Effect of Surface Roughness on Early Stage Oxidation Behavior of Ni-Base Superalloy IN 625
Abstract
:1. Introduction
2. Materials and Methods
- (a)
- Image acquisition by optical microscope,
- (b)
- Converting images into binary scale images (alloy—white, resin—black),
- (c)
- Digitalization of binary scale images into 2-D x-y data sets with constant x step,
- (d)
- Performing fractal analysis using Sfrax 1.0 software [16].
- D (Fractal Dimension), given by the Equation (1):D = 1 + |slope|,
- LSFC (Length-Scale Fractal Complexity), given by the Equation (2):LSFC = 1000 (D − 1),
- Relative length at a given scale (LR)
- Smooth-rough crossover (SRC)
3. Results
3.1. Surface Roughness Evaluation
3.1.1. Standard Roughness Evaluation
3.1.2. Microroughness Evaluation Using Fractal Analysis
3.2. Post-Exposure Analyses
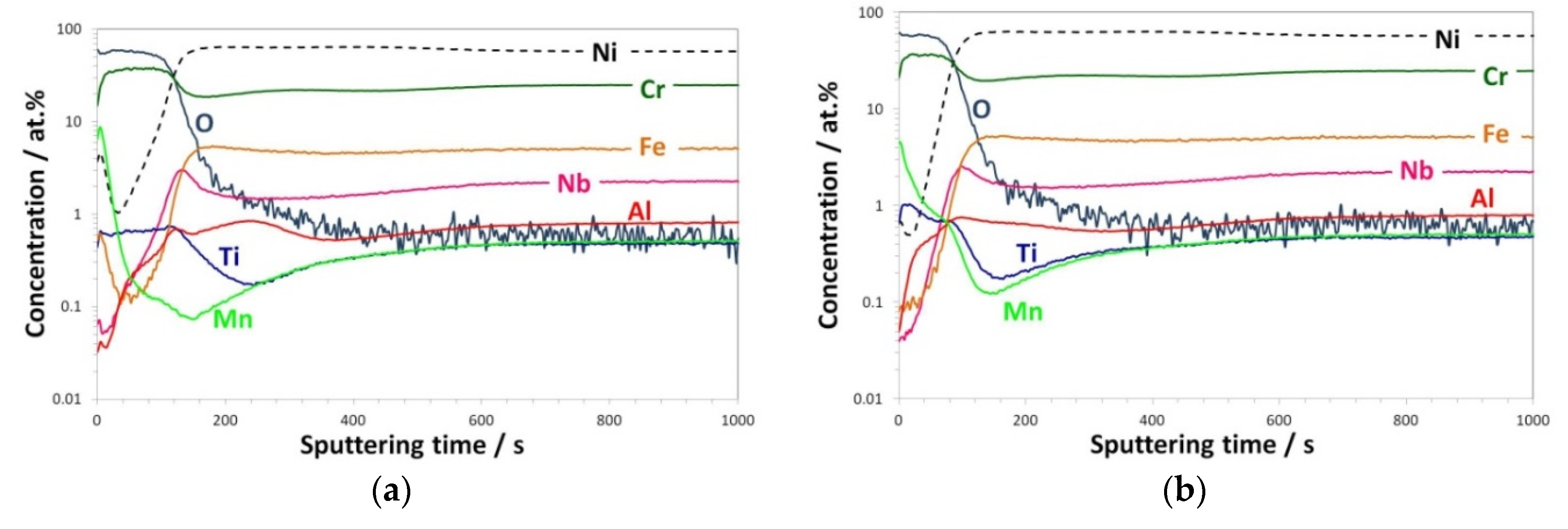
3.2.1. GD-OES Depth Profiles
3.2.2. SEM Analysis
3.2.3. Oxidation Kinetics
4. Discussion
Funding
Conflicts of Interest
References
- Davis, J.R. ASM Specialty Handbook, Heat Resistant Materials; ASM International: Materials Park, OH, USA, 1997. [Google Scholar]
- Giggins, C.S.; Pettit, F.S. Oxidation of Ni-Cr-Al Alloys Between 1000 °C and 1200 °C. J. Electrochem. Soc. Solid State Sci. 1971, 118, 1782–1790. [Google Scholar] [CrossRef]
- Yuan, K.; Eriksson, R.; Peng, R.L.; Li, X.-H.; Johansson, S.; Wang, Y.-D. Modelling of microstructural evolution and lifetime prediction of MCrAlY coatings on nickel based superalloys during high temperature oxidation. Surf. Coat. Technol. 2013, 232, 204–215. [Google Scholar] [CrossRef]
- Pomeroy, M.J. Coatings for gas turbine materials and long term stability issues. Mater. Des. 2013, 26, 223–231. [Google Scholar] [CrossRef]
- Giggins, C.S.; Pettit, F.S. The effect of Alloy Grain-Size and Surface Deformation on the selective Oxidation of Chromium in Ni-Cr Alloys at temperatures of 900 °C and 1100 °C. Trans. Metall. Soc. AIME 1969, 245, 2509–2514. [Google Scholar]
- Leistikow, S.; Wolf, I.; Grabke, H.J. Effect of cold work on the oxidation behavior and carburization resistance of Alloy 800. Werkstoffe und Korrosion 1987, 38, 556–562. [Google Scholar] [CrossRef]
- Sudbrack, C.K.; Beckett, D.L.; Mackay, R.A. Effect of Surface Preparation on the 815 °C Oxidation of Single Crystal Ni-Based Superalloys. JOM 2015, 67, 2589–2598. [Google Scholar] [CrossRef]
- Nowak, W.J.; Wierzba, B.; Sieniawski, J. Surface preparation effect on oxidation kinetics of Ni-base superalloy. J. Phys. Conf. Ser. 2018, 936, 012002. [Google Scholar] [CrossRef]
- Garcia-Fresnillo, L.; Chyrkin, A.; Böhme, C.; Barnikel, J.; Schmitz, F.; Quadakkers, W.J. Oxidation behaviour and microstructural stability of alloy 625 during long-term exposure in steam. J. Mater. Sci. 2014, 49, 6127–6142. [Google Scholar] [CrossRef]
- Staszewska, K.; Scendo, M. Mechanism and Kinetics Oxidation of Inconel 617 and 625 Alloys; Technical Issues; ADVSEO sp. z o.o.: Szczecin, Poland, 2016; pp. 82–89. [Google Scholar]
- Kumar, L.; Venkataramani, R.; Sundararaman, M.; Mukhopadhayay, P.; Garg, S.P. Studies on the Oxidation Behavior of Inconel 625 Between 873 and 1523 K. Oxid. Met. 1996, 45, 221–244. [Google Scholar] [CrossRef]
- Vesel, A.; Drenik, A.; Elersic, K.; Mozetic, M.; Kovac, J.; Gyergyek, T.; Stockel, J.; Varju, J.; Panek, R.; Balat-Pichelin, M. Oxidation of Inconel 625 superalloy upon treatment with oxygen or hydrogen plasma at high temperature. Appl. Surf. Sci. 2014, 305, 674–682. [Google Scholar] [CrossRef]
- Gademawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- PN-EN ISO 4287:1999—Wersja Polska. Specyfikacje Geometrii Wyrobów—Struktura Geometryczna Powierzchni: Metoda Profilowa—Terminy, Definicje I Parametry Struktury Geometrycznej Powierzchni; Polski Komitet Normalizacyjny: Warszawa, Poland, 1999. [Google Scholar]
- Nowak, W.; Naumenko, D.; Mor, G.; Mor, F.; Mack, D.E.; Vassen, R.; Singheiser, L.; Quadakkers, W.J. Effect of processing parameters on MCrAlY bondcoat roughness and lifetime of APS-TBC systems. Surf. Coat. Technol. 2014, 260, 82–89. [Google Scholar] [CrossRef]
- Sfrax 1.0. Available online: http://www.surfract.com/sfrax.html (accessed on 28 June 2018).
- Brown, C.A.; Savary, G. Describing ground surface texture using contact profilometry and fractal analysis. Wear 1991, 141, 211–226. [Google Scholar] [CrossRef]
- Brown, C.A.; Johnsen, W.A.; Butland, R.M. Scale-Sensitive Fractal Analysis of Turned Surfaces. Ann. CIRP 1996, 45, 515–518. [Google Scholar] [CrossRef]
- Pfeifer, J.P.; Holzbrecher, H.; Quadakkers, W.J.; Speier, J. Quantitative analysis of oxide films on ODS-alloys using MCs+-SIMS and e-beam SNMS. J. Anal. Chem. 1993, 346, 186–191. [Google Scholar] [CrossRef]
- Quadakkers, W.J.; Elschner, A.; Speier, W.; Nickel, H. Composition and growth mechanisms of alumina scales on FeCrAl-based alloys determined by SNMS. App. Surf. Sci. 1991, 52, 271–287. [Google Scholar] [CrossRef]
- Nowak, W.J. Characterization of oxidized Ni-base superalloys by GD-OES. JAAS 2017, 32, 1730–1738. [Google Scholar] [CrossRef]
- Kear, B.H.; Pettit, F.S.; Fornwalt, D.E.; Lemaire, L.P. On the Transient Oxidation of a Ni-15Cr-6Al Alloy. Oxid. Met. 1971, 3, 557–569. [Google Scholar] [CrossRef]
- Jalowicka, A.; Nowak, W.; Naumenko, D.; Singheiser, L.; Quadakkers, W.J. Effect of nickel base superalloys composition on oxidation resistance in SO2 containing, high pO2 environments. Mater. Corros. 2014, 65, 178–187. [Google Scholar] [CrossRef]
- Nowak, W.J.; Wierzba, B.; Sieniawski, J. Effect of Ti and Ta on oxidation kinetic of chromia forming Ni-base superalloys in Ar-O2 based atmosphere. High Temp. Mater. Process. 2018. [Google Scholar] [CrossRef]
- Wierzba, B.; Skibiński, W. The interdiffusion in copper-nickel alloys. J. Alloys Compd. 2016, 687, 104–108. [Google Scholar] [CrossRef]




| Elements (wt %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ni | Cr | Co | Mo | Nb | Al | Ti | Fe | Mn | C |
| BASE | 21.5 | 1 | 9 | 3.65 | 0.4 | 0.4 | 5 | 0.5 | 0.1 |
| Parameter | Polishing (1 μm) | Grinding (220 grit) | ||
|---|---|---|---|---|
| Average Value | Standard Deviation | Average Value | Standard Deviation | |
| Ra | 0.039 | 0.007 | 1.067 | 0.065 |
| Rz | 0.227 | 0.021 | 7.632 | 0.547 |
| Rmax | 0.288 | 0.056 | 9.189 | 0.800 |
| Parameter | Polishing (1 µm) | Grinding (220 grit) |
|---|---|---|
| D | 1.0018 | 1.0084 |
| LSFC | 1.8 | 8.4 |
| LR 1 µm | 1.02 | 1.05 |
| SRC | 15.8 | 12.7 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, W.J. Effect of Surface Roughness on Early Stage Oxidation Behavior of Ni-Base Superalloy IN 625. Appl. Syst. Innov. 2018, 1, 32. https://doi.org/10.3390/asi1030032
Nowak WJ. Effect of Surface Roughness on Early Stage Oxidation Behavior of Ni-Base Superalloy IN 625. Applied System Innovation. 2018; 1(3):32. https://doi.org/10.3390/asi1030032
Chicago/Turabian StyleNowak, Wojciech J. 2018. "Effect of Surface Roughness on Early Stage Oxidation Behavior of Ni-Base Superalloy IN 625" Applied System Innovation 1, no. 3: 32. https://doi.org/10.3390/asi1030032





