Molecular Mechanisms and Current Treatment Strategy of Sarcopenia and Cachexia
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cellular Pathology".
Viewed by 96410Editor
Topical Collection Information
Dear Colleagues,
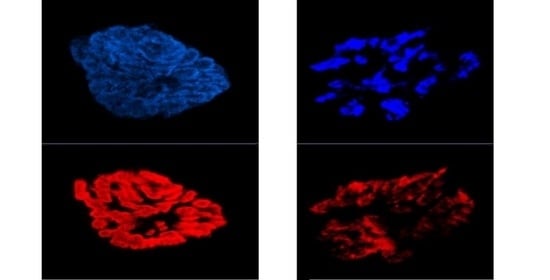
Skeletal muscle is the most abundant tissue in the body, comprising 40–50% of body mass and playing vital roles in locomotion, heat production during periods of cold stress, and overall metabolism. That skeletal muscle consists of the largest pool of proteins in the whole organism, which highlights why this specific tissue is highly sensitive under conditions that act to alter the balance between protein synthesis and degradation.
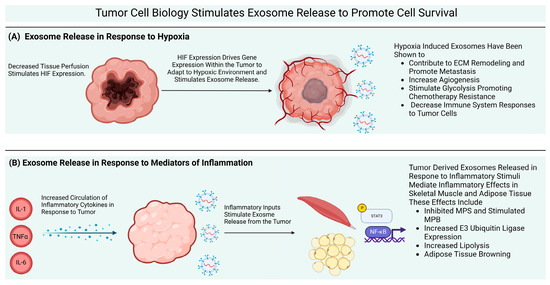
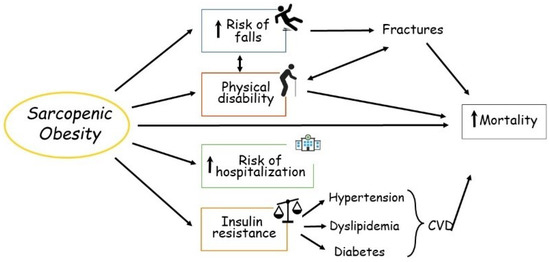
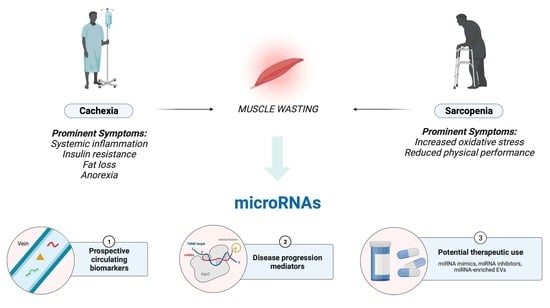
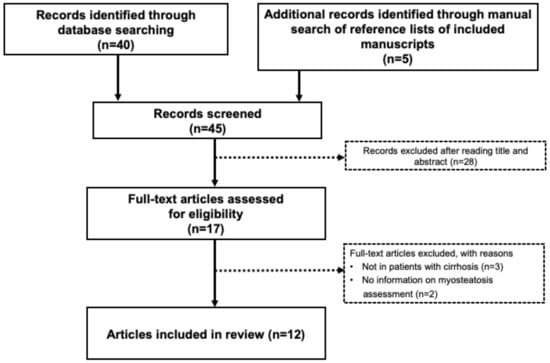
Two common but distinct conditions characterized by a loss of skeletal muscle mass are sarcopenia and cachexia. Muscle wasting is an inevitable part of aging (sarcopenia). Cachexia is associated not only with chronic diseases, most commonly cancer, but also with other inflammatory conditions, such as chronic obstructive pulmonary disease (COPD), heart failure, chronic kidney disease, AIDS, and sepsis.
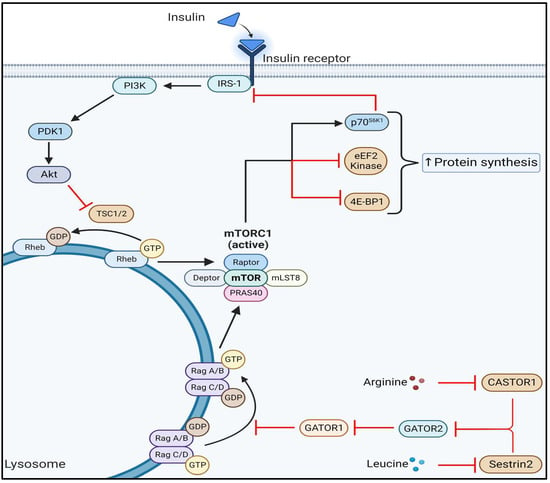
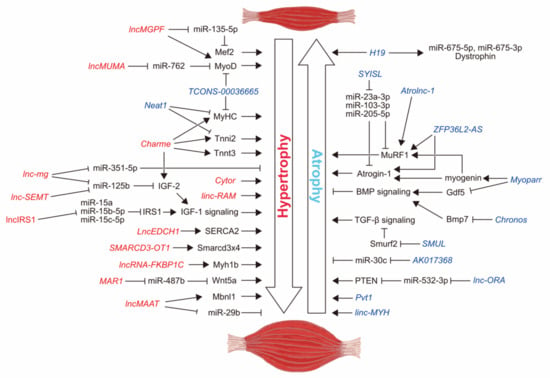
Sarcopenia and cachexia occur due to a multifactorial process that involves physical activity, nutritional intake, metabolic homeostasis, oxidative stress, hormonal changes, and life span. The specific contribution of each of these factors is unknown, but more recent studies have indicated an apparent functional defect in autophagy-dependent signaling in both sarcopenic and cachectic muscles. In contrast, many investigators have failed to demonstrate age-related enhancement in the ubiquitin-proteasome system in the case of sarcopenia. Since many researchers try to elucidate the molecular mechanism of sarcopenia and cachexia, recent understanding is very broad.
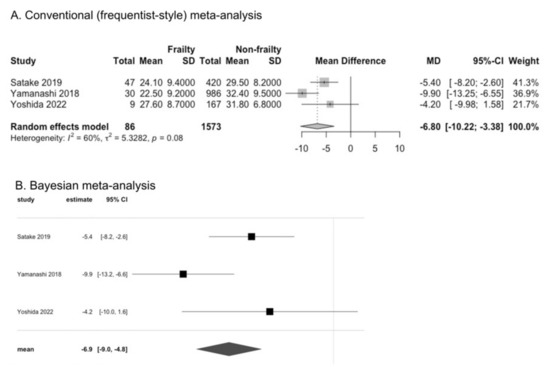
A recent review indicated the effectiveness of exercise training and some hormonal, nutritional, and pharmacological approaches for these two conditions. In addition, sarcopenia has been most attenuated by mild caloric restriction (CR) in all mammals. In contrast, treatment with ghrelin is well known to exhibit positive effects on cancer cachexia.
This Topical Collection aims to provides recent research advances dealing with molecular mediators modulating muscle mass in both sarcopenia and cachexia. In addition, this topic includes recent attenuating strategies (hormone, pharmacology, nutrition, etc.) for this wasting.
We look forward to your contributions.
Prof. Dr. Kunihiro Sakuma
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- skeletal muscle
- muscle fiber
- sarcopenia
- cachexia
- autophagy
- mitophagy
- apoptosis
- inflammation
- ubiquitin-proteasome system
- myostatin
- ribosome
- mitochondria
- Akt
- mTOR
- FOXO
- amino acids
- supplementation
- hormonal treatment
- caloric restriction
- satellite cell