Effect of Chitin Nanocrystal Deacetylation on a Nature-Mimicking Interface in Carbon Fiber Composites
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Preparation of Graphene Oxide (GO)
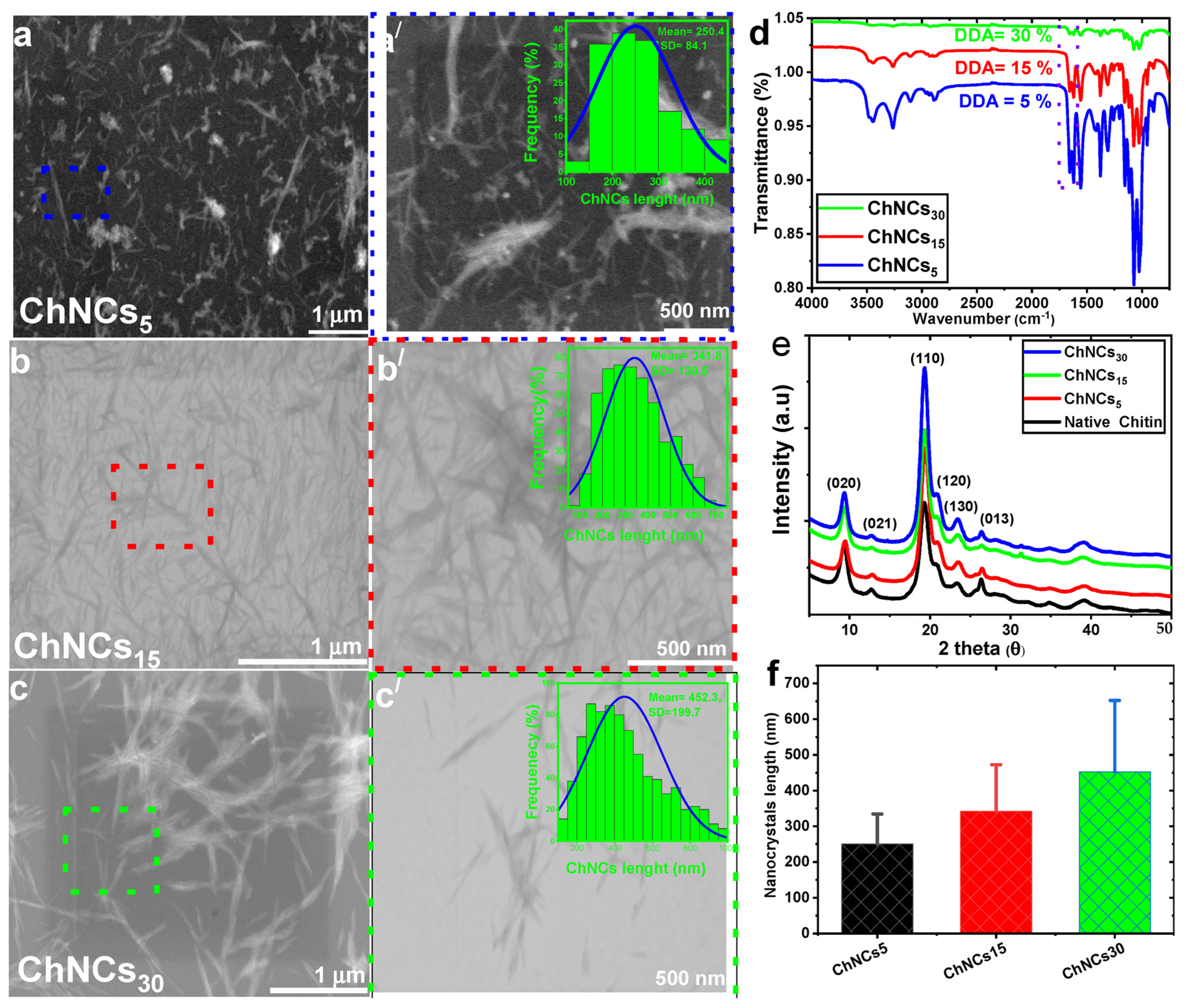
2.3. Preparation of Chitin Nanocrystals (ChNCs)
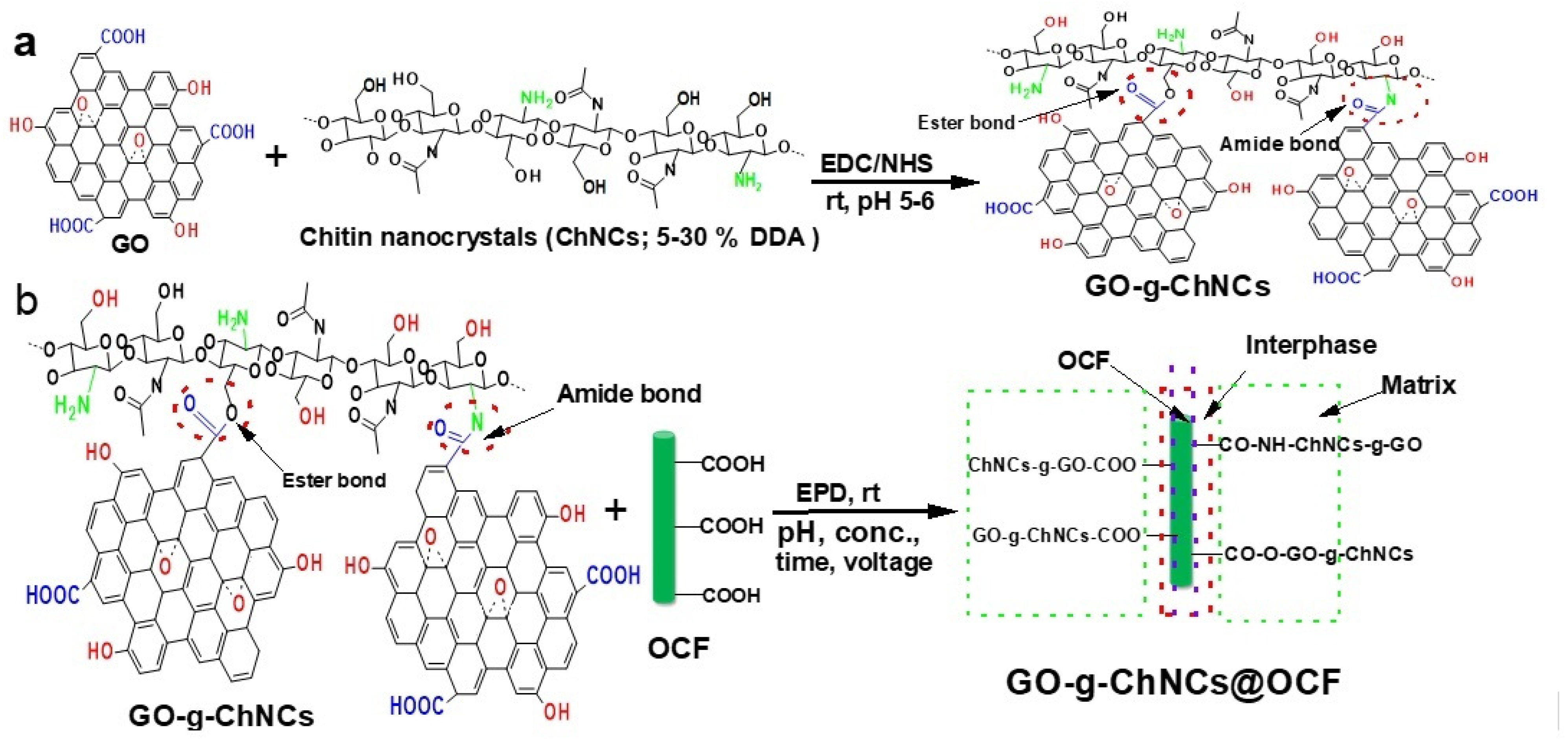
2.4. GO/ChNC Adduct Synthesis
2.5. Electrophoretic Coating
2.6. Characterization of OCF Coated with a GO/CHNC Adduct
3. Results and Discussions
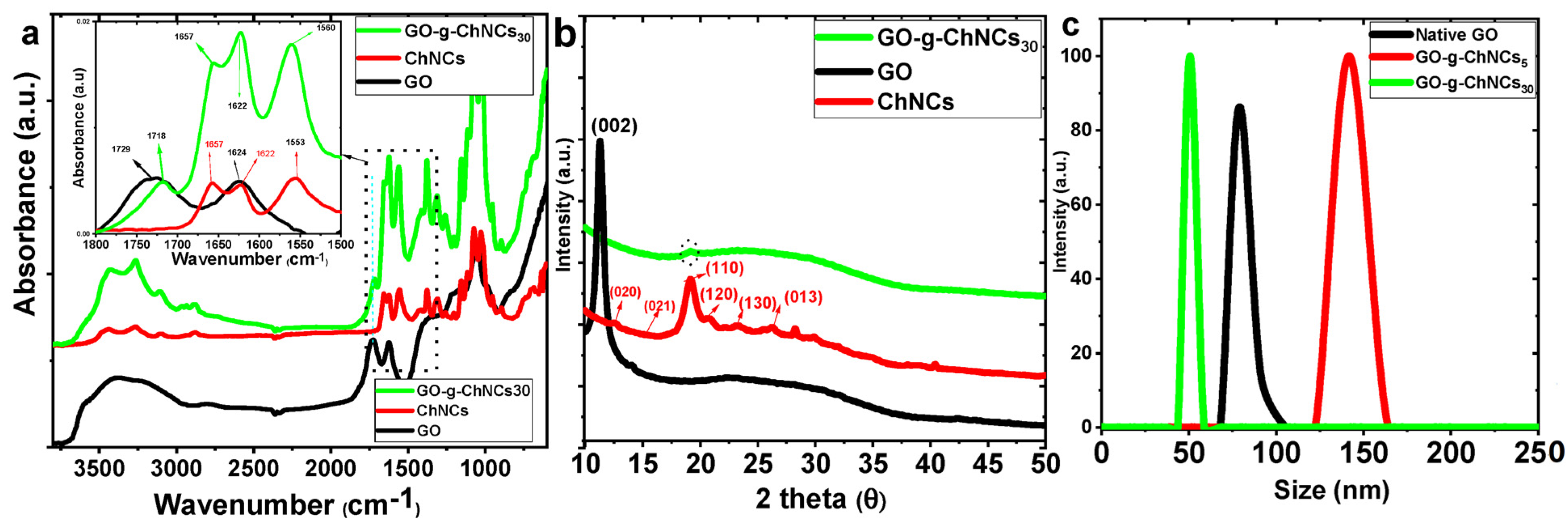
3.1. Effect of DDA on GO/ChNC Adduct Formation
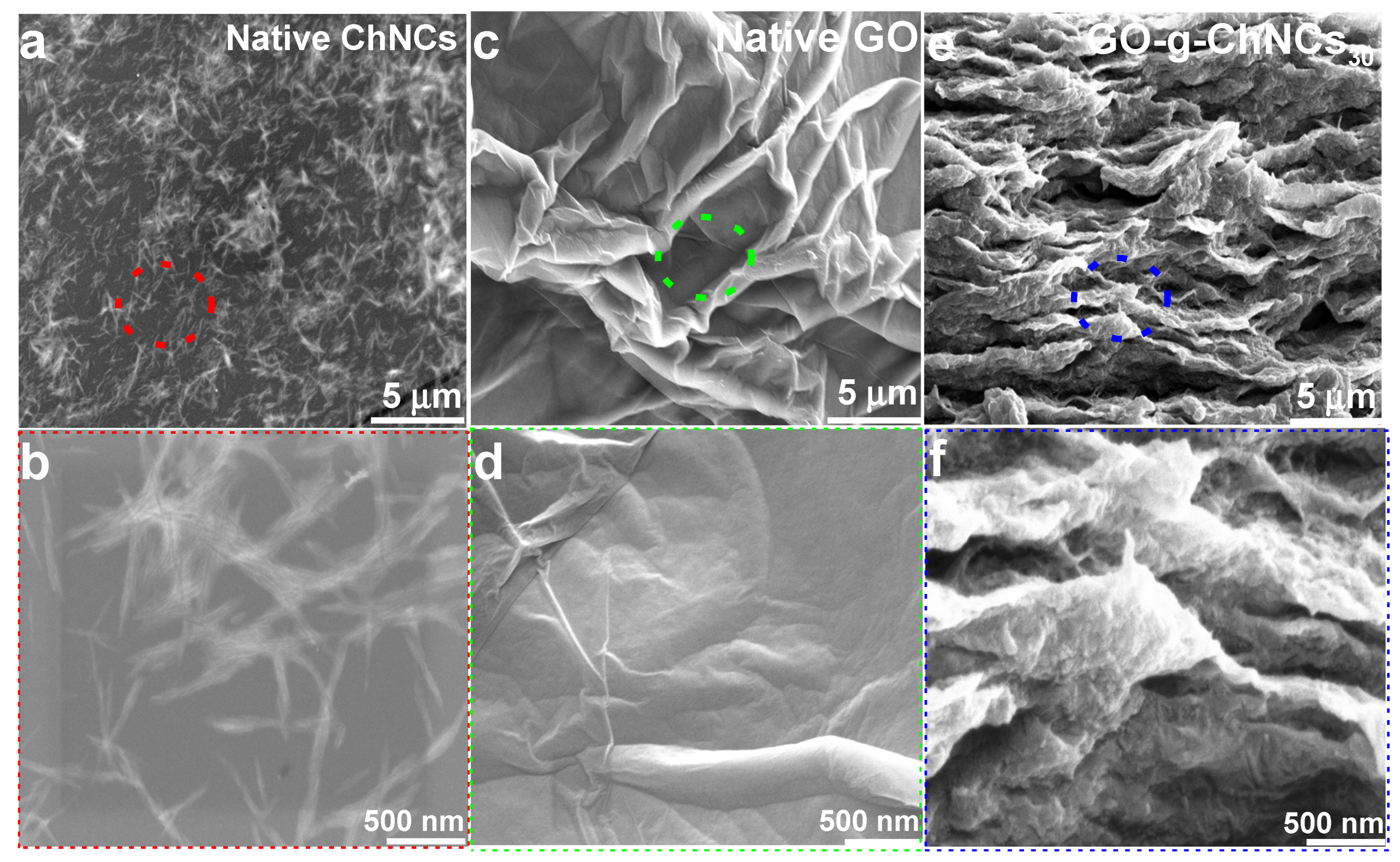
3.2. Effect of the GO/ChNC Adduct Composition and EPD Variations on the Structure of the Coating
3.3. Short-Beam Testing (SBS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Gong, S.; Zhang, Q.; Ming, P.; Wan, S.; Peng, J.; Jiang, L.; Cheng, Q. Graphene-based artificial nacre nanocomposites. Chem. Soc. Rev. 2016, 45, 2378–2395. [Google Scholar] [CrossRef] [PubMed]
- Bonderer, L.J.; Studart, A.R.; Gauckler, L.J. Bioinspired Design and Assembly of Platelet Reinforced Polymer Films. Science 2008, 319, 1069–1073. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Peng, J.; Li, Y.; Hu, H.; Jiang, L.; Cheng, Q. Use of Synergistic Interactions to Fabricate Strong, Tough, and Conductive Artificial Nacre Based on Graphene Oxide and Chitosan. ACS Nano 2015, 9, 9830–9836. [Google Scholar] [CrossRef]
- Wang, R.Z.; Suo, Z.; Evans, A.G.; Yao, N.; Aksay, I.A. Deformation mechanisms in nacre. J. Mater. Res. 2001, 16, 2485–2493. [Google Scholar] [CrossRef]
- Chen, K.; Tang, X.; Yue, Y.; Zhao, H.; Guo, L. Strong and Tough Layered Nanocomposites with Buried Interfaces. ACS Nano 2016, 10, 4816–4827. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Gong, S.; Gao, Y.; Xie, X.; Jiang, L.; Cheng, Q. Bioinspired Ternary Artificial Nacre Nanocomposites Based on Reduced Graphene Oxide and Nanofibrillar Cellulose. ACS Appl. Mater. Interfaces 2016, 8, 10545–10550. [Google Scholar] [CrossRef]
- Yao, K.; Huang, S.; Tang, H.; Xu, Y.; Buntkowsky, G.; Berglund, L.A.; Zhou, Q. Bioinspired Interface Engineering for Moisture Resistance in Nacre-Mimetic Cellulose Nanofibrils/Clay Nanocomposites. ACS Appl. Mater. Interfaces 2017, 9, 20169–20178. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Zhao, R.; Miao, Y.; Liu, Z. A novel graphene-based micro/nano architecture with high strength and conductivity inspired by multiple creatures. Sci. Rep. 2021, 11, 1387. [Google Scholar] [CrossRef]
- Xiong, R.; Kim, H.S.; Zhang, L.; Korolovych, V.F.; Zhang, S.; Yingling, Y.G.; Tsukruk, V.V. Wrapping Nanocellulose Nets around Graphene Oxide Sheets. Angew. Chem. Int. Ed. 2018, 57, 8508–8513. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.-H.; Bergström, L. Transparent and Flexible Nacre-Like Hybrid Films of Aminoclays and Carboxylated Cellulose Nanofibrils. Adv. Funct. Mater. 2018, 28, 1703277. [Google Scholar] [CrossRef]
- Xu, D.; Wang, S.; Berglund, L.A.; Zhou, Q. Surface Charges Control the Structure and Properties of Layered Nanocomposite of Cellulose Nanofibrils and Clay Platelets. ACS Appl. Mater. Interfaces 2021, 13, 4463–4472. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, P.; Walther, A.; Malho, J.-M.; Kainlauri, M.; Ikkala, O.; Linder, M.B. Genetic Engineering of Biomimetic Nanocomposites: Diblock Proteins, Graphene, and Nanofibrillated Cellulose. Angew. Chem. Int. Ed. 2011, 50, 8688–8691. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, C.; Yuan, W.; Shi, G. Strong composite films with layered structures prepared by casting silk fibroin–graphene oxide hydrogels. Nanoscale 2013, 5, 3780–3786. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiong, P.; Mo, M.; Cheng, Y.; Zheng, Y. Electrophoretic-deposited novel ternary silk fibroin/graphene oxide/hydroxyapatite nanocomposite coatings on titanium substrate for orthopedic applications. Front. Mater. Sci. 2016, 10, 270–280. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, K.; Ren, J.; Fan, Y.; Ling, S. Strong, ductile and lightweight bionanocomposites constructed by bioinspired hierarchical assembly. Compos. Commun. 2020, 17, 97–103. [Google Scholar] [CrossRef]
- Tran, T.H.; Nguyen, H.-L.; Hwang, D.S.; Lee, J.Y.; Cha, H.G.; Koo, J.M.; Hwang, S.Y.; Park, J.; Oh, D.X. Five different chitin nanomaterials from identical source with different advantageous functions and performances. Carbohydr. Polym. 2019, 205, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, R.; Abdel-Mohsen, A.; Zboncak, M.; Frankova, J.; Lepcio, P.; Kobera, L.; Steinhart, M.; Pavlinak, D.; Spotaz, Z.; Sklenářévá, R.; et al. Hyaluronan biofilms reinforced with partially deacetylated chitin nanowhiskers: Extraction, fabrication, in-vitro and antibacterial properties of advanced nanocomposites. Carbohydr. Polym. 2020, 235, 115951. [Google Scholar] [CrossRef] [PubMed]
- Kelnar, I.; Kovářová, J.; Tishchenko, G.; Kaprálková, L.; Pavlová, E.; Carezzi, F.; Morganti, P. Chitosan/Chitin nanowhiskers composites: Effect of plasticisers on the mechanical behaviour. J. Polym. Res. 2015, 22, 5. [Google Scholar] [CrossRef]
- Uddin, A.J.; Fujie, M.; Sembo, S.; Gotoh, Y. Outstanding reinforcing effect of highly oriented chitin whiskers in PVA nanocomposites. Carbohydr. Polym. 2012, 87, 799–805. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Sun, S.; Li, X.; Zhao, F.; Jiang, B.; Huang, Y. Electrophoretic deposition of graphene oxide on continuous carbon fibers for reinforcement of both tensile and interfacial strength. Compos. Sci. Technol. 2016, 135, 46–53. [Google Scholar] [CrossRef]
- Jiang, J.; Yao, X.; Xu, C.; Su, Y.; Zhou, L.; Deng, C. Influence of electrochemical oxidation of carbon fiber on the mechanical properties of carbon fiber/graphene oxide/epoxy composites. Compos. Part A Appl. Sci. Manuf. 2017, 95, 248–256. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Tong, L.; Song, Q.; Li, K.; Li, J.; Peng, Q.; He, X.; Wang, R.; Jiao, W.; et al. The role of grafting force and surface wettability in interfacial enhancement of carbon nanotube/carbon fiber hierarchical composites. Carbon 2014, 69, 239–246. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, D.; Liu, L.; Huang, Y.; Long, J.; Wu, G.; Wu, Z.; Umar, A.; Guo, J.; Zhang, X.; et al. Effects of Graphene Oxide Modified Sizing Agents on Interfacial Properties of Carbon Fibers/Epoxy Composites. J. Nanosci. Nanotechnol. 2015, 15, 9807–9811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.L.; Gao, B.; Ma, Q.H.; Zhang, J.; Cui, H.Z.; Liu, L. Directly grafting graphene oxide onto carbon fiber and the effect on the mechanical properties of carbon fiber composites. Mater. Des. 2016, 93, 364–369. [Google Scholar] [CrossRef]
- Qian, H.; Greenhalgh, E.S.; Shaffer, M.S.P.; Bismarck, A. Carbon nanotube-based hierarchical composites: A review. J. Mater. Chem. 2010, 20, 4751–4762. [Google Scholar] [CrossRef]
- Li, Y.; Peng, Q.; He, X.; Hu, P.; Wang, C.; Shang, Y.; Wang, R.; Jiao, W.; Lv, H. Synthesis and characterization of a new hierarchical reinforcement by chemically grafting graphene oxide onto carbon fibers. J. Mater. Chem. 2012, 22, 18748–18752. [Google Scholar] [CrossRef]
- Asadi, A.; Miller, M.; Moon, R.J.; Kalaitzidou, K. Improving the interfacial and mechanical properties of short glass fiber/epoxy composites by coating the glass fibers with cellulose nanocrystals. Express Polym. Lett. 2016, 10, 587–597. [Google Scholar] [CrossRef]
- Uribe, B.E.B.; Chiromito, E.M.S.; Carvalho, A.J.F.; Tarpani, J.R. Low-cost, environmentally friendly route for producing CFRP laminates with microfibrillated cellulose interphase. Express Polym. Lett. 2017, 11, 47–59. [Google Scholar] [CrossRef]
- Reale Batista, M.D.; Drzal, L.T. Carbon fiber/epoxy matrix composite interphases modified with cellulose nanocrystals. Compos. Sci. Technol. 2018, 164, 274–281. [Google Scholar] [CrossRef]
- Lee, J.U.; Park, B.; Kim, B.-S.; Bae, D.-R.; Lee, W. Electrophoretic deposition of aramid nanofibers on carbon fibers for highly enhanced interfacial adhesion at low content. Compos. Part A Appl. Sci. Manuf. 2016, 84, 482–489. [Google Scholar] [CrossRef]
- Zhang, B.; Lian, T.; Shao, X.; Tian, M.; Ning, N.; Zhang, L.; Wang, W. Surface Coating of Aramid Fiber by a Graphene/Aramid Nanofiber Hybrid Material to Enhance Interfacial Adhesion with Rubber Matrix. Ind. Eng. Chem. Res. 2021, 60, 2472–2480. [Google Scholar] [CrossRef]
- Wisnom, M.R. The role of delamination in failure of fibre-reinforced composites. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 1850–1870. [Google Scholar] [CrossRef] [PubMed]
- Gerard, J.-F. Characterization and role of an elastomeric interphase on carbon fibers reinforcing an epoxy matrix. Polym. Eng. Sci. 1988, 28, 568–577. [Google Scholar] [CrossRef]
- Agarwal, B.D.; Bansal, R.K. Effect of an interfacial layer on the properties of fibrous composites: A theoretical analysis. J. Fiber Sci. Technol. 1979, 12, 149–158. [Google Scholar] [CrossRef]
- Lee, S.; Ryu, S. Theoretical study of the effective modulus of a composite considering the orientation distribution of the fillers and the weakened interface. Eur. J. Mech. A/Solids 2018, 72, 79–89. [Google Scholar] [CrossRef]
- Kausar, A.; Siddiq, M. Epoxy composites reinforced with multi-walled carbon nanotube/poly(ethylene glycol)methylether-coated aramid fiber. J. Polym. Eng. 2016, 36, 465–471. [Google Scholar] [CrossRef]
- Yao, X.; Gao, X.; Jiang, J.; Xu, C.; Deng, C.; Wang, J. Comparison of carbon nanotubes and graphene oxide coated carbon fiber for improving the interfacial properties of carbon fiber/epoxy composites. Compos. B Eng. 2018, 132, 170–177. [Google Scholar] [CrossRef]
- Wang, P.; Yang, J.; Liu, W.; Tang, X.-Z.; Zhao, K.; Lu, X.; Xu, S. Tunable crack propagation behavior in carbon fiber reinforced plastic laminates with polydopamine and graphene oxide treated fibers. Mater. Des. 2017, 113, 68–75. [Google Scholar] [CrossRef]
- De Luca, F.; Sernicola, G.; Shaffer, M.S.P.; Bismarck, A. “Brick-and-Mortar” Nanostructured Interphase for Glass-Fiber-Reinforced Polymer Composites. ACS Appl. Mater. Interfaces 2018, 10, 7352–7361. [Google Scholar] [CrossRef]
- Yang, X.; Du, H.; Li, S.; Wang, Z.; Shao, L. Codepositing Mussel-Inspired Nanohybrids onto One-Dimensional Fibers under “Green” Conditions for Significantly Enhanced Surface/Interfacial Properties. ACS Sustain. Chem. Eng. 2018, 6, 4412–4420. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, M.; Shang, L.; Liu, L.; Li, M.; Ao, Y. A nature-inspired interface design strategy of carbon fiber composites by growing brick-and-mortar structure on carbon fiber. Compos. Sci. Technol. 2020, 200, 108382. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, S.; Huang, J.; Zhao, G.; Liu, Y. Interfacial modification of basalt fiber filling composites with graphene oxide and polydopamine for enhanced mechanical and tribological properties. RSC Adv. 2018, 8, 12222–12231. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Kelnar, I.; Kaprálková, L.; Němeček, P.; Dybal, J.; Abdel-Rahman, R.M.; Vyroubalová, M.; Nevoralová, M.; Abdel-Mohsen, A.M. The Effects of the Deacetylation of Chitin Nanowhiskers on the Performance of PCL/PLA Bio-Nanocomposites. Polymers 2023, 15, 3071. [Google Scholar] [CrossRef]
- Kelnar, I.; Kaprálková, L.; Němeček, P.; Janata, M.; Dybal, J.; Svoboda, J.; Padovec, Z.; Abdel-Mohsen, A. Nature-mimicking rigid tough interface in fibrous composites: Effect of polymer/GO combination. Mater. Today Commun. 2022, 33, 104883. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, X.; Ye, Z.; Deng, H.; Zhu, J. Dopamine-dependent graphene oxide modification and its effects on interfacial adhesion of carbon fiber composites. Surf. Interfaces 2022, 31, 102086. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.; Abdel-Rahman, R.; Kubena, I.; Kobera, L.; Spotz, Z.; Zboncak, M.; Prikryl, R.; Brus, J.; Jancar, J. Chitosan-glucan complex hollow fibers reinforced collagen wound dressing embedded with aloe vera. Part I: Preparation and characterization. Carbohydr. Polym. 2020, 230, 115708. [Google Scholar] [CrossRef]
- Aly, A.S.; Abdel-Mohsen, A.M.; Hrdina, R.; Abou-Okeil, A. Preparation and Characterization of Polyethylene Glycol/Dimethyl Siloxane Adduct and Its Utilization as Finishing Agent for Cotton Fabric. J. Nat. Fibers 2011, 8, 176–188. [Google Scholar] [CrossRef]
- Lin, Z.; Ma, Y.; Hu, C.; Zhang, Q. Molecular intercalated graphene oxide with finely controllable interlayer spacing for fast dye separation, Colloids Surf. A: Physicochem. Eng. Asp. 2023, 677, 132437. [Google Scholar] [CrossRef]
- Dong, S.; Wang, B.; Liu, D.; He, M.; Chen, M.; Zhao, J.; Jin, W. Tailoring the interlayer channel structure of graphene oxide membrane with conjugated cationic dyes for butanol dehydration. Sep. Purif. Technol. 2023, 325, 124728. [Google Scholar] [CrossRef]
- Xu, W.L.; Fang, C.; Zhou, F.; Song, Z.; Liu, Q.; Qiao, R.; Yu, M. Self-Assembly: A Facile Way of Forming Ultrathin, High-Performance Graphene Oxide Membranes for Water Purification. Nano Lett. 2017, 17, 2928–2933. [Google Scholar] [CrossRef]
- Zheng, N.; Huang, Y.; Liu, H.-Y.; Gao, J.; Mai, Y.-W. Improvement of interlaminar fracture toughness in carbon fiber/epoxy composites with carbon nanotubes/polysulfone interleaves. Compos. Sci. Technol. 2017, 140, 8–15. [Google Scholar] [CrossRef]
- Riccardi, C.C.; Adabbo, H.E.; Williams, R.J.J. Curing reaction of epoxy resins with diamines. J. Appl. Polym. Sci. 1984, 29, 2481–2492. [Google Scholar] [CrossRef]
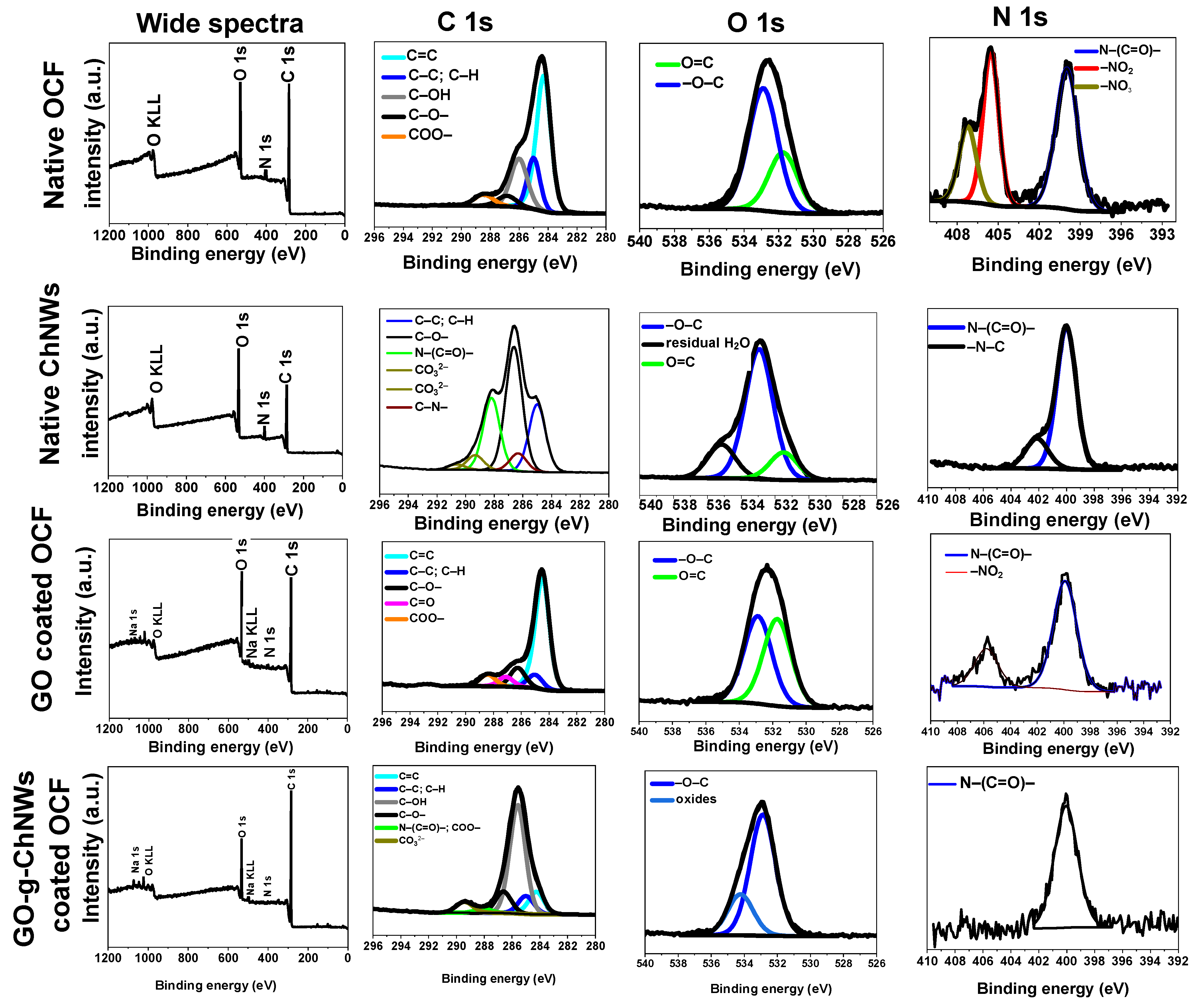
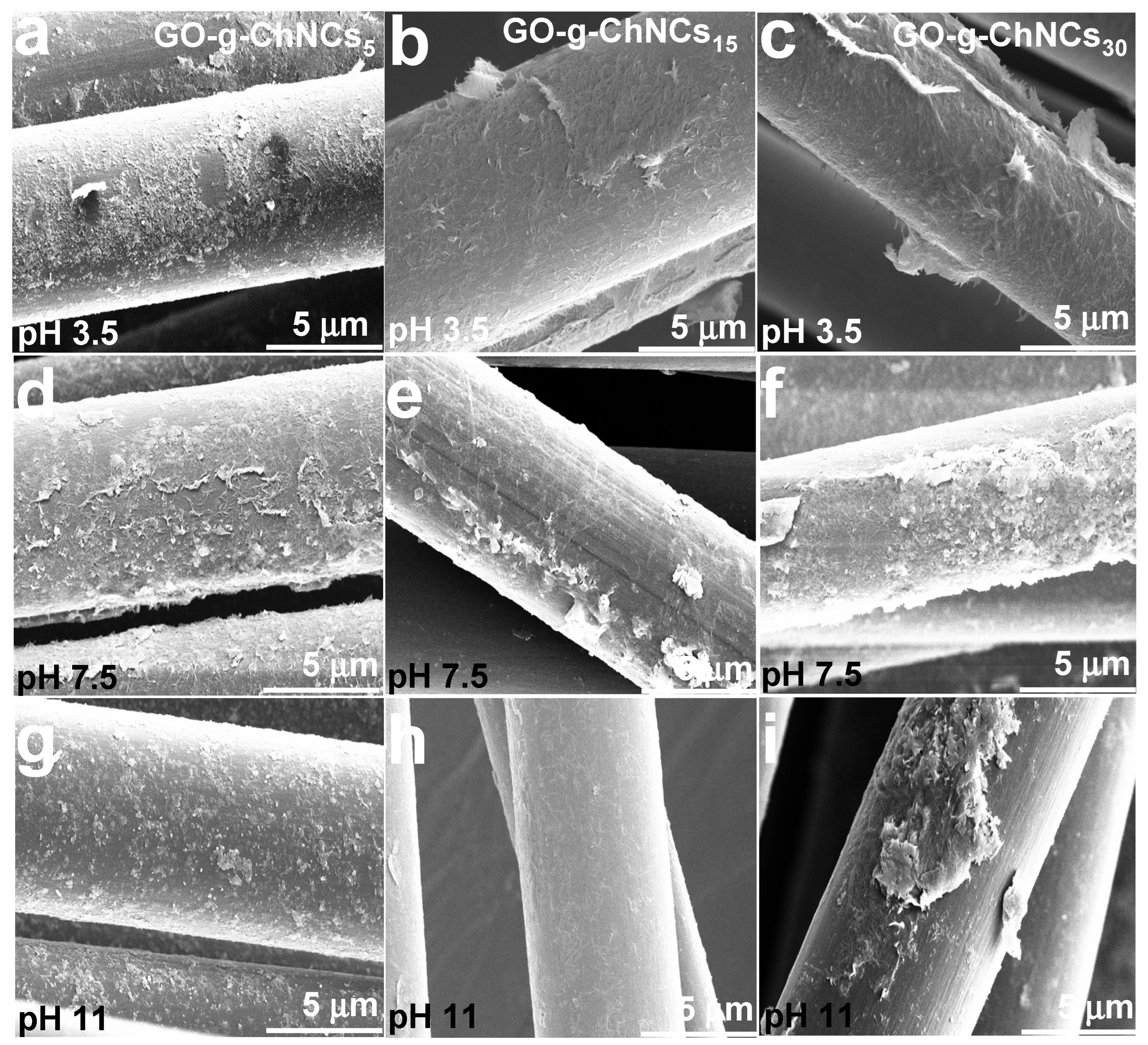
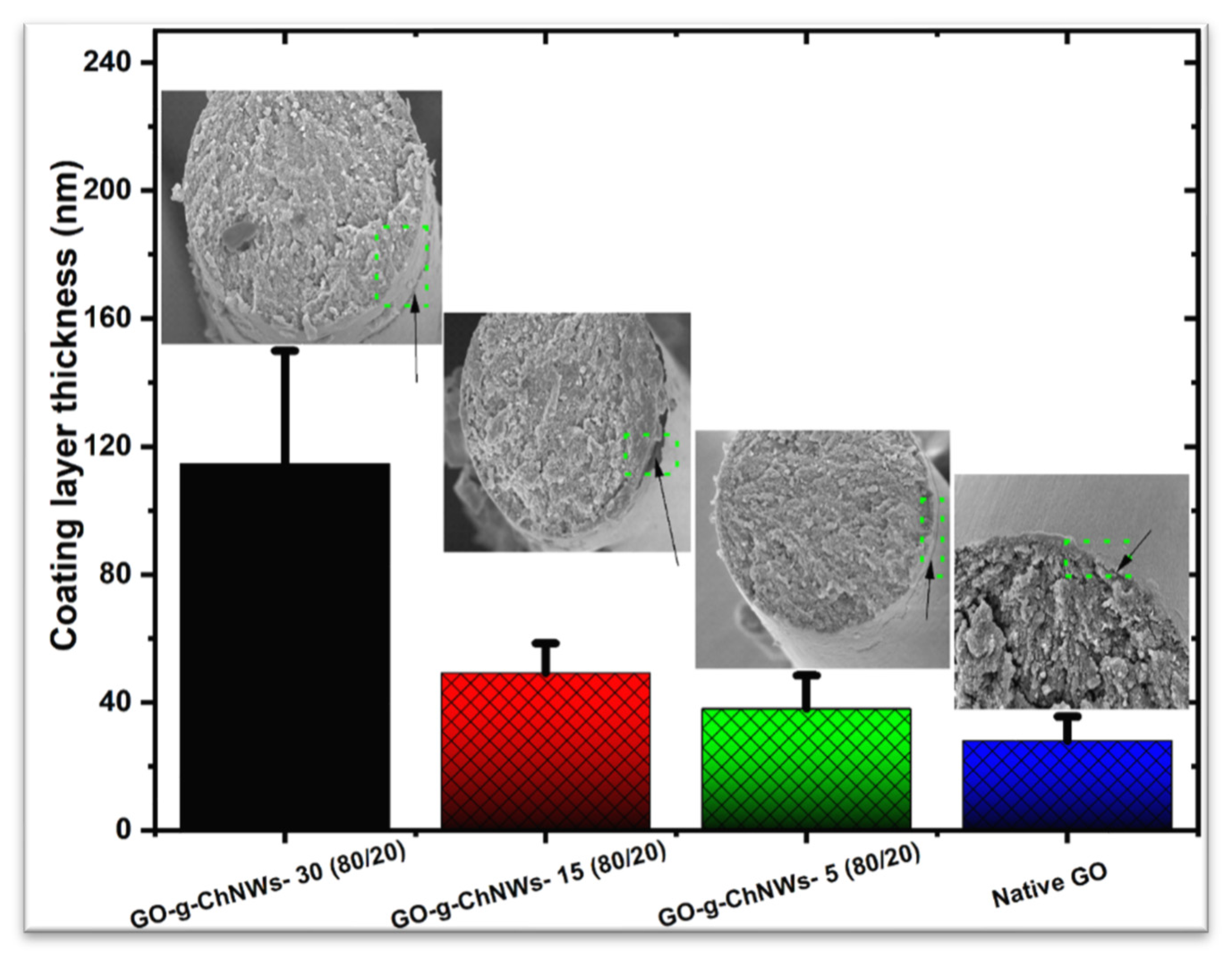


| Number | GO (% wt) | ChNCs (% wt) | DDA of ChNCs * (%) | Abbreviation |
|---|---|---|---|---|
| 1 | 95 | 5 | 5 | GO/ChNCs5 adduct |
| 2 | 90 | 10 | 5 | GO/ChNCs5 adduct |
| 3 | 80 | 20 | 5 | GO/ChNCs5 adduct |
| 4 | 50 | 50 | 5 | GO/ChNCs5 adduct |
| 5 | 95 | 5 | 15 | GO/ChNCs15 adduct |
| 6 | 90 | 10 | 15 | GO/ChNCs15 adduct |
| 7 | 80 | 20 | 15 | GO/ChNCs15 adduct |
| 8 | 50 | 50 | 15 | GO/ChNCs15 adduct |
| 9 | 95 | 5 | 30 | GO/ChNCs30 adduct |
| 10 | 90 | 10 | 30 | GO/ChNCs30 adduct |
| 11 | 80 | 20 | 30 | GO/ChNCs30 adduct |
| 12 | 50 | 50 | 30 | GO/ChNCs30 adduct |
| Adduct | GO (% wt) | ChNCs (% wt) | DDA * (%) | Modulus (MPa) | SBS (MPa) |
|---|---|---|---|---|---|
| GO | 100 | - | - | 6640 ± 950 | 51.8 ± 1.1 |
| GO/ChNCs5 | 95 | 5 | 5 | 8460 ± 450 | 47.8 ± 1.5 |
| GO/ChNCs15 | 95 | 5 | 15 | 8000 ± 50 | 52.2 ± 1.5 |
| GO/ChNCs30 | 95 | 5 | 30 | 7750 ± 250 | 47.4 ± 2.0 |
| GO/ChNCs5 | 90 | 10 | 5 | 8490 ± 120 | 46.7 ± 4.0 |
| GO/ChNCs15 | 90 | 10 | 15 | 8400 ± 240 | 52.4 ± 2.5 |
| GO/ChNCs30 | 90 | 10 | 30 | 9100 ± 460 | 47.5 ± 1.5 |
| GO/ChNCs5 | 80 | 20 | 5 | 7100 ± 150 | 51.8 ± 1.5 |
| GO/ChNCs15 | 80 | 20 | 15 | 8930 ± 850 | 51.9 ± 1.0 |
| GO/ChNCs30 | 80 | 20 | 30 | 6900 ± 100 | 52.1 ± 4.5 |
| GO/ChNCs5 | 50 | 50 | 5 | 7250 ± 550 | 53.2 ± 1.0 |
| GO/ChNCs15 | 50 | 50 | 15 | 6750 ± 100 | 54.9 ± 1.0 |
| GO/ChNCs30 | 50 | 50 | 30 | 7890 ± 120 | 57.2 ± 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Mohsen, A.M.; Abdel-Rahman, R.M.; Kalina, L.; Vishakha, V.; Kaprálková, L.; Němeček, P.; Jančář, J.; Kelnar, I. Effect of Chitin Nanocrystal Deacetylation on a Nature-Mimicking Interface in Carbon Fiber Composites. J. Compos. Sci. 2024, 8, 163. https://doi.org/10.3390/jcs8050163
Abdel-Mohsen AM, Abdel-Rahman RM, Kalina L, Vishakha V, Kaprálková L, Němeček P, Jančář J, Kelnar I. Effect of Chitin Nanocrystal Deacetylation on a Nature-Mimicking Interface in Carbon Fiber Composites. Journal of Composites Science. 2024; 8(5):163. https://doi.org/10.3390/jcs8050163
Chicago/Turabian StyleAbdel-Mohsen, Abdellatif M., Rasha M. Abdel-Rahman, Lukáš Kalina, Vishakha Vishakha, Ludmila Kaprálková, Pavel Němeček, Josef Jančář, and Ivan Kelnar. 2024. "Effect of Chitin Nanocrystal Deacetylation on a Nature-Mimicking Interface in Carbon Fiber Composites" Journal of Composites Science 8, no. 5: 163. https://doi.org/10.3390/jcs8050163






