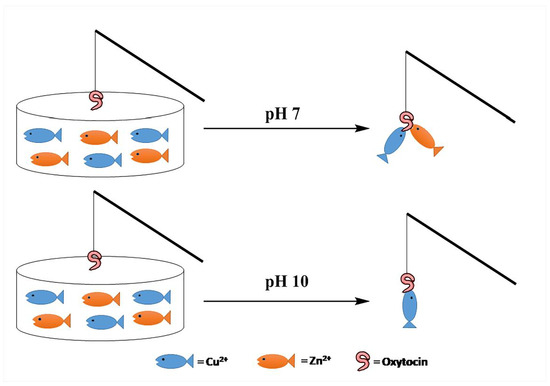
pH Controlled Impedimetric Sensing of Copper(II) Ion Using Oxytocin as Recognition Element
Abstract
:1. Introduction
2. Experimental Section
2.1. Material and Methods
2.2. UV-Visible Spectroscopy Titrations
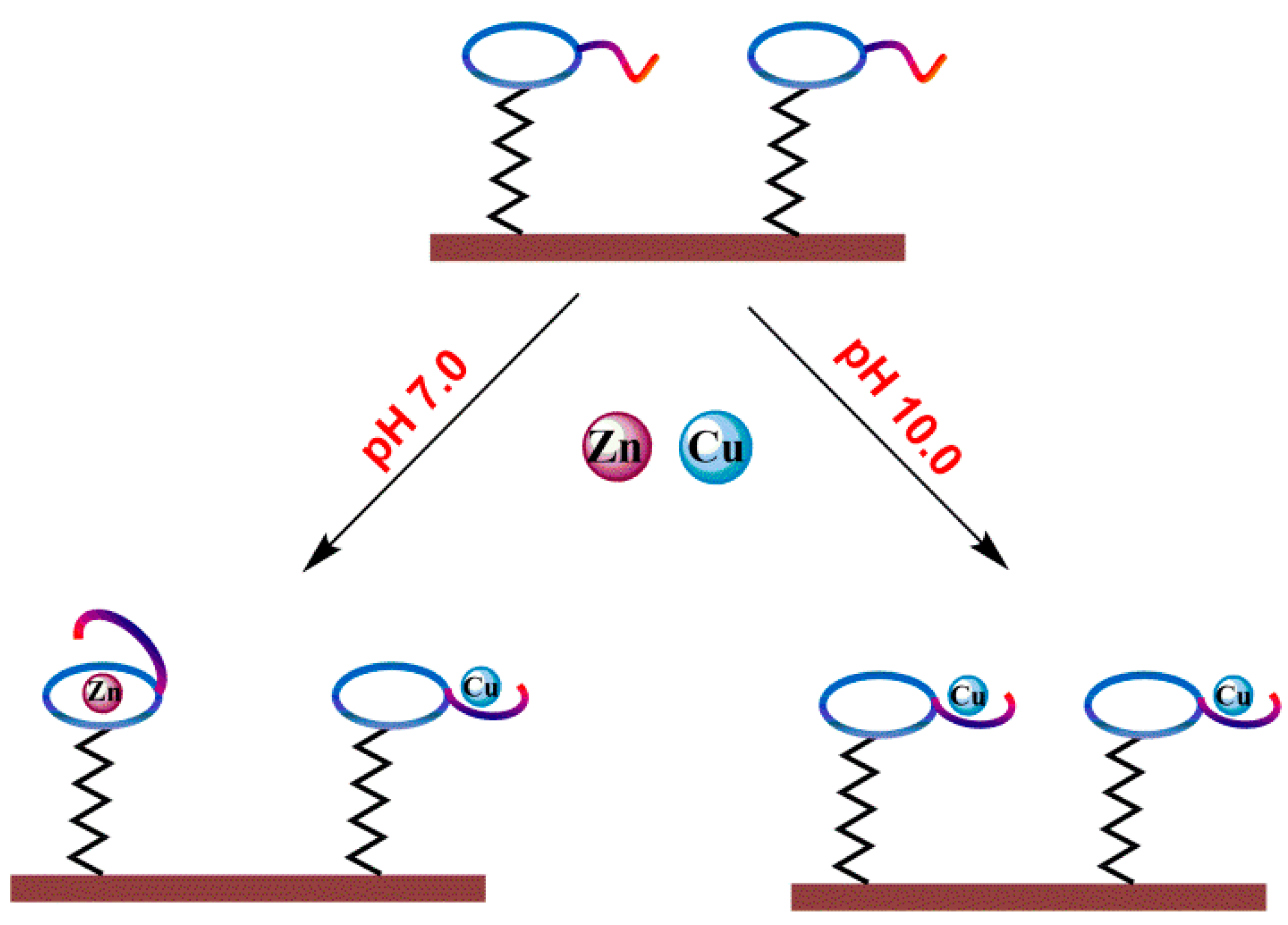
2.3. Fabrication of OT-Modified Electrode
2.4. Electrochemical Impedance Studies
3. Results and Discussion
3.1. Job’s Plot
3.2. Impedimetric Detection of Cu2+
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, C.M.; Lin, L.Y. Immobilization of metallothionien as a selective biosensor chip for the detection of metal ions by surface plasmon resonance. Biosens. Bioelectron. 2004, 20, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Edith, C.; Gary, D.W.; Hibbert, D.B.; Gooding, J.J. Exploring the use of the tripeptide Gly-Gly-his as a selective recognition element for the fabrication of electrochemical copper sensors. Analyst 2002, 128, 712–718. [Google Scholar] [CrossRef]
- Chow, E.; Goading, J.J. Peptide modified electrodes as electrochemical metal ion sensors. Electroanalysis 2006, 18, 1437–1448. [Google Scholar] [CrossRef]
- Bontidean, I.; Berggren, C.; Johansson, G.; Csöregi, E.; Mattiasson, B.; Lloyd, J.R.; Jakeman, K.J.; Brown, N.L. Detection of heavy metal ions at femtomolar levels using protein-based biosensors. Anal. Chem. 1998, 70, 4162–4169. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M.; Holcombe, J.A. Poly(L-cysteine) as an electrochemically modifiable ligand for trace metal chelation. Anal. Chem. 2005, 77, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Mervinetsky, E.; Alshanski, I.; Hamo, Y.; Sandonas, L.M.; Dianat, A.; Buchwald, J.; Gutierrez, R.; Cuniberti, G.; Hurevich, M.; Yitzchaik, S. Copper Induced Conformational Changes of Tripeptide Monolayer Based Impedimetric Biosensor. Sci. Rep. 2017, 7, 9498. [Google Scholar] [CrossRef] [PubMed]
- Godwin, H.A.; Berg, J.M. A fluorescent zinc probe based on metal-induced peptide folding. J. Am. Chem. Soc. 1996, 118, 6514–6515. [Google Scholar] [CrossRef]
- Szunyogh, D.; Gyurcsik, B.; Larsen, F.H.; Stachura, M.; Thulstrup, P.W.; Hemmingsen, L.; Jancsó, A. ZnII and HgII binding to a designed peptide that accommodates different coordination geometries. Dalton Trans. 2015, 44, 12576–12588. [Google Scholar] [CrossRef] [PubMed]
- Marx, G.; Gilon, C. The Molecular Basis of Memory. Part 2: Chemistry of the Tripartite Mechanism. ACS Chem. Neurosci. 2013, 4, 983–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Seuthe, A.B.; Ehrler, O.T.; Zhang, X.; Wyttenbach, T.; Hsu, J.F. Oxytocin-Receptor Binding: Why Divalent Metals Are Essential. J. Am. Chem. Soc. 2005, 127, 2024–2025. [Google Scholar]
- Tadi, K.K.; Alshanksi, I.; Mervinetsky, E.; Marx, G.; Petrou, P.; Dimitrios, K.M.; Gilon, C.; Hurevich, M.; Yitzchaik, S. Oxytocin-Monolayer-Based Impedimetric Biosensor for Zinc and Copper Ions. ACS Omega 2017, 2, 8770–8778. [Google Scholar] [CrossRef] [PubMed]
- Wyttenbach, T.; Liu, D.; Bowers, M.T. Interactions of the hormone oxytocin with divalent metal ions. J. Am. Chem. Soc. 2008, 130, 5993–6000. [Google Scholar] [CrossRef] [PubMed]
- Jewett, J.C.; Bertozzi, C.R. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 2010, 39, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Renny, J.S.; Tomasevich, L.L.; Tallmadge, E.H.; Collum, D.B. Method of Continuous Variations: Applications of Job Plots to the Study of Molecular Associations in Organometallic Chemistry. Angew. Chem. Int. Ed. Engl. 2013, 52, 11998–12013. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadi, K.K.; Alshanski, I.; Hurevich, M.; Yitzchaik, S. pH Controlled Impedimetric Sensing of Copper(II) Ion Using Oxytocin as Recognition Element. Surfaces 2018, 1, 90-95. https://doi.org/10.3390/surfaces1010008
Tadi KK, Alshanski I, Hurevich M, Yitzchaik S. pH Controlled Impedimetric Sensing of Copper(II) Ion Using Oxytocin as Recognition Element. Surfaces. 2018; 1(1):90-95. https://doi.org/10.3390/surfaces1010008
Chicago/Turabian StyleTadi, Kiran Kumar, Israel Alshanski, Mattan Hurevich, and Shlomo Yitzchaik. 2018. "pH Controlled Impedimetric Sensing of Copper(II) Ion Using Oxytocin as Recognition Element" Surfaces 1, no. 1: 90-95. https://doi.org/10.3390/surfaces1010008