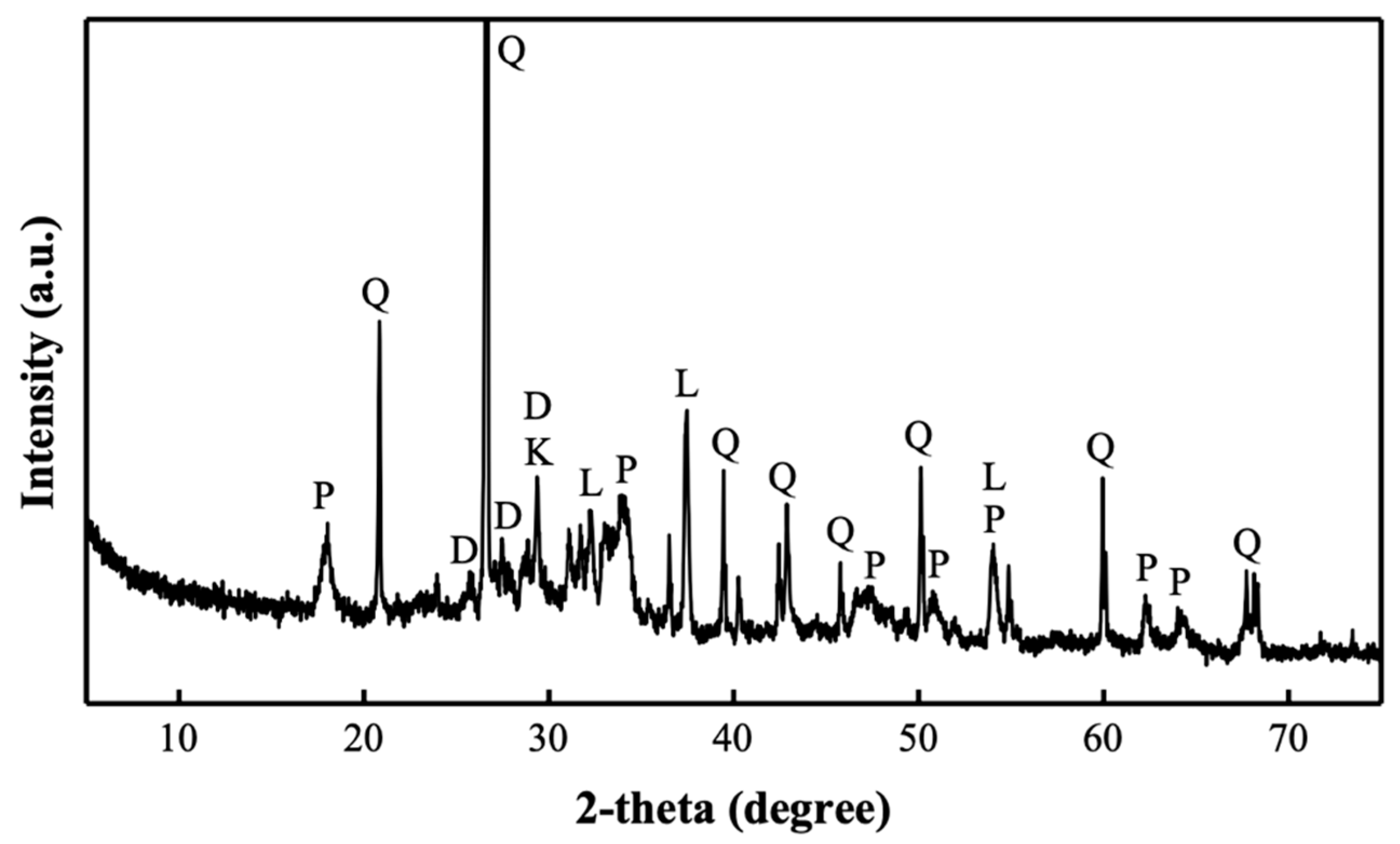
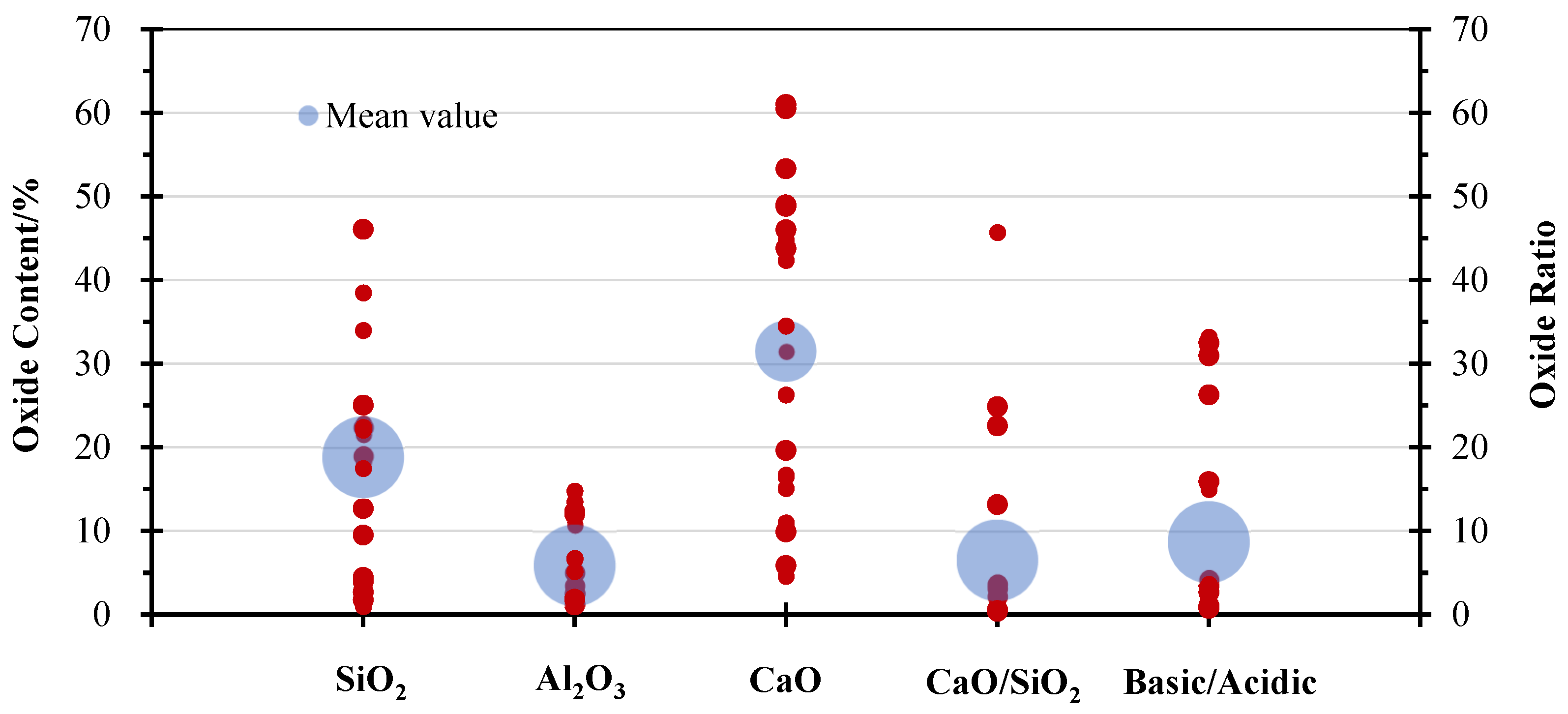



| Ash Category | Biomass Source | Specific Surface Area/(m2/g) | Density/(g/cm3) | Chemical Composition/wt% | CaO/SiO2 Ratio | Basic Oxide/Acidic Oxide Ratio | Mineralogical Composition | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOI | SiO2 | Al2O3 | CaO | MgO | Fe2O3 | Na2O | K2O | P2O5 | ||||||||
| BBA | Forest biomass Jaen, Spain | - | - | 5.58 | 46.10 | 12.04 | 19.65 | 3.71 | 4.78 | 0.78 | 4.59 | 1.12 | 0.43 | 0.77 | Quartz, calcite, lime, aluminosilicates | [58] |
| BBA | Cedar chips Taiwan, China | 0.67 | - | - | 18.33 | 3.88 | 26.29 | 5.04 | 2.48 | - | 11.42 | - | 1.43 | 1.92 | Quartz, calcite, portlandite | [70] |
| BWA | Pine sawdust and chips Cacador, Brazil | 0.30 | 1.86 | 74.31 | 9.51 | 2.67 | 5.87 | 1.69 | 2.65 | - | 1.42 | 0.89 | 0.62 | 1.08 | Quartz, calcite | [27] |
| BWA | Pine sawdust and chips Cacador, Brazil | 0.10 | 2.34 | 31.50 | 25.06 | 12.28 | 9.90 | 2.60 | 8.05 | 1.33 | 3.99 | 1.60 | 0.40 | 0.99 | Quartz, calcite | [27] |
| BWA | Pine sawdust and chips Cacador, Brazil | 0.21 | 2.09 | 58.59 | 21.56 | 10.73 | 4.60 | 1.36 | 7.82 | 0.12 | 3.03 | 0.85 | 0.21 | 0.77 | Quartz, calcite | [27] |
| Treated BFA | Wood pellet plant | 0.44 | 2.7 | - | 3.97 | 1.18 | 53.33 | 8.67 | 1.44 | 0.09 | 4.19 | 5.01 | 13.16 | 15.91 | - | [71] |
| Non-treated BFA | Wood pellet plant | 0.37 | 2.6 | - | 3.22 | 1.07 | 42.38 | 4.87 | 1.31 | 0.11 | 4.57 | 3.23 | 13.43 | 15.01 | - | [71] |
| BBA | Radviliskis, Lithuania | 0.40 | - | 4.06 | 22.40 | 2.51 | 49.00 | 8.29 | 2.18 | 0.28 | 8.69 | 5.05 | 2.19 | 2.67 | Quartz, portlandite, anorthoclase, gehlenite | [72,73,74] |
| BWA | Eucalyptus chips Nestle SA, Brazil | 13.84 | 1.98 | 29.72 | 1.76 | 5.06 | 43.80 | 8.36 | 2.43 | 0.28 | 4.64 | 3.32 | 24.89 | 32.51 | Calcite, lime, portlandite, periclase, sylvite | [65] |
| BFA | Krosno, Poland | 1.88 | 2.58 | 5.88 | 12.70 | 3.50 | 46.05 | 3.70 | 3.40 | - | 9.00 | 4.0 | 3.66 | 4.19 | Portlandite, quartz, calcite, syngenite, sylvite, kalsilite, hydroxylapatite | [75] |
| BFA | Pine chips Vilnius, Lithuania | 0.49 | 0.68 | - | 22.91 | 2.63 | 31.50 | 3.57 | 2.32 | 0.26 | 4.00 | 2.71 | 1.37 | 1.65 | Quartz, portlandite, lime, calcite | [61] |
| BFA | Portugal | - | 3.05 | 6.39 | 38.5 | 14.8 | 16.7 | 3.44 | 5.94 | 1.53 | 5.97 | 1.12 | 0.43 | 0.91 | Mica, quartz, calcite, microcline | [64] |
| BWA | - | - | - | - | 19.00 | 2.51 | 60.50 | 1.97 | 3.28 | - | 4.44 | - | 3.18 | 3.42 | Quartz, calcite | [76] |
| BWA | Timber wood Perak, Malaysia | 6.26 | 2.40 | 18.00 | 2.70 | 1.30 | 61.00 | 8.70 | 1,30 | - | 12.00 | 2.70 | 22.59 | 26.30 | Quartz, calcite, portlandite, mullite, potassium aluminate | [77] |
| BWA | Michoacan state, Mexico | - | - | - | 0.98 | 1.51 | 44.81 | 3.81 | 1.19 | 1.64 | 6.89 | 1.72 | 45.72 | 33.20 | Quartz, calcite | [78] |
| BFA | Portugal | - | - | 2.66 | 34.00 | 13.5 | 16.5 | 3.1 | 5.0 | 1.5 | 5.5 | - | 0.49 | 2.45 | Quartz, calcite | [79] |
| BBA | Olive tree and forest, Jaén, Spain | - | 2.546 | 5.58 | 46.10 | 12.04 | 19.65 | 3.71 | 4.78 | 0.78 | 4.59 | 1.12 | 0.43 | 0.77 | Lime, quartz, calcite, aluminosilicates | [80] |
| BFA | Pitea, Sweden | - | - | 29.70 | 22.40 | 6.75 | 15.10 | 2.69 | 2.62 | 1.46 | 8.25 | 2.81 | 0.49 | 3.64 | Quartz, calcite, arcanite, sylvite | [5] |
| BFA | Olive tree and forest, Jaén, Spain | - | - | 9.99 | 22.08 | 6.65 | 34.54 | 4.77 | 3.64 | 1.91 | 7.99 | 2.33 | 0.43 | 2.08 | Quartz, calcite, alumininosilicate, sylvite | [81] |
| BBA | Driftwood waste from biofuel | - | 2.68 | 12.24 | 4.45 | 1.85 | 48.83 | 6.62 | 5.32 | 0.46 | 10.42 | 2.45 | 0.49 | 3.97 | - | [82] |
| BWA | Wood chips from deciduous trees Arłamów, Poland | - | - | - | 17.50 | 5.16 | 10.99 | 1.56 | 1.48 | - | 17.22 | - | 0.43 | 1.01 | - | [83] |
| Maximum | Maximal value | 13.84 | 3.05 | 74.31 | 46.10 | 14.80 | 61.00 | 8.70 | 8.05 | 1.91 | 17.22 | 5.05 | 45.72 | 33.20 | - | - |
| Minimum | Minimal value | 0.10 | 0.68 | 2.66 | 0.98 | 1.07 | 4.60 | 1.36 | 1.19 | 0.09 | 1.42 | 0.85 | 0.21 | 0.77 | - | - |
| Mean value | Mean value | 1.92 | 2.29 | 21.01 | 18.82 | 5.89 | 31.48 | 4.39 | 3.61 | 0.84 | 6.80 | 2.47 | 6.50 | 8.68 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Pundienė, I.; Pranckevičienė, J.; Kligys, M.; Girskas, G.; Korjakins, A. A Review of Biomass Wood Ash in Alkali-Activated Materials: Treatment, Application, and Outlook. J. Compos. Sci. 2024, 8, 161. https://doi.org/10.3390/jcs8050161
Du Y, Pundienė I, Pranckevičienė J, Kligys M, Girskas G, Korjakins A. A Review of Biomass Wood Ash in Alkali-Activated Materials: Treatment, Application, and Outlook. Journal of Composites Science. 2024; 8(5):161. https://doi.org/10.3390/jcs8050161
Chicago/Turabian StyleDu, Yiying, Ina Pundienė, Jolanta Pranckevičienė, Modestas Kligys, Giedrius Girskas, and Aleksandrs Korjakins. 2024. "A Review of Biomass Wood Ash in Alkali-Activated Materials: Treatment, Application, and Outlook" Journal of Composites Science 8, no. 5: 161. https://doi.org/10.3390/jcs8050161








