Disjoining Pressure Derived from the Lennard–Jones Potential, Diffusion Equation, and Diffusion Coefficient for Submonolayer Liquid Film
Abstract
:1. Introduction
2. Disjoining Pressure for Submonolayer Liquid Film
2.1. Analytical Model and Assumptions
2.2. Disjoining Pressure Derived from Attractive Term in LJP
2.3. Disjoining Pressure Derived from LJP
3. Diffusion Equation Incorporating the Rigorous Disjoining Pressure
3.1. Diffusion Flow Model for Submonolayer Film
3.2. Derivation of the Diffusion Equation
4. Comparison of the Diffusion Coefficient Between Theories and Experiment
4.1. Theoretical Diffusion Coefficient
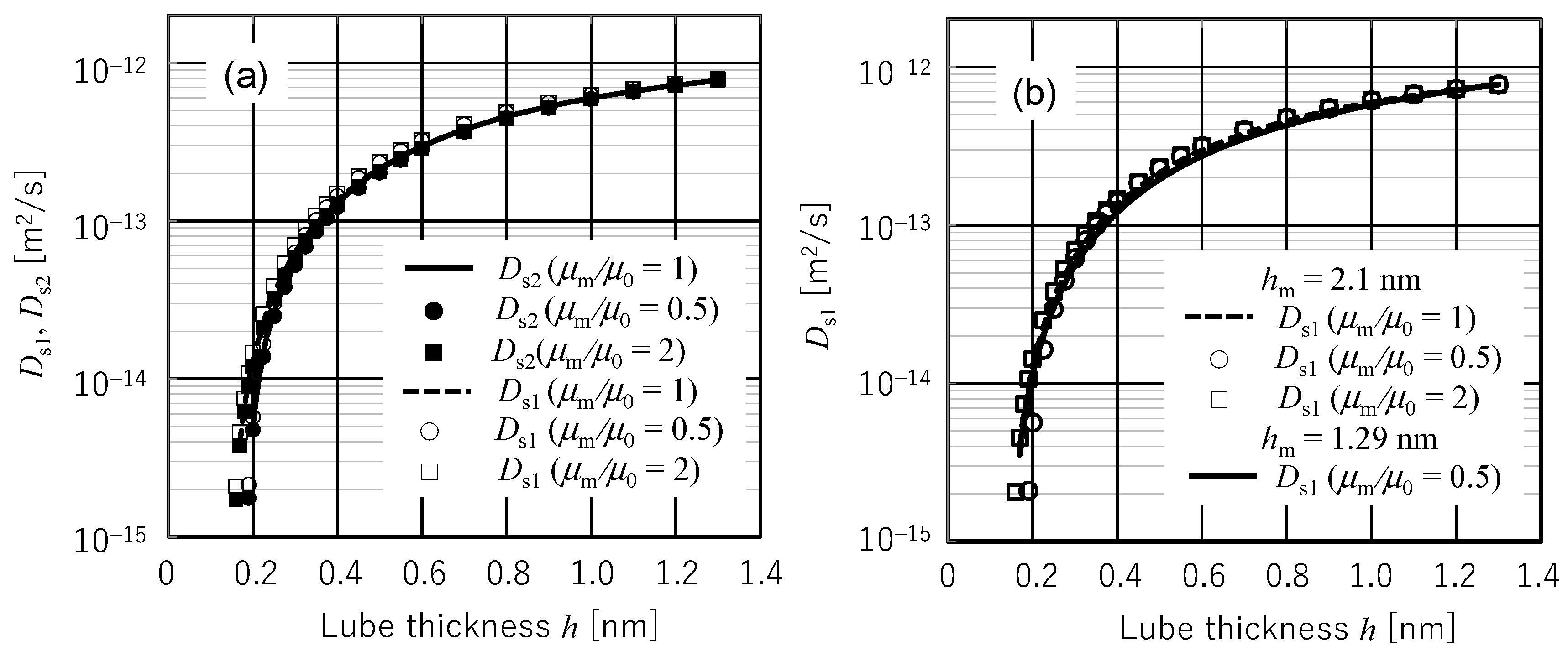
4.2. Calculated Diffusion Coefficients and Comparison with Experimental Ones
5. Conclusions
- The disjoining pressure for a submonolayer liquid film model derived from the LJP and diffusion equation for submonolayer liquid film incorporating the new disjoining pressure is a rigorous mathematical solution for the disjoining pressure and diffusion equation proposed previously. It was found that the previous submonolayer theory is a good approximated expression of the rigorous submonolayer theory in the region of film thickness above d0.
- From the axiomatic condition that the disjoining pressure must be zero at infinite film thickness, the mean vdW distance d0 of the liquid–solid interface must be 0.637 times the molecular equilibrium distance in LJP.
- The conventional disjoining pressure for a multilayer continuous liquid film including the vdW distance d0 cannot be derived from only the adhesive term of the LJP. The disjoining pressure rigorously derived from the LJP contains an additional term. Although the disjoining pressure derived from the LJP gives almost the same value as the conventional version in the region of film thickness larger than 0.5d0, it exhibits different behavior for a film thickness smaller than 0.5d0 and becomes zero at zero film thickness in contrast to the conventional disjoining pressure that has a maximum value at zero film thickness. It is considered that the disjoining pressure derived from the LJP is more reasonable.
- Four diffusion coefficients Ds2 (rigorous submonolayer theory), Ds1 (approximated submonolayer theory), Dc2 (rigorous continuous layer theory), and Dc1 (conventional continuous layer theory) have been compared with Waltman’s experimental diffusion coefficients by identifying the multiplication factor A/μm from the experimental diffusion coefficient value at 1.29 nm film thickness. When the presumable value of 0.3 nm is used for d0, Ds2 and Ds1 showed excellent agreement with the experimental data in the entire range of measured film thickness although Ds1 exhibited a slightly higher value in the region of film thickness less than d0. In contrast, Dc2 and Dc1 showed quantitatively and qualitatively different behaviors from the experimental data. This is because Dc2 and Dc1 take maximum values at a film thickness of ~3d0. As a result, it can be concluded that the spreading, diffusion, and replenishment behaviors of submonolayer liquid film can be evaluated by submonolayer theory if the amplification factor A/μm is identified from an arbitrarily chosen experimental value.
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Hamaker constant for a multi-layer lubricant film coated on a solid surface [J] |
| D | Diffusion coefficient [m2/s] |
| O-xyz | Rectangular coordinate system for lubricant flow analysis |
| d | Mean distance between the liquid film and solid surface [m] |
| d0 | Mean van der Waals distance between the liquid film and solid surface [m] |
| h | Local thickness of the liquid film [m] |
| hm | Thickness of the monolayer of liquid film [m] |
| p | Pressure in the liquid film [Pa] |
| rμ | = μm/μ0 |
| t | Time [s] |
| u | Local velocity within the liquid film [m] |
| Π | Disjoining pressure [Pa] |
| α | = rμ − 1 |
| μz | Local viscosity of the submonolayer liquid film at a height of z [Pa·s] |
| μ0 | Effective viscosity at the solid surface [Pa·s] |
| μm | Viscosity of the liquid film for monolayer and multilayer liquid film [Pa·s] |
| ρl | Density of the bulk liquid [kg/m3] |
| ρh | Density of the submonolayer liquid film [kg/m3] |
References
- Xu, J.; Shimizu, Y.; Furukawa, M.; Li, J.; Sano, Y.; Shiramatsu, T.; Aoki, Y.; Matsumoto, H.; Kuroki, K.; Kohira, H. Contact/clearance sensor for HDI subnanometer regime. IEEE Trans. Magn. 2014, 50, 114–118. [Google Scholar] [CrossRef]
- Kurita, T.; Shiramatsu, K.; Miyake, A.; Kato, M.; Soga, H.; Tanaka, S.; Saegusa, S.; Suk, M. Active flying-height control slider using MEMS thermal actuator. Microsyst. Technol. 2006, 12, 369–375. [Google Scholar] [CrossRef]
- Canchi, S.V.; Bogy, D.B. Slider-lubricant interactions and lubricant distribution for contact and near contact recording conditions. IEEE Trans. Magn. 2011, 47, 1842–1848. [Google Scholar] [CrossRef]
- Marchon, B. Lubricant Design Attributes Subnanometer Head-Disk Clearance. IEEE Trans. Magn. 2009, 45, 872–876. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Murthy, A.N.; Pit, R.; Bogy, D.B. Angstrom scale wear of the air-bearing sliders in hard disk drives. Tribol. Lett. 2014, 54, 273–278. [Google Scholar] [CrossRef]
- Matthes, L.; Brunner, R.; Knigge, B.; Talke, F.E. Head wear of thermal flying height control sliders as a function of bonded lubricant ratio, temperature, and relative humidity. Tribol. Lett. 2015, 60, 39. [Google Scholar] [CrossRef]
- de Gennes, P.G. Wetting: Statics and dynamics. Rev. Mod. Phys. 1985, 57 Pt 1, 827–863. [Google Scholar] [CrossRef]
- Oron, A.; Davis, S.H.; Bankoff, S.G. Long-scale evolution of thin liquid films. Rev. Mod. Phys. 1997, 69, 931–980. [Google Scholar] [CrossRef]
- Mate, C.M. Application of disjoining and capillary pressure to liquid lubricant films in magnetic recording. J. Appl. Phys. 1992, 72, 3084–3090. [Google Scholar] [CrossRef]
- Karis, T.E.; Tyndall, G.W. Calculation of spreading profiles for molecularly-thin films from surface energy gradients. J. Non-Newton. Fluid Flow 1999, 82, 287–302. [Google Scholar] [CrossRef]
- Tyndall, G.W.; Karis, T.E.; Jhon, M.S. Spreading profiles of molecularly thin perfluoro-polyether films. Tribol. Trans. 1999, 42, 463–470. [Google Scholar] [CrossRef]
- Marchon, H.; Karis, T.E. Poiseuille flow at a nanometer scale. Europhys. Lett. 2006, 74, 294–298. [Google Scholar] [CrossRef]
- Waltman, R.J.; Tyndall, G.W.; Pacansky, J. Computer-modeling study of the interactions of Zdol with amorphous carbon surfaces. Langmuir 1999, 15, 6470–6483. [Google Scholar] [CrossRef]
- Mate, C.M. Spreading kinematics of lubricant droplets on magnetic recording disks. Tribol. Lett. 2013, 51, 385–395. [Google Scholar] [CrossRef]
- Scarpulla, M.A.; Mate, C.M.; Carter, M.D. Air shear driven flow of thin perfluoropolyether polymer films. J. Chem. Phys. 2003, 118, 3368–3375. [Google Scholar] [CrossRef]
- Ono, K. Replenishment speed of depleted scar in submonolayer lubricant. Tribol. Lett. 2013, 52, 123–195. [Google Scholar] [CrossRef]
- Ono, K. Diffusion equation for spreading and replenishment in submonolayer lubricant film. Tribol. Lett. 2015, 57, 13. [Google Scholar] [CrossRef]
- Waltman, R.J. Z-Tetraol reflow in heat-assisted magnetic recording. Tribol. Online 2016, 11, 50–60. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 2nd ed.; Academic Press: London, UK, 1992. [Google Scholar]
- Mate, C.M. Anomalous diffusion kinetics of the precursor film that spreads from polymer droplets. Langmuir 2012, 28, 16821–16827. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Gui, J.; Grannen, K.J.; Smoliar, L.A.; Marchon, B.; Jhon, M.S.; Bauer, C.L. Spreading of PFPE lubricants on carbon surfaces: effect of hydrogen and nitrogen content. Tribol. Lett. 1999, 6, 9–14. [Google Scholar] [CrossRef]
- Ma, X.; Gui, J.; Smoliar, L.; Grannen, K.; Marchon, B.; Bauer, C.L.; Jhon, M.S. Complex terraced spreading of perfluoropolyalkylether films on carbon surfaces. Phys. Rev. E 1999, 59, 723–727. [Google Scholar] [CrossRef]
- Fukuda, M.; Zhang, H.; Ishiguro, T.; Fukuzawa, K. Structure-based coarse-graining for inhomogeneous liquid polymer systems. J. Chem. Phys. 2013, 139, 054901. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Ohkubo, S.; Fukui, S. Correction expression of the van der Waals pressure for multilayered system with application to analyses of static characteristics of flying head sliders with an ultrasmall spacing. Microsyst. Technol. 2005, 11, 824–829. [Google Scholar] [CrossRef]
- Waltman, R.J.; Deng, H.; Wang, G.J.; Zhu, H.; Tyndall, G.W. The effect of PFPE film thickness and molecular polarity on the pick-up of disk lubricant by a low-flying slider. Tribol. Lett. 2010, 39, 211–219. [Google Scholar] [CrossRef]
- Waltman, R.J.; Newman, J.; Guo, X.-C.; Burns, J.; Witta, C.; Amo, M. The effect of UV irradiation on the Z-Tetraol boundary lubricant. Tribol. Online 2012, 7, 70–80. [Google Scholar] [CrossRef]
- Tani, H.; Mitsutome, T.; Tagawa, N. Adhesion and friction behavior of magnetic disks with ultrathin perfluoropolyether lubricant films having different end-groups measured suing pin-on-disk test. IEEE Trans. Magn. 2013, 49, 2638–2644. [Google Scholar] [CrossRef]




| Diffusion Coefficient | Dc1 | Dc2 | Ds1 | Ds2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| hm [nm] | 2.1 | 2.1 | 2.1 | 1.29 | 2.1 | ||||
| rμ (=μm/μ0) | 1 | 1 | 1 | 0.5 | 2 | 0.5 | 1 | 0.5 | 2 |
| A/μm [×10−19 m3/s] | 0.432 | 0.432 | 2.385 | 4.384 | 1.362 | 2.528 | 2.385 | 4.385 | 1.362 |
| μm [Pa∙s] (A = 0.47 × 10−19 J) | 1.089 | 1.089 | 0.197 | 0.107 | 0.345 | 0.186 | 0.197 | 0.107 | 0.345 |
| μm [Pa∙s] (A = 1.9 × 10−19 J) | 4.403 | 4.402 | 0.797 | 0.433 | 1.395 | 0.751 | 0.796 | 0.433 | 1.395 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono, K. Disjoining Pressure Derived from the Lennard–Jones Potential, Diffusion Equation, and Diffusion Coefficient for Submonolayer Liquid Film. Surfaces 2018, 1, 122-137. https://doi.org/10.3390/surfaces1010010
Ono K. Disjoining Pressure Derived from the Lennard–Jones Potential, Diffusion Equation, and Diffusion Coefficient for Submonolayer Liquid Film. Surfaces. 2018; 1(1):122-137. https://doi.org/10.3390/surfaces1010010
Chicago/Turabian StyleOno, Kyosuke. 2018. "Disjoining Pressure Derived from the Lennard–Jones Potential, Diffusion Equation, and Diffusion Coefficient for Submonolayer Liquid Film" Surfaces 1, no. 1: 122-137. https://doi.org/10.3390/surfaces1010010




