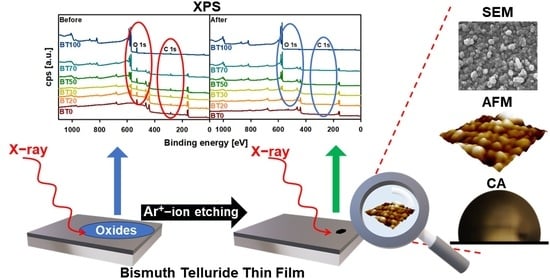
Etching and Compositional Ratio Effect on the Surface Properties of Bismuth Telluride Thin Films
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ishibe, T.; Komatsubara, Y.; Ishikawa, K.; Takigawa, S.; Naruse, N.; Mera, Y.; Yamashita, Y.; Ohishi, Y.; Nakamura, Y. Boosting Thermoelectric Performance in Epitaxial GeTe Film/Si by Domain Engineering and Point Defect Control. ACS Appl. Mater. Interfaces 2023, 15, 26104. [Google Scholar] [CrossRef]
- Han, C.; Tan, G.; Varghese, T.; Kanatzidis, M.G.; Zhang, Y. High-Performance PbTe Thermoelectric Films by Scalable and Low-Cost Printing. ACS Energy Lett. 2018, 3, 818–822. [Google Scholar] [CrossRef]
- El-Makaty, F.M.; Ahmed, H.K.; Youssef, K.M. The Effect of Different Nanofiller Materials on the Thermoelectric Behavior of Bismuth Telluride. Mater. Des. 2021, 209, 109974. [Google Scholar] [CrossRef]
- Nour, A.; Hamida, R.S.; El-Dissouky, A.; Soliman, H.M.A.; Refaat, H.M. One-Pot Facile Synthesis of Hexagonal Bi2Te3 Nanosheets and Its Novel Nanocomposites: Characterization, Anticancer, Antibacterial, and Antioxidant Activities. Colloids Surf. B 2023, 225, 113230. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, L.; Chen, F.; Liu, J. Synergistic Antibacterial Mechanism of Bi2Te3 Nanoparticles Combined with Ineffective β-Lactam Antibiotic Cefotaxime Against Methicillin-Resistant Staphylococcus aureus. J. Inorg. Biochem. 2019, 196, 110687. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Shi, X.-L.; Li, M.; Hu, B.; Chen, W.; Liu, W.-D.; Lyu, W.; MacLeod, J.; Chen, Z.-G. Advances in Bismuth-Telluride-based Thermoelectric Devices: Progress and Challenges. eScience 2023, 3, 100122. [Google Scholar] [CrossRef]
- Mamur, H.; Bhuiyan, M.R.A.; Korkmaz, F.; Nil, M. A Review on Bismuth Telluride (Bi2Te3) Nanostructure for Thermoelectric Applications. Renew. Sustain. Energy Rev. 2018, 82, 4159–4169. [Google Scholar] [CrossRef]
- Chen, R.; Wei, L.; Yan, Y.; Chen, G.; Yang, X.; Liu, Y.; Zhang, M.; Liu, X.; Cheng, Y.; Sun, J.; et al. Bismuth Telluride Functionalized Bismuth Oxychloride Used for Enhancing Antibacterial Activity and Wound Healing Efficacy with Sunlight Irradiation. Biomater. Sci. 2022, 10, 467–473. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.X.; Qi, X.L.; Dai, X.; Fang, Z.; Zhang, S.C. Topological Insulators in Bi2Se3, Bi2Te3 and Sb2Te3 with a Single Dirac Cone on the Surface. Nat. Phys. 2009, 6, 438–442. [Google Scholar] [CrossRef]
- Steiner, H.; Volobuev, V.; Caha, O.; Bauer, G.; Springholz, G.; Holý, V. Structure and Composition of Bismuth Telluride Topological Insulators Grown by Molecular Beam Epitaxy. J. Appl. Crystallogr. 2014, 6, 1889–1900. [Google Scholar] [CrossRef]
- Ashalley, E.; Chen, H.; Tong, X.; Li, H.; Wang, Z.M. Bismuth Telluride Nanostructures: Preparation, Thermoelectric Properties and Topological Insulating Effect. Front. Mater. Sci. 2015, 9, 103–125. [Google Scholar] [CrossRef]
- Choi, S.; Kang, J.; Park, J.; Kang, Y.-C. Tin Nitride Thin Films Fabricated by Reactive Radio Frequency Magnetron Sputtering at Various Nitrogen Gas Ratios. Thin Solid Films 2014, 571, 84–89. [Google Scholar] [CrossRef]
- Choi, S.; Park, J.; Kang, J.; Johnson, A.T.C.; Kang, Y.-C. Surface Characterization of PZT Thin Films Obtained at Various O2 Gas Ratios. Vacuum 2016, 128, 234–239. [Google Scholar] [CrossRef]
- Zeng, Z.; Yang, P.; Hu, Z. Temperature and Size Effects on Electrical Properties and Thermoelectric Power of Bismuth Telluride Thin Films Deposited by Co-Sputtering. Appl. Surf. Sci. 2013, 268, 472–476. [Google Scholar] [CrossRef]
- Singkaselit, K.; Sakulkalavek, A.; Sakdanuphab, R. Effects of Annealing Temperature on the Structural, Mechanical and Electrical Properties of Flexible Bismuth Telluride Thin Films Prepared by High-Pressure RF Magnetron Sputtering. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 035002. [Google Scholar] [CrossRef]
- Rogacheva, E.I.; Budnik, A.V.; Dobrotvorskaya, M.V.; Fedorov, A.G.; Krivonogov, S.I.; Mateychenko, P.V.; Nashchekina, O.N.; Sipatov, A.Y. Growth and structure of thermally evaporated Bi2Te3 thin films. Thin Solid Films 2016, 612, 128–134. [Google Scholar] [CrossRef]
- Le, P.H.; Liao, C.-N.; Luo, C.W.; Leu, J. Thermoelectric Properties of Nanostructured Bismuth-Telluride Thin Films Grown Using Pulsed Laser Deposition. J. Alloys Compd. 2014, 615, 546–552. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Zeng, Z.; Hu, Z. Two-Step Molecular Beam Epitaxy Growth of Bismuth Telluride Nanoplate Thin Film with Enhanced Thermoelectric Properties. ECS Solid State Lett. 2014, 3, P99. [Google Scholar] [CrossRef]
- Golia, S.; Arora, M.; Sharma, R.K.; Rastogi, A.C. Electrochemically Deposited Bismuth Telluride Thin Films. Curr. Appl. Phys. 2003, 3, 195–197. [Google Scholar] [CrossRef]
- Choi, J.; Park, J.; Kang, J.; Kehayias, C.E.; Oh, J.W.; Kang, Y.-C. Annealing Temperature Effect on the Surface Properties of the MoSe Thin Films. Phys. Status Solidi A 2023, 220, 2300477. [Google Scholar] [CrossRef]
- Yoon, H.; Lee, J.; Park, J.; Oh, J.W.; Kang, Y.-C. Physicochemical Properties of SnTe Thin Films Dependent on Compositional Ratios. Phys. Status Solidi A 2022, 219, 2200059. [Google Scholar] [CrossRef]
- Stevie, F.A.; Garcia, R.; Shallenberger, J.; Newman, J.G.; Donley, C.L. Sample Handling, Preparation and Mounting for XPS and Other Surface Analytical Techniques. J. Vac. Sci. Technol. A 2020, 38, 063202. [Google Scholar] [CrossRef]
- Kim, D.; Park, J.; Choi, J.; Oh, J.W.; Kang, Y.-C. Compositional Ratio Effect on the Physicochemical Properties of SnSe Thin Films. Phys. B Condens. Matter 2021, 612, 412890. [Google Scholar] [CrossRef]
- Choi, A.; Park, J.; Kang, J.; Jonas, O.; Kim, D.; Kim, H.; Oh, J.W.; Kang, Y.-C. Surface Characterization and Investigation on Antibacterial Activity of CuZn Nanofibers Prepared by Electrospinning. Appl. Surf. Sci. 2020, 508, 144883. [Google Scholar] [CrossRef]
- Jeong, E.; Park, J.; Choi, S.; Kang, J.; Kang, Y.-C. Surface Characteristics of MoN x Thin Films Obtained by Reactive rf Magnetron Sputtering in UHV System. Bull. Korean Chem. Soc. 2015, 36, 2446. [Google Scholar] [CrossRef]
- Park, J.; Seo, S.Y.; Kang, Y.-C. Tuning the Oxidation States and Crystallinity of Copper Oxide Nanofibers by Calcination. J. Vac. Sci. Technol. B 2014, 32, 04E104. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Dixit, A.; Rao, M.S.R.; Naik, R. Influence of Ca Doping on X-Ray Photoelectron Core-Level Spectra of Magnetoelectric Bulk BiFeO3. Surf. Interface Anal. 2021, 53, 798–807. [Google Scholar] [CrossRef]
- Soriano, L.; Galan, L.; Rueda, F. An XPS Study of Cs2Te Photocathode Materials. Surf. Interface Anal. 1990, 16, 193–198. [Google Scholar] [CrossRef]
- Qiao, Y.; Lai, W.; Huang, K.; Yu, T.; Wang, Q.; Gao, L.; Yang, Z.; Ma, Z.; Sun, T.; Liu, M.; et al. Engineering the Local Microenvironment over Bi Nanosheets for Highly Selective Electrocatalytic Conversion of CO2 to HCOOH in Strong Acid. ACS Catal. 2022, 12, 2357–2364. [Google Scholar] [CrossRef]
- Kogo, G.; Lee, H.; Ibrahim, A.H.; Bo, X.; Pradhan, S.K.; Bahoura, M. Highly-Efficient Thermoelectric PN-Junction Device based on Bismuth Telluride (Bi2Te3) and Molybdenum Disulfide (MoS2) Thin Films Fabricated by RF Magnetron Sputtering Technique. J. Appl. Phys. 2018, 124, 16. [Google Scholar] [CrossRef]
- Zhu, H.-T.; Luo, J.; Fan, H.-M.; Zhang, H.; Liang, J.-K.; Rao, G.-H.; Li, J.-B.; Liu, G.-Y.; Du, Z.-M. Tri-wing Bismuth Telluride Nanoribbons with Quasi-Periodic Rough Surfaces. J. Mater. Chem. 2011, 21, 12375–12380. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, G.; Li, C.; Han, Z.; Hao, S.; Hong, W.; Xing, W. Non-Integer Induced Spontaneous Polarization of Highly Efficient Perovskite-based NBTO SCN Photocatalysts. J. Mater. Chem. A 2017, 5, 22984–22987. [Google Scholar] [CrossRef]
- Gomez-Iriarte, G.A.; Pentón-Madrigal, A.; de Oliveira, L.A.S.; Sinnecker, J.P. XPS Study in BiFeO3 Surface Modified by Argon Etching. Materials 2022, 15, 4285. [Google Scholar] [CrossRef] [PubMed]
- Shevchik, N.J.; Cardona, M.; Tejeda, J. X-Ray and Far-UV Photoemission from Amorphous and Crystalline Films of Se and Te. Phys. Rev. B 1973, 8, 2833. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, C.; Yu, C.; Chen, Q. General Solution-based Route to V-VI Semiconductors Nanorods from Hydrolysate. J. Nanopart. Res. 2007, 9, 269–274. [Google Scholar] [CrossRef]
- Zeng, C.; Ramos-Ruiz, A.; Field, J.A.; Sierra-Alvarez, R. Cadmium Telluride (CdTe) and Cadmium Selenide (CdSe) Leaching Behavior and Surface Chemistry in Response to pH and O2. J. Environ. Manag. 2015, 154, 78–85. [Google Scholar] [CrossRef]
- Wei, T.; Wei, J.; Zhang, K.; Zhang, L. Image Lithography in Telluride Suboxide Thin Film through Controlling “Virtual” Bandgap. Photon. Res. 2017, 5, 22–26. [Google Scholar] [CrossRef]
- Swartz, W.E.; Wynne, K.J.; Hercules, D.M. X-ray Photoelectron Spectroscopic Investigation of Group VIA Elements. Anal. Chem. 1971, 43, 1884–1887. [Google Scholar] [CrossRef]
- Bahl, M.K.; Watson, R.L.; Irgolic, K.J. X-ray Photoemission Studies of Telluride and Some of Its Compounds. J. Chem. Phys. 1977, 66, 5526. [Google Scholar] [CrossRef]
- Fan, K.; Ji, Y.; Zou, H.; Zhang, J.; Zhu, B.; Chen, H.; Daniel, Q.; Luo, Y.; Yu, J.; Sun, L. Hollow Iron-Vanadium Composite Spheres: A Highly Efficient Iron-based Water Oxidation Electrocatalyst without the Need for Nickel or Cobalt. Angew. Chem. Int. Ed. 2017, 56, 3289–3293. [Google Scholar] [CrossRef]
- Arteaga, G.; Rivera-Gavidia, L.M.; Martinez, S.J.; Rizo, R.; Pastor, E.; Garcia, G. Methanol Oxidation on Graphenic-supported Platinum Catalysts. Surfaces 2019, 2, 16–31. [Google Scholar] [CrossRef]
- Rinke, M.T.; Zhang, L.; Eckert, H. Structural Integration of Tellurium Oxide into Mixed-Network-Former Glasses: Connectivity Distribution in the System NaPO3-TeO2. ChemPhysChem 2007, 8, 1988–1998. [Google Scholar] [CrossRef]
- Chu, D.; Wu, Y.; Wang, L. Synthesis and Characterization of Novel Coral Spherical Bismuth Oxide. Results Chem. 2022, 4, 100448. [Google Scholar] [CrossRef]
- Li, J.; Wu, X.; Wan, Z.; Chen, H.; Zhang, G. Full Spectrum Light Driven Photocatalytic in-situ Epitaxy of One-Unit-Cell Bi2O2CO3 layers on Bi2O4 nanocrystals for Highly Efficient Photocatalysis and Mechanism Unveiling. Appl. Catal. B Environ. 2019, 243, 667–677. [Google Scholar] [CrossRef]
- Yu, M.; Peng, X.; Luo, Y.; Yin, S. Applying Research on Creep Constitutive Model of LC4 Aluminium Alloy Based on BC-RBFNN. J. Appl. Phys. 2010, 108, 083704. [Google Scholar]
- Bernede, J.C.; Alaoui, Z.K.; Manai, N. Films of Molybdenum Trioxide Obtained from MoTe2 Oriented Films. Thin Solid Films 1993, 235, 25–29. [Google Scholar] [CrossRef]
- Jensen, H.; Soloviev, A.; Li, Z.; Sogaard, E.G. XPS and FTIR Investigation of the Surface Properties of Different Prepared Titania Nano-Powders. Appl. Surf. Sci. 2005, 246, 239–249. [Google Scholar] [CrossRef]
- Soykan, C.; Gocmez, H. The Physical Properties of Bismuth Replacement in Lead Halogen Perovskite Solar Cells: CH3NH3Pb1–xBixI3 Compounds by Ab-Initio Calculations. Results Phys. 2019, 13, 102278. [Google Scholar] [CrossRef]
- Stanworth, J.E. Tellurite Glasses. Nature 1952, 4301, 581–582. [Google Scholar] [CrossRef]
- Jupnik, H. Photoelectric Properties of Bismuth. Phys. Rev. 1941, 60, 884. [Google Scholar] [CrossRef]
- Katkova, M.A.; Ilichev, V.A.; Konev, A.N.; Batenkin, M.A.; Pestova, I.I.; Vitukhnovsky, A.G.; Bochkarev, M.N. Modification of Anode Surface in Organic Light-Emitting Diodes by Chalcogenes. Appl. Surf. Sci. 2008, 254, 2216–2219. [Google Scholar] [CrossRef]
- Kirichenko, E.A.; Kaminsky, O.I.; Zaytsev, A.V.; Makarevich, K.S.; Pyachin, S.A. Photocatalytic Properties of the α-Bi2O3/Bi Composition in the Visible Region Depending on Metallic Bismuth Concentration and Degree of Imperfection of the Bismuth Oxide Crystal Lattice. Opt. Spectrosc. 2020, 128, 315–322. [Google Scholar] [CrossRef]
- Smyth, C.M.; Zhou, G.; Barton, A.T.; Wallace, R.M.; Hinkle, C.L. Controlling the Pd Metal Contact Polarity to Trigonal Tellurium by Atomic Hydrogen-Removal of the Native Tellurium Oxide. Adv. Mater. Interfaces 2021, 8, 2002050. [Google Scholar] [CrossRef]
- Ryu, B. Work Function of Bismuth Telluride: First-Principles Approach. J. Korean Phys. Soc. 2018, 72, 122–128. [Google Scholar] [CrossRef]
- Palatnik, L.S. Diffraction Effects of X-ray and Electron Scattering from One-and Two-Dimensional Superlattices. Thin Solid Films 1980, 66, 3–10. [Google Scholar] [CrossRef]
- Zhang, H.; Song, D.; Huang, F.; Zhang, J.; Jiang, Y.-P. Critical Behavior in the Epitaxial Growth of Two-Dimensional Tellurium Films on SrTiO3 (001) Substrates. Chin. Phys. B 2023, 32, 066802. [Google Scholar] [CrossRef]
- Kumari, L.; Lin, S.-J.; Lin, J.-H.; Ma, Y.-R. Effects of Deposition Temperature and Thickness on the Structural Properties of Thermal Evaporated Bismuth Thin Films. Appl. Surf. Sci. 2007, 253, 5931–5938. [Google Scholar] [CrossRef]
- Siciliano, T.; Di Giulio, M.; Tepore, M.; Filippo, E.; Micocci, G.; Tepore, A. Tellurium Sputtered Thin Films as NO2 Gas Sensors. Sens. Actuators B Chem. 2008, 135, 250–254. [Google Scholar] [CrossRef]
- Zheng, Z.H.; Chen, T.B.; Cai, Z.K.; Liu, P.J.; Liang, G.X.; Zhang, D.P.; Cai, X.M. Optimization in Fabricating Bismuth Telluride Thin Films by Ion Beam Sputtering Deposition. Thin Solid Films 2012, 520, 5245–5248. [Google Scholar] [CrossRef]
- Kim, D.-H.; Byon, E.; Lee, G.-H.; Cho, S. Effect of Deposition Temperature on the Structural and Thermoelectric Properties of Bismuth Telluride Thin Films Grown by Co-Pputtering. Thin Solid Films 2006, 510, 148–153. [Google Scholar] [CrossRef]
- Queiroga, L.R.; Marcolino, G.F.; Santos, M.; Rodrigues, G.; dos Santos, C.E.; Brito, P. Influence of machining parameters on surface roughness and susceptibility to hydrogen embrittlement of austenitic stainless steels. Int. J. Hydrogen Energy 2019, 44, 29027–29033. [Google Scholar] [CrossRef]
- Tian, Y.; Guo, S.; Lin, Q. Anisotropic Nano-Column Arrays of Bismuth and Its Conductivity. J. Nanosci. Nanotechnol. 2013, 13, 776–780. [Google Scholar] [CrossRef]
- Bhol, P.; Jagdale, P.B.; Jadhav, A.H.; Saxena, M.; Samal, A.K. All-Solid-State Supercapacitors Based on Cobalt Magnesium Telluride Microtubes Decorated with Tellurium Nanotubes. ChemSusChem 2023, 1, e202301009. [Google Scholar] [CrossRef] [PubMed]
- Ketenoğlu, D.İ.; Ünal, B. Influence of Surface Roughness on the Electrical Conductivity of Semiconducting Thin Films. Phys. A Stat. Mech. Appl. 2013, 392, 3008–3017. [Google Scholar] [CrossRef]











| BT0 | BT20 | BT30 | BT50 | BT70 | BT100 | ||
|---|---|---|---|---|---|---|---|
| RF sputtering power [W] | Bi | 5 | 8 | 10 | 12 | 15 | – |
| Te | – | 10 | 15 | 20 | 25 | 30 | |
| Relative Atomic Percentage [%] | ||||
|---|---|---|---|---|
| Sample | XPS | EDX | ||
| Bi | Te | Bi | Te | |
| BT0 | 100.0 | – | 100.0 | – |
| BT20 | 58.4 | 41.6 | 75.5 | 24.5 |
| BT30 | 53.0 | 47.0 | 68.1 | 31.9 |
| BT50 | 33.5 | 66.5 | 50.3 | 49.7 |
| BT70 | 18.6 | 81.4 | 27.0 | 73.0 |
| BT100 | – | 100.0 | – | 100.0 |
| Relative Atomic Percentage [%] | ||||||
|---|---|---|---|---|---|---|
| Sample | Before Ar+-Ion Etching | After Ar+-Ion Etching | ||||
| Bi | Te | O | Bi | Te | O | |
| BT0 | 18.5 | – | 81.5 | 52.1 | – | 47.9 |
| BT20 | 18.8 | 13.4 | 67.8 | 69.2 | 22.6 | 8.2 |
| BT30 | 13.1 | 11.6 | 75.3 | 44.1 | 30.1 | 25.8 |
| BT50 | 11.7 | 23.2 | 65.1 | 23.8 | 55.6 | 20.6 |
| BT70 | 9.2 | 40.2 | 50.6 | 19.4 | 75.3 | 5.3 |
| BT100 | – | 50.4 | 49.6 | – | 89.1 | 10.9 |
| BT20 | BT30 | BT50 | BT70 | |
|---|---|---|---|---|
| RF sputtering power of Te/Bi [W] | 1.25 | 1.50 | 1.67 | 1.67 |
| Work Function [eV] | |
|---|---|
| Bi | 4.20~4.40 [50] |
| Te | 4.80 [51] |
| Bismuth oxide | 5.07 [52] |
| Tellurium oxide | 4.64 [53] |
| Bismuth telluride | 5.10~5.30 [54] |
| Relative Atomic Percentage of Bi and Te with Different Oxidation State [%] | Weighted Average Work Function [eV] | ||||
|---|---|---|---|---|---|
| Te4+–O | Te0 | Bi3+–O | Bi0 | ||
| BT0 | – | – | 83.3 | 16.7 | 4.92 |
| BT20 | 19.6 | 1.9 | 59.8 | 18.6 | 4.81 |
| BT30 | 20.6 | 1.9 | 74.6 | 3.0 | 4.95 |
| BT50 | 55.3 | 5.3 | 39.4 | – | 4.81 |
| BT70 | 44.1 | 43.0 | 12.9 | – | 4.76 |
| BT100 | 56.5 | 43.5 | – | – | 4.71 |
| Sample | Phase | θ [°] | FWHM [°] | Lattice Strain [10−3] |
|---|---|---|---|---|
| BT0 | r-Bi(003) | 11.30 | 0.75 | 1.70 |
| r-Bi(012) | 13.64 | 0.60 | ||
| r-Bi(104) | 19.07 | 0.94 | ||
| r-Bi(110) | 19.87 | 0.58 | ||
| r-Bi(015) | 22.40 | 0.85 | ||
| r-Bi(006) | 23.07 | 0.80 | ||
| BT100 | h-Te(100) | 11.52 | 0.50 | 3.00 |
| h-Te(101) | 13.79 | 0.20 | ||
| h-Te(012) | 19.14 | 0.23 | ||
| h-Te(110) | 20.24 | 0.50 | ||
| h-Te(111) | 21.69 | 0.34 | ||
| h-Te(003) | 22.55 | 0.08 | ||
| h-Te(021) | 24.84 | 0.30 |
| Sample | Surface Free Energy [mN/m] | ||
|---|---|---|---|
| BT0 | 20.0 | 0.6 | 20.6 |
| BT20 | 10.2 | 6.1 | 16.3 |
| BT30 | 9.7 | 6.8 | 16.4 |
| BT50 | 8.8 | 8.5 | 17.3 |
| BT70 | 10.4 | 9.4 | 19.8 |
| BT100 | 28.9 | 1.0 | 29.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mun, J.; Han, S.; Yoon, H.-S.; Kang, J.; Jonas, O.; Park, J.; Kang, Y.-C. Etching and Compositional Ratio Effect on the Surface Properties of Bismuth Telluride Thin Films. Surfaces 2024, 7, 181-195. https://doi.org/10.3390/surfaces7010012
Mun J, Han S, Yoon H-S, Kang J, Jonas O, Park J, Kang Y-C. Etching and Compositional Ratio Effect on the Surface Properties of Bismuth Telluride Thin Films. Surfaces. 2024; 7(1):181-195. https://doi.org/10.3390/surfaces7010012
Chicago/Turabian StyleMun, Jeongho, Sangmin Han, Hee-Seung Yoon, Jisoo Kang, Oliver Jonas, Juyun Park, and Yong-Cheol Kang. 2024. "Etching and Compositional Ratio Effect on the Surface Properties of Bismuth Telluride Thin Films" Surfaces 7, no. 1: 181-195. https://doi.org/10.3390/surfaces7010012