How Perinatal Stress Affects Brain Plasticity in Ontogenesis
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cells of the Nervous System".
Viewed by 9060Editors
2. Head of the Laboratory of Biochemistry, Saint-Petersburg Institute of Bioregulation and Gerontology, Saint-Petersburg, Russia
Interests: nervous and immune systems; neurons and glial cells; neuroinflammation; neurodegeneration; reproduction; placenta; fetus; neurotrophins; hyperhomocysteinemia; brain development and plasticity
Interests: adaptation; alzheimer animal models; apoptosis; cellular models; cerebral ischemia; dementia; depression; epilepsy; excitotoxicity; neuroinflammation; hippocampus; traumatic brain injury; epileptogenesis
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
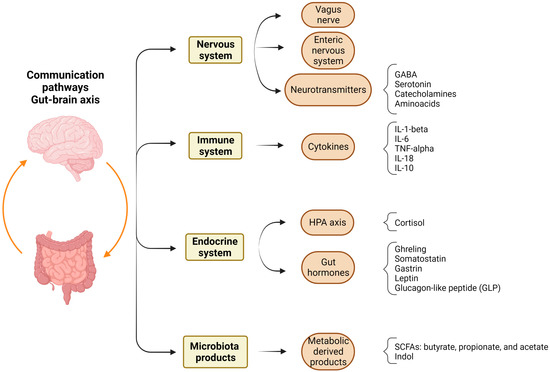
Neuroplasticity (brain plasticity or neural plasticity) is the remarkable capacity of the brain to alter and adapt to changing environments. This dynamic process allowing one to adapt to different experiences and learn is also a factor in recovery from brain injuries, since rehabilitation is aimed at rebuilding connections between neurons or the “rewiring” of the brain. Neuroplasticity can be observed on multiple scales, with adaptive behavior, learning, and memory being at the top of the neuroplasticity hierarchy. The base of this pyramid is formed of molecules and their interactions, which underlie subcellular/synaptic, cellular, and neuronal circuits as well as different network levels. Long-term plasticity occurs as a result of changes in gene expression that are triggered by signaling cascades during altered neuronal activity. Cerebral pathologies are often associated with limitations of the adaptive capacity of neuroplasticity or aberrant excessive neuroplasticity.
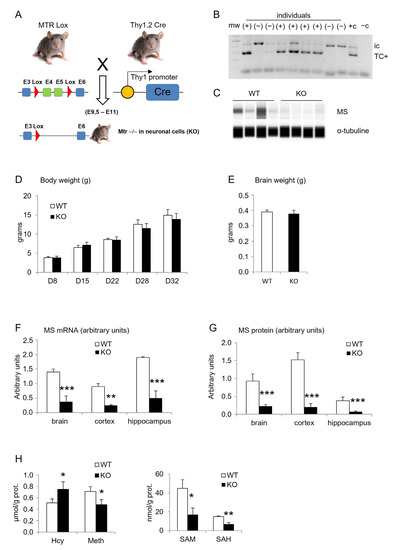
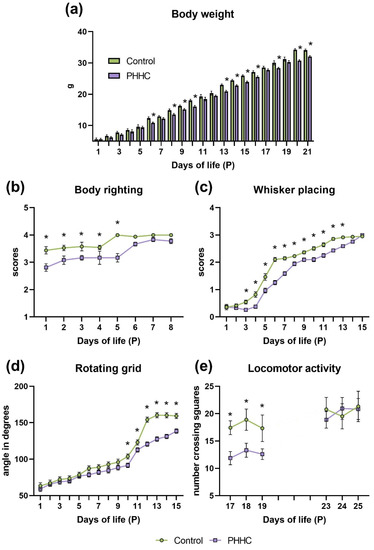
Early life stress (due to different forms of abuse and neglect as well as the effects of pathological factors experienced by the developing child) is associated with the disturbed development of the brain. Recent advances strongly support the essential role of perinatal stress in delayed psychological, psychiatric, and neurological sequelae during ontogenesis, specifically in adolescence and adulthood. A number of animal models of perinatal stress have been developed and used to study the mechanisms of its detrimental effects on the brain, with these studies having major translational significance. The brain plays a key role in the development, well-being, and survival of organisms; therefore, various stress factors during prenatal and early postnatal periods (fetal or maternal hypoxia, hyperhomocysteinemia, etc.) that affect the development of neuroplasticity mechanisms are of critical importance. The perinatal period of brain development is extremely important, since it involves the formation of the main brain structures associated with neurogenesis and gliogenesis, the maturation of synapses, as well as other essential events regulated by endocrine and immune systems and potentially vulnerable to early-life stress. Stress-induced changes involve all levels of neuroplasticity, including synaptic plasticity. This Topical Collection is aimed at the accumulation of new data regarding the effects of perinatal stress on the development of brain plasticity mechanisms at all levels of brain organization. The goal of this collection is to highlight the translational potential of these data to elucidate connections between perinatal stress and negative health outcomes.
Prof. Dr. Alexander V. Arutjunyan
Prof. Dr. Natalia V. Gulyaeva
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- neuroplasticity
- synaptic plasticity
- maladaptive plasticity
- prenatal stress
- perinatal stress
- early life stress
- development
- ontogenesis
- neuropsychiatric disorders
- neurogenesis