Hot-Moments of Soil CO2 Efflux in a Water-Limited Grassland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Instrumentation
2.2.1. Eddy Covariance
2.2.2. Soil CO2 Efflux (Fs)
2.3. Empirical Modeling of Soil CO2 Efflux (Fs)
2.4. Time Series Analyses
3. Results
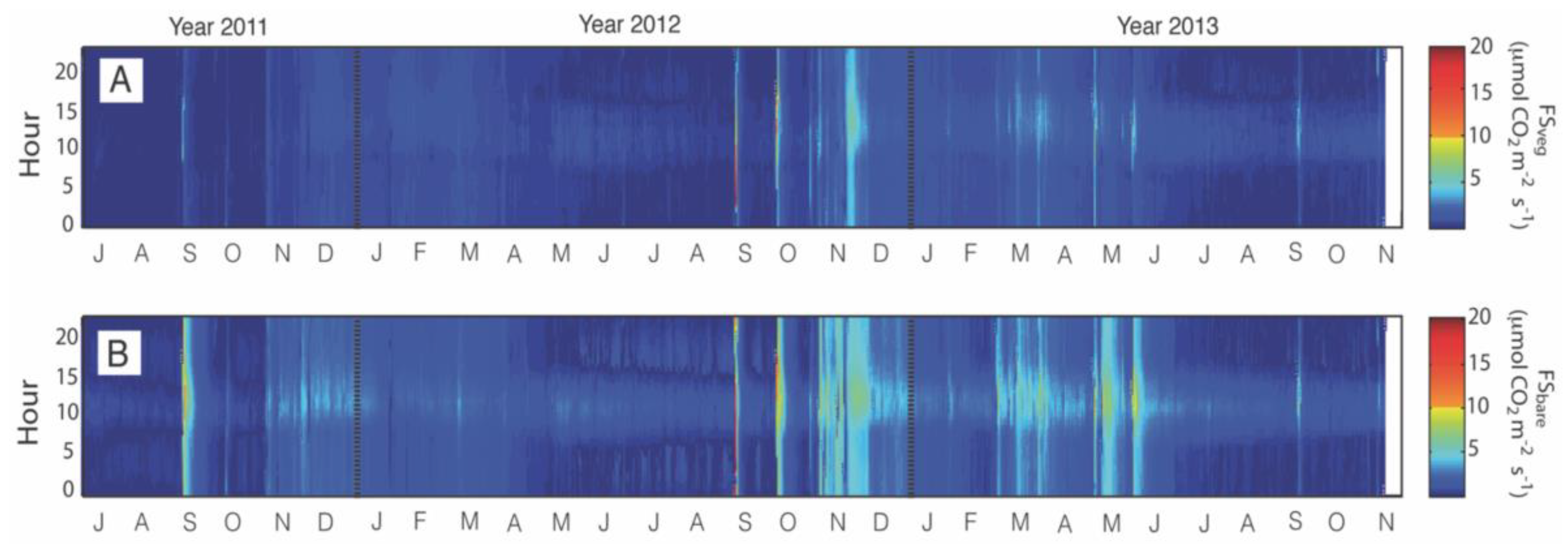
3.1. Description of Temporal Patterns
3.2. Influence of Soil Temperature and Soil Moisture on Fs
3.3. Modeling Daily Fs
3.3.1. Model 1
3.3.2. Support Vector Machine (SVM)
3.3.3. Model 2
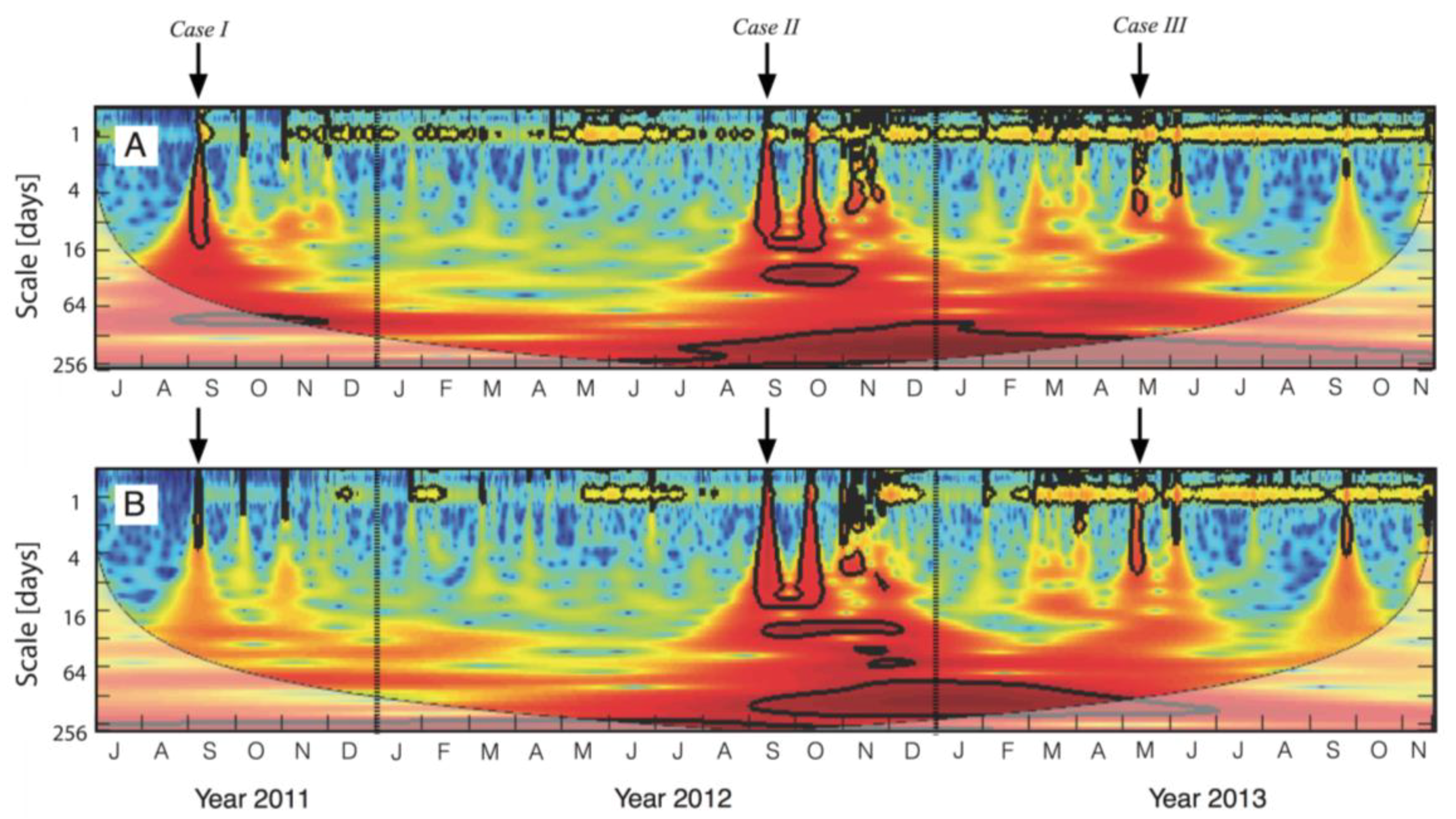
3.4. Responses to Different Precipitation Pulses
4. Discussion
4.1. Can We Model Daily Fs Using Temperature and Soil Moisture Information?
4.2. How Discrete PPs Influence Hot-Moments of Fs?
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poulter, B.; Frank, D.; Ciais, P.; Myneni, R.B.; Andela, N.; Bi, J.; Broquet, G.; Canadell, J.G.; Chevallier, F.; Liu, Y.Y.; et al. Contribution of semi-arid ecosystems to interannual variability of the global carbon cycle. Nature 2014, 509, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Ahlstrom, A.; Raupach, M.R.; Schurgers, G.; Smith, B.; Arneth, A.; Jung, M.; Reichstein, M.; Canadell, J.G.; Friedlingstein, P.; Jain, A.K.; et al. The dominant role of semi-arid ecosystems in the trend and variability of the land CO2 sink. Science 2015, 348, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Huxman, T.E.; Smith, M.D.; Fay, P.A.; Knapp, A.K.; Shaw, M.R.; Loik, M.E.; Smith, S.D.; Tissue, D.T.; Zak, J.C.; Weltzin, J.F.; et al. Convergence across biomes to a common rain-use efficiency. Nature 2004, 429, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Sala, O.E.; Parton, W.J.; Joyce, L.A.; Lauenroth, W.K. Primary production of the central grassland region of the united-states. Ecology 1988, 69, 40–45. [Google Scholar] [CrossRef]
- Knapp, A.K.; Smith, M.D. Variation among biomes in temporal dynamics of aboveground primary production. Science 2001, 291, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Noy-Meir, I. Desert ecosystems: Environment and producers. Annu. Rev. Ecol. Syst. 1973, 4, 25–51. [Google Scholar] [CrossRef]
- Reynolds, J.F.; Kemp, P.R.; Ogle, K.; Fernandez, R.J. Modifying the ‘pulse-reserve’ paradigm for deserts of north america: Precipitation pulses, soil water, and plant responses. Oecologia 2004, 141, 194–210. [Google Scholar] [CrossRef] [PubMed]
- Huxman, T.E.; Snyder, K.A.; Tissue, D.; Leffler, A.J.; Ogle, K.; Pockman, W.T.; Sandquist, D.R.; Potts, D.L.; Schwinning, S. Precipitation pulses and carbon fluxes in semiarid and arid ecosystems. Oecologia 2004, 141, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Thomey, M.L.; Collins, S.L.; Vargas, R.; Johnson, J.E.; Brown, R.F.; Natvig, D.O.; Friggens, M.T. Effect of precipitation variability on net primary production and soil respiration in a chihuahuan desert grassland. Glob. Chang. Biol. 2011, 17, 1505–1515. [Google Scholar] [CrossRef]
- Xu, L. Seasonal variation in carbon dioxide exchange over a mediterranean annual grassland in California. Agric. For. Meteorol. 2004, 123, 79–96. [Google Scholar] [CrossRef]
- Ogle, K.; Reynolds, J.F. Plant responses to precipitation in desert ecosystems: Integrating functional types, pulses, thresholds, and delays. Oecologia 2004, 141, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Sala, O.E.; Lauenroth, W.K. Small rainfall events: An ecological role in semi-arid regions. Oecologia 1982, 53, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Schwinning, S.; Sala, O. Hierarchy of responses to resource pulses in arid and semi-arid ecosystems. Oecologia 2004, 141, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Curiel Yuste, J.; Baldocchi, D.D.; Gershenson, A.; Goldstein, A.; Misson, L.; Wong, S. Microbial soil respiration and its dependency on carbon inputs, soil temperature and moisture. Glob. Chang. Biol. 2007, 13, 2018–2035. [Google Scholar] [CrossRef] [Green Version]
- Vargas, R.; Carbone, M.S.; Reichstein, M.; Baldocchi, D.D. Frontiers and challenges in soil respiration research: From measurements to model-data integration. Biogeochemistry 2011, 102, 1–13. [Google Scholar] [CrossRef]
- Baldocchi, D.; Tang, J.W.; Xu, L.K. How switches and lags in biophysical regulators affect spatial-temporal variation of soil respiration in an oak-grass savanna. J. Geophys. Res. Biogeosci. 2006, 111. [Google Scholar] [CrossRef] [Green Version]
- Christensen, J.H.; Hewitson, B.; Busuioc, A.; Chen, A.; Gao, X.; Held, R.; Jones, R.; Kolli, R.K.; Kwon, W.K.; Laprise, R.; et al. Regional climate projections, climate change, 2007: The physical science basis. In Contribution of Working Group i to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; University Press: Cambridge, UK, 2007; pp. 847–940. [Google Scholar]
- Vargas, R.; Collins, S.L.; Thomey, M.L.; Johnson, J.E.; Brown, R.F.; Natvig, D.O.; Friggens, M.T. Precipitation variability and fire influence the temporal dynamics of soil CO2 efflux in an arid grassland. Glob. Chang. Biol. 2012, 18, 1401–1411. [Google Scholar] [CrossRef]
- Heisler-White, J.L.; Knapp, A.K.; Kelly, E.F. Increasing precipitation event size increases aboveground net primary productivity in a semi-arid grassland. Oecologia 2008, 158, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Vicca, S.; Bahn, M.; Estiarte, M.; van Loon, E.E.; Vargas, R.; Alberti, G.; Ambus, P.; Arain, M.A.; Beier, C.; Bentley, L.P.; et al. Can current moisture responses predict soil CO2 efflux under altered precipitation regimes? A synthesis of manipulation experiments. Biogeosciences 2014, 11, 2991–3013. [Google Scholar] [CrossRef] [Green Version]
- Knapp, A.K.; Avolio, M.L.; Beier, C.; Carroll, C.J.; Collins, S.L.; Dukes, J.S.; Fraser, L.H.; Griffin-Nolan, R.J.; Hoover, D.L.; Jentsch, A.; et al. Pushing precipitation to the extremes in distributed experiments: Recommendations for simulating wet and dry years. Glob. Chang. Biol. 2017, 23, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Anderson-Teixeira, K.J.; Delong, J.P.; Fox, A.M.; Brese, D.A.; Litvak, M.E. Differential responses of production and respiration to temperature and moisture drive the carbon balance across a climatic gradient in new mexico. Glob. Chang. Biol. 2011, 17, 410–424. [Google Scholar] [CrossRef]
- Huxman, T.E.; Cable, J.M.; Ignace, D.D.; Eilts, J.A.; English, N.B.; Weltzin, J.; Williams, D.G. Response of net ecosystem gas exchange to a simulated precipitation pulse in a semi-arid grassland: The role of native versus non-native grasses and soil texture. Oecologia 2004, 141, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.K.; Baldocchi, D.D.; Tang, J.W. How soil moisture, rain pulses, and growth alter the response of ecosystem respiration to temperature. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef] [Green Version]
- Jenerette, G.D.; Scott, R.L.; Huxman, T.E. Whole ecosystem metabolic pulses following precipitation events. Funct. Ecol. 2008, 22, 924–930. [Google Scholar] [CrossRef] [Green Version]
- Leon, E.; Vargas, R.; Bullock, S.H.; Lopez, E.; Panosso, A.R.; La Scala, N. Hot spots, hot moments and spatio-temporal controls on soil CO2 efflux in a water-limited ecosystem. Soil Biol. Biogeochem. 2014, 77, 12–21. [Google Scholar] [CrossRef]
- López-Ballesteros, A.; Serrano-Ortiz, P.; Sánchez-Cañete, E.v.; Oyonarte, C.; Kowalski, A.S.; Pérez-Priego, Ó.; Domingo, F. Rain pulses enhance the net CO2 release of a semi-arid grassland in se spain. J. Geophys. Res. Biogeosci. 2015. [Google Scholar] [CrossRef]
- Yan, L.M.; Chen, S.P.; Huang, J.H.; Lin, G.H. Water regulated effects of photosynthetic substrate supply on soil respiration in a semiarid steppe. Glob. Chang. Biol. 2011, 17, 1990–2001. [Google Scholar] [CrossRef]
- Kim, D.G.; Vargas, R.; Bond-Lamberty, B.; Turetsky, M.R. Effects of soil rewetting and thawing on soil gas fluxes: A review of current literature and suggestions for future research. Biogeosciences 2012, 9, 2459–2483. [Google Scholar] [CrossRef] [Green Version]
- Ogle, K.; Barber, J.J.; Barron-Gafford, G.A.; Bentley, L.P.; Young, J.M.; Huxman, T.E.; Loik, M.E.; Tissue, D.T. Quantifying ecological memory in plant and ecosystem processes. Ecol. Lett. 2015, 18, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Potts, D.L.; Huxman, T.E.; Enquist, B.J.; Weltzin, J.F.; Williams, D.G. Resilience and resistance of ecosystem functional response to a precipitation pulse in a semi-arid grassland. J. Ecol. 2006, 94, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Austin, A.T.; Vivanco, L. Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 2006, 442, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.Y.; Baldocchi, D.D.; Hatala, J.A.; Detto, M.; Yuste, J.C. Are rain-induced ecosystem respiration pulses enhanced by legacies of antecedent photodegradation in semi-arid environments? Agric. For. Meteorol. 2012, 154, 203–213. [Google Scholar] [CrossRef]
- Petrakis, S.; Seyfferth, A.; Kan, J.J.; Inamdar, S.; Vargas, R. Influence of experimental extreme water pulses on greenhouse gas emissions from soils. Biogeochemistry 2017, 133, 147–164. [Google Scholar] [CrossRef]
- Almagro, M.; Lopez, J.; Querejeta, J.I.; Martinez-Mena, M. Temperature dependence of soil CO2 efflux is strongly modulated by seasonal patterns of moisture availability in a mediterranean ecosystem. Soil Biol. Biochem. 2009, 41, 594–605. [Google Scholar] [CrossRef]
- Moyano, F.E.; Vasilyeva, N.; Bouckaert, L.; Cook, F.; Craine, J.; Yuste, J.C.; Don, A.; Epron, D.; Formanek, P.; Franzluebbers, A.; et al. The moisture response of soil heterotrophic respiration: Interaction with soil properties. Biogeosciences 2012, 9, 1173–1182. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Ortiz, P.; Oyonarte, C.; Perez-Priego, O.; Reverter, B.R.; Sanchez-Canete, E.P.; Were, A.; Ucles, O.; Morillas, L.; Domingo, F. Ecological functioning in grass-shrub mediterranean ecosystems measured by eddy covariance. Oecologia 2014, 175, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Aubinet, M.; Grelle, A.; Ibrom, A.; Rannik, U.; Moncrieff, J.; Foken, T.; Kowalski, A.S.; Martin, P.H.; Berbigier, P.; Bernhofer, C.; et al. Estimates of the annual net carbon and water exchange of forests: The euroflux methodology. Adv. Ecol. Res. 2000, 30, 113–175. [Google Scholar]
- Reichstein, M.; Falge, E.; Baldocchi, D.; Papale, D.; Aubinet, M.; Berbigier, P.; Bernhofer, C.; Buchmann, N.; Gilmanov, T.; Granier, A.; et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: Review and improved algorithm. Glob. Chang. Biol. 2005, 11, 1424–1439. [Google Scholar] [CrossRef]
- Sanchez-Canete, E.P.; Kowalski, A.S. Comment on “using the gradient method to determine soil gas flux: A review” by m. Maier and h. Schack-kirchner. Agric. For. Meteorol. 2014, 197, 254–255. [Google Scholar] [CrossRef]
- Puigdefabregas, J.; Sole, A.; Gutierrez, L.; del Barrio, G.; Boer, M. Scales and processes of water and sediment redistribution in drylands: Results from the rambla honda field site in southeast spain. Earth-Sci. Rev. 1999, 48, 39–70. [Google Scholar] [CrossRef]
- Oyonarte, C.; Rey, A.; Raimundo, J.; Miralles, I.; Escribano, P. The use of soil respiration as an ecological indicator in arid ecosystems of the se of spain: Spatial variability and controlling factors. Ecol. Indic. 2012, 14, 40–49. [Google Scholar] [CrossRef]
- Rey, A.; Pegoraro, E.; Oyonarte, C.; Were, A.; Escribano, P.; Raimundo, J. Impact of land degradation on soil respiration in a steppe (Stipa tenacissima L.) semi-arid ecosystem in the se of spain. Soil Biol. Biochem. 2011, 43, 393–403. [Google Scholar] [CrossRef]
- Fan, J.; Jones, S.B. Soil surface wetting effects on gradient-based estimates of soil carbon dioxide efflux. Vadose Zone J. 2014, 13. [Google Scholar] [CrossRef]
- Jones, H.G. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology; Cambridge University Press: New York, NY, USA, 1992; p. 51. [Google Scholar]
- Moldrup, P.; Olesen, T.; Schjonning, P.; Yamaguchi, T.; Rolston, D.E. Predicting the gas diffusion coefficient in undisturbed soil from soil water characteristics. Soil Sci. Soc. Am. J. 2000, 64, 94–100. [Google Scholar] [CrossRef]
- Vargas, R.; Allen, M.F. Dynamics of fine root, fungal rhizomorphs, and soil respiration in a mixed temperate forest: Integrating sensors and observations. Vadose Zone J. 2008, 7, 1055–1064. [Google Scholar] [CrossRef]
- Barron-Gafford, G.A.; Scott, R.L.; Jenerette, G.D.; Huxman, T.E. The relative controls of temperature, soil moisture, and plant functional group on soil CO2 efflux at diel, seasonal, and annual scales. J. Geophys. Res. 2011, 116. [Google Scholar] [CrossRef]
- Burges, C.J.C. A tutorial on support vector machines for pattern recognition. Data Min. Knowl. Discov. 1998, 2, 121–167. [Google Scholar] [CrossRef]
- Smola, A.J.; Scholkopf, B. A tutorial on support vector regression. Stat. Comput. 2004, 14, 199–222. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, C.E.; Williams, C.K.I. Gaussian Processes for Machine Learning; Adaptive Computation and Machine Learning Series; The MIT Press: Cambridge, MA, USA, 2005; pp. 1–247. [Google Scholar]
- Keerthi, S.S.; Lin, C.J. Asymptotic behaviors of support vector machines with gaussian kernel. Neural Comput. 2003, 15, 1667–1689. [Google Scholar] [CrossRef] [PubMed]
- Torrence, C.; Compo, G.P. A practical guide to wavelet analysis. Bull. Am. Meteorol. Soc. 1998, 79, 61–78. [Google Scholar] [CrossRef]
- Vargas, R.; Baldocchi, D.D.; Bahn, M.; Hanson, P.J.; Hosman, K.P.; Kulmala, L.; Pumpanen, J.; Yang, B. On the multi-temporal correlation between photosynthesis and soil co2 efflux: Reconciling lags and observations. New Phytol. 2011, 191, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Kang, S.; Vargas, R.; Zhang, Y.; Hao, X. Multiscale spectral analysis of temporal variability in evapotranspiration over irrigated cropland in an arid region. Agric. Water Manag. 2013, 130, 79–89. [Google Scholar] [CrossRef]
- Katul, G.; Lai, C.T.; Schafer, K.; Vidakovic, B.; Albertson, J.; Ellsworth, D.; Oren, R. Multiscale analysis of vegetation surface fluxes: From seconds to years. Adv. Water Resour. 2001, 24, 1119–1132. [Google Scholar] [CrossRef]
- Vargas, R.; Detto, M.; Baldocchi, D.D.; Allen, M.F. Multiscale analysis of temporal variability of soil CO2 production as influenced by weather and vegetation. Glob. Chang. Biol. 2010, 16, 1589–1605. [Google Scholar] [CrossRef]
- Dietze, M.C.; Vargas, R.; Richardson, A.D.; Stoy, P.C.; Barr, A.G.; Anderson, R.S.; Arain, M.A.; Baker, I.T.; Black, T.A.; Chen, J.M.; et al. Characterizing the performance of ecosystem models across time scales: A spectral analysis of the north american carbon program site-level synthesis. J. Geophys. Res. Biogeosci. 2011, 116. [Google Scholar] [CrossRef]
- Stoy, P.C.; Dietze, M.C.; Richardson, A.D.; Vargas, R.; Barr, A.G.; Anderson, R.S.; Arain, M.A.; Baker, I.T.; Black, T.A.; Chen, J.M.; et al. Evaluating the agreement between measurements and models of net ecosystem exchange at different times and timescales using wavelet coherence: An example using data from the north american carbon program site-level interim synthesis. Biogeosciences 2013, 10, 6893–6909. [Google Scholar] [CrossRef] [Green Version]
- Grinsted, A.; Moore, J.C.; Jevrejeva, S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process. Geophys. 2004, 11, 561–566. [Google Scholar] [CrossRef] [Green Version]
- Ng, E.K.W.; Chan, J.C.L. Geophysical applications of partial wavelet coherence and multiple wavelet coherence. J. Atmos. Ocean. Technol. 2012, 29, 1845–1853. [Google Scholar] [CrossRef]
- Scott, R.L.; Serrano-Ortiz, P.; Doming, F.; Hamerlynck, E.P.; Kowalski, A.S. Commonalities of carbon dioxide exchange in semiarid regions with monsoon and mediterranean climates. J. Arid Environ. 2012, 84, 71–79. [Google Scholar] [CrossRef]
- Phillips, C.L.; Bond-Lamberty, B.; Desai, A.R.; Lavoie, M.; Risk, D.; Tang, J.W.; Todd-Brown, K.; Vargas, R. The value of soil respiration measurements for interpreting and modeling terrestrial carbon cycling. Plant Soil 2017, 413, 1–25. [Google Scholar] [CrossRef]
- Gomez-Casanovas, N.; Anderson-Teixeira, K.; Zeri, M.; Bernacchi, C.J.; DeLucia, E.H. Gap filling strategies and error in estimating annual soil respiration. Glob. Chang. Biol. 2013, 19, 1941–1952. [Google Scholar] [CrossRef] [PubMed]
- Thiessen, U.; van Brakel, R.; de Weijer, A.P.; Melssen, W.J.; Buydens, L.M.C. Using support vector machines for time series prediction. Chemom. Intell. Lab. Syst. 2003, 69, 35–49. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Bunn, A.G.; Thomson, A.M. Multi-year lags between forest browning and soil respiration at high northern latitudes. PLoS ONE 2012, 7, e50441. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.; Oyonarte, C.; Moran-Lopez, T.; Raimundo, J.; Pegoraro, E. Changes in soil moisture predict soil carbon losses upon rewetting in a perennial semiarid steppe in se spain. Geoderma 2017, 287, 135–146. [Google Scholar] [CrossRef]
- Cleverly, J.; Boulain, N.; Villalobos-Vega, R.; Grant, N.; Faux, R.; Wood, C.; Cook, P.G.; Yu, Q.; Leigh, A.; Eamus, D. Dynamics of component carbon fluxes in a semi-arid acacia woodland, central australia. J. Res. Biogeosci. 2013, 118, 1168–1185. [Google Scholar] [CrossRef]
- Knapp, A.K.; Beier, C.; Briske, D.D.; Classen, A.T.; Luo, Y.; Reichstein, M.; Smith, M.D.; Smith, S.D.; Bell, J.E.; Fay, P.A.; et al. Consequences of more extreme precipitation regimes for terrestrial ecosystems. Bioscience 2008, 58, 811–821. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas, R.; Sánchez-Cañete P., E.; Serrano-Ortiz, P.; Curiel Yuste, J.; Domingo, F.; López-Ballesteros, A.; Oyonarte, C. Hot-Moments of Soil CO2 Efflux in a Water-Limited Grassland. Soil Syst. 2018, 2, 47. https://doi.org/10.3390/soilsystems2030047
Vargas R, Sánchez-Cañete P. E, Serrano-Ortiz P, Curiel Yuste J, Domingo F, López-Ballesteros A, Oyonarte C. Hot-Moments of Soil CO2 Efflux in a Water-Limited Grassland. Soil Systems. 2018; 2(3):47. https://doi.org/10.3390/soilsystems2030047
Chicago/Turabian StyleVargas, Rodrigo, Enrique Sánchez-Cañete P., Penélope Serrano-Ortiz, Jorge Curiel Yuste, Francisco Domingo, Ana López-Ballesteros, and Cecilio Oyonarte. 2018. "Hot-Moments of Soil CO2 Efflux in a Water-Limited Grassland" Soil Systems 2, no. 3: 47. https://doi.org/10.3390/soilsystems2030047




