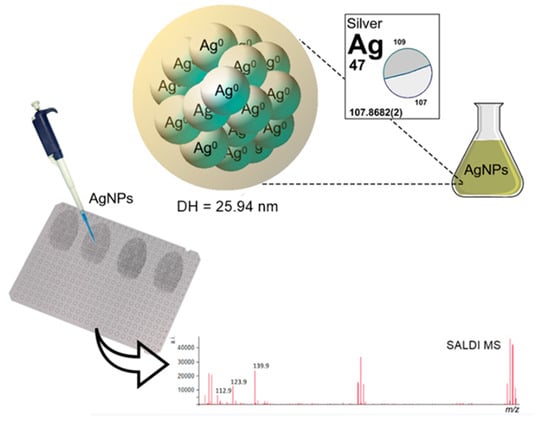
Surface-Assisted Laser Desorption/Ionization Mass Spectrometry Analysis of Latent Fingermarks Using Greenly Synthesized Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Silver Nanoparticles
2.3. Dynamic Light Scattering (DLS) and Zeta Potential
2.4. Instrument and Sample Preparation for LDI-TOF MS
3. Results
3.1. Silver Nanoparticle Characterization
3.2. Greenly Synthesized AgNPs for SALDI-TOF MS
3.3. SALDI-TOF MS Analysis of Latent Fingermarks
3.4. Additional Findings and Challenges
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dilag, J.; Kobus, H.J.; Ellis, A.V. Nanotechnology as a new tool for fingermark detection: A review. Curr. Nanosci. 2011, 7, 153–159. [Google Scholar] [CrossRef]
- Choi, M.J.; McDonagh, A.M.; Maynard, P.; Roux, C. Metal-containing nanoparticles and nano-structured particles in fingermark detection. Forensic Sci. Int. 2008, 179, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Moret, S.; Bécue, A.; Champod, C. Functionalised silicon oxide nanoparticles for fingermark detection. Forensic Sci. Int. 2016, 259, 10–18. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Review of some interesting surface plasmon resonance-enhanced properties of noble metal nanoparticles and their applications to biosystems. Plasmonics 2007, 2, 107–118. [Google Scholar] [CrossRef]
- Hodes, G. When small is different: Some recent advances in concepts and applications of Nanoscale Phenomena. Adv. Mater. 2007, 19, 639–655. [Google Scholar] [CrossRef]
- Becue, A.; Champod, C.; Margot, P. Use of gold nanoparticles as molecular intermediates for the detection of fingermarks. Forensic Sci. Int. 2007, 168, 169–176. [Google Scholar] [CrossRef]
- Sametband, M.; Shweky, I.; Banin, U.; Mandler, D.; Almog, J. Application of nanoparticles for the enhancement of latent fingerprints. Chem. Commun. 2007, 11, 1142–1144. [Google Scholar] [CrossRef] [PubMed]
- Girod, A.; Ramotowski, R.; Weyermann, C. Composition of fingermark residue: A qualitative and quantitative review. Forensic Sci. Int. 2012, 22, 10–24. [Google Scholar] [CrossRef]
- Kaplan-Sandquist, K.; LeBeau, M.A.; Miller, M.L. Chemical analysis of pharmaceuticals and explosives in fingermarks using matrix-assisted laser desorption ionization/time-of-flight mass spectrometry. Forensic Sci. Int. 2014, 235, 68–77. [Google Scholar] [CrossRef]
- Rowell, F.; Hudson, K.; Seviour, J. Detection of drugs and their metabolites in dusted latent fingermarks by mass spectrometry. Analyst 2009, 134, 701–707. [Google Scholar] [CrossRef]
- Becue, A. Emerging fields in fingermark (meta) detection–a critical review. Anal. Methods 2016, 8, 7983–8003. [Google Scholar] [CrossRef]
- Theaker, B.J.; Hudson, K.E.; Rowell, F.J. Doped hydrophobic silica nano-and micro-particles as novel agents for developing latent fingerprints. Forensic Sci. Int. 2008, 174, 26–34. [Google Scholar] [CrossRef]
- Moret, S.; Bécue, A.; Champod, C. Nanoparticles for fingermark detection: An insight into the reaction mechanism. Nanotechnology 2014, 25, 425502. [Google Scholar] [CrossRef]
- Leśniewski, A. Hybrid organic–inorganic silica based particles for latent fingermarks development: A review. Synth. Met. 2016, 222, 124–131. [Google Scholar] [CrossRef]
- Prabakaran, E.; Pillay, K. A novel approach of fluorescent porous graphite carbon nitride based silica gel powder for latent fingerprint detection. Appl. Nanosci. 2019, 9, 255–277. [Google Scholar] [CrossRef]
- Lim, A.Y.; Ma, Z.; Ma, J.; Rowell, F. Separation of fingerprint constituents using magnetic silica nanoparticles and direct on-particle SALDI-TOF-mass spectrometry. J. Chromatogr. B 2011, 879, 2244–2250. [Google Scholar] [CrossRef]
- Benton, M.; Chua, M.J.; Gu, F.; Rowell, F.; Ma, J. Environmental nicotine contamination in latent fingermarks from smoker contacts and passive smoking. Forensic Sci. Int. 2010, 200, 28–34. [Google Scholar] [CrossRef]
- Barros, R.M.; Clemente, M.C.; Martins, G.A.; Silva, L.P. Application of mesocellular siliceous foams (MCF) for surface-assisted laser desorption ionization mass spectrometry (SALDI-MS) analysis of fingermarks. Sci. Justice 2018, 58, 264–270. [Google Scholar] [CrossRef]
- Gao, D.; Li, F.; Song, J.; Xu, X.; Zhang, Q.; Niu, L. One step to detect the latent fingermarks with gold nanoparticles. Talanta 2009, 80, 479–483. [Google Scholar] [CrossRef]
- De la Hunty, M.; Moret, S.; Chadwick, S.; Lennard, C.; Spindler, X.; Roux, C. Understanding physical developer (PD): Part II–is PD targeting eccrine constituents? Forensic Sci. Int. 2015, 257, 488–495. [Google Scholar] [CrossRef]
- De la Hunty, M.; Moret, S.; Chadwick, S.; Lennard, C.; Spindler, X.; Roux, C. An effective physical developer (PD) method for use in Australian laboratories. Aust. J. Forensic Sci. 2018, 50, 666–671. [Google Scholar] [CrossRef]
- Houlgrave, S.; Andress, M.; Ramotowski, R. Comparison of different physical developer working solutions—Part I: Longevity studies*. J. Forensic Identif. 2011, 61, 621–639. [Google Scholar]
- Ramya, M.; Subapriya, M.S. Green synthesis of silver nanoparticles. Int. J. Pharm. Bio-Med. Sci. 2011, 1, 55–61. [Google Scholar]
- Nizioł, J.; Ruman, T. Surface-transfer mass spectrometry imaging on a monoisotopic silver nanoparticle enhanced target. Anal. Chem. 2013, 85, 12070–12076. [Google Scholar] [CrossRef] [PubMed]
- Nizioł, J.; Rode, W.; Laskowska, B.; Ruman, T. Novel monoisotopic 109AgNPET for laser desorption/ionization mass spectrometry. Anal. Chem. 2013, 85, 1926–1931. [Google Scholar] [CrossRef]
- Nizioł, J.; Rode, W.; Zieliński, Z.; Ruman, T. Matrix-free laser desorption–ionization with silver nanoparticle-enhanced steel targets. Int. J. Mass Spectrom. 2013, 335, 22–32. [Google Scholar] [CrossRef]
- Prysiazhnyi, V.; Dycka, F.; Kratochvil, J.; Stranak, V.; Popok, V.N. Effect of Ag nanoparticle size on ion formation in nanoparticle assisted LDI MS. Appl. Nano 2020, 1, 3–13. [Google Scholar] [CrossRef]
- Sekuła, J.; Nizioł, J.; Rode, W.; Ruman, T. Silver nanostructures in laser desorption/ionization mass spectrometry and mass spectrometry imaging. Analyst 2015, 140, 6195–6209. [Google Scholar] [CrossRef]
- Suvith, V.S.; Philip, D. Catalytic degradation of methylene blue using biosynthesized gold and silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Sithara, R.; Selvakumar, P.; Arun, C.; Anandan, S.; Sivashanmugam, P. Economical synthesis of silver nanoparticles using leaf extract of Acalypha hispida and its application in the detection of Mn(II) ions. J. Adv. Res. 2017, 8, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.J.; Finub, J.S.; Narayanan, A. Synthesis of silver nanoparticles using Piper longum leaf extracts and its cytotoxic activity against Hep-2 cell line. Colloids Surf. B Biointerfaces 2012, 91, 212–214. [Google Scholar] [CrossRef]
- Chung, I.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-mediated synthesis of silver nanoparticles: Their characteristic properties and therapeutic applications. Nanoscale Res. Lett. 2016, 11, 40. [Google Scholar] [CrossRef]
- Khatoon, N.; Mazumder, J.A.; Sardar, M. Biotechnological applications of green synthesized silver nanoparticles. J. Nanosci. Curr. Res. 2017, 2, 107. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Bonatto, C.C.; Silva, L.P. Higher temperatures speed up the growth and control the size and optoelectrical properties of silver nanoparticles greenly synthesized by cashew nutshells. Ind. Crops Prod. 2014, 58, 46–54. [Google Scholar] [CrossRef]
- Amendola, V.; Bakr, O.M.; Stellacci, F. A study of the surface plasmon resonance of silver nanoparticles by the discrete dipole approximation method: Effect of shape, size, structure, and assembly. Plasmonics 2010, 5, 85–97. [Google Scholar] [CrossRef]
- Yadav, R.; Preet, S. Comparative assessment of green and chemically synthesized glutathione capped silver nanoparticles for antioxidant, mosquito larvicidal and eco-toxicological activities. Sci. Rep. 2023, 13, 8152. [Google Scholar] [CrossRef]
- Bergman, N.; Shevchenko, D.; Bergquist, J. Approaches for the analysis of low molecular weight compounds with laser desorption/ionization techniques and mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Lauzon, N.; Dufresne, M.; Chauhan, V.; Chaurand, P. Development of laser desorption imaging mass spectrometry methods to investigate the molecular composition of latent fingermarks. J. Am. Soc. Mass Spectrom. 2015, 26, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Fournelle, F.; Chaurand, P. Silver spray deposition for AgLDI imaging MS of cholesterol and other olefins on thin tissue sections. J. Mass Spectrom. 2020, 55, e4428. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, R.; Bleay, S.; Wolstenholme, R.; Clench, M.R.; Francese, S. Towards the integration of matrix assisted laser desorption ionisation mass spectrometry imaging into the current fingermark examination workflow. Forensic Sci. Int. 2013, 232, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Francese, S.; Bradshaw, R.; Ferguson, L.S.; Wolstenholme, R.; Clench, M.R.; Bleay, S. Beyond the ridge pattern: Multi-informative analysis of latent fingermarks by MALDI mass spectrometry. Analyst 2013, 138, 4215–4228. [Google Scholar] [CrossRef]
- Lauzon, N.; Dufresne, M.; Beaudoin, A.; Chaurand, P. Forensic analysis of latent fingermarks by silver-assisted LDI imaging MS on nonconductive surfaces. J. Mass Spectrom. 2017, 52, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; Prasad, L.; Lukose, S.; Agarwal, P. Latent fingerprint development by using silver nanoparticles and silver nitrate—A comparative study. J. Forensic Sci. 2021, 66, 1065–1074. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, R.M.; Bonatto, C.C.; Ramada, M.H.S.; Silva, L.P. Surface-Assisted Laser Desorption/Ionization Mass Spectrometry Analysis of Latent Fingermarks Using Greenly Synthesized Silver Nanoparticles. Surfaces 2023, 6, 341-350. https://doi.org/10.3390/surfaces6040024
Barros RM, Bonatto CC, Ramada MHS, Silva LP. Surface-Assisted Laser Desorption/Ionization Mass Spectrometry Analysis of Latent Fingermarks Using Greenly Synthesized Silver Nanoparticles. Surfaces. 2023; 6(4):341-350. https://doi.org/10.3390/surfaces6040024
Chicago/Turabian StyleBarros, Rodrigo M., Cínthia C. Bonatto, Marcelo H. S. Ramada, and Luciano P. Silva. 2023. "Surface-Assisted Laser Desorption/Ionization Mass Spectrometry Analysis of Latent Fingermarks Using Greenly Synthesized Silver Nanoparticles" Surfaces 6, no. 4: 341-350. https://doi.org/10.3390/surfaces6040024