Cellular and Molecular Pathophysiology of Vascular Proliferative Diseases
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cellular Pathology".
Viewed by 42361
Share This Topical Collection
Editor
 Dr. Lahouaria Hadri
Dr. Lahouaria Hadri
 Dr. Lahouaria Hadri
Dr. Lahouaria Hadri
E-Mail
Website
Collection Editor
Icahn School of Medicine at Mount Sinai, New York, NY, USA
Interests: vascular remodeling; pulmonary arterial hypertension; vascular pulmonary injury; gene therapy; calcium signaling; epigenetic modifications
Topical Collection Information
Dear Colleagues,
Excessive cellular proliferation contributes to the pathobiology of vascular obstructive diseases (e.g., atherosclerosis, in-stent restenosis, transplant vasculopathy, vessel bypass graft failure, and pulmonary vascular diseases). Remodeling of the injured vessel, endothelium dysfunction, proliferation and migration of vascular smooth muscle cells (VSMC), and accumulation of extracellular matrix proteins are the main traits of these diseases.
Current advances in the study of pathogenesis have revealed that vascular proliferative diseases involve a combination of multiple causative factors (genomic, epigenomic, and environmental factors), which consequentially trigger signaling cascades of downstream effects, resulting in complex and heterogeneous phenotypes. Therefore, investigating the mechanism pathways underlying vascular dysfunction and remodeling can help design the potential key targets and find the most effective treatment. The main aim of this Special Issue is to publish cutting-edge research using cutting-edge tools and technologies that can significantly advance the understanding of vascular diseases and transform future therapies.
Dr. Lahouaria Hadri
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- vascular remodeling
- coronary artery diseases
- pulmonary vascular diseases
- lung injury
- endothelial cell dysfunction
- smooth muscle cell dedifferentiation
- epigenetics
- chromatin regulation
Published Papers (7 papers)
Open AccessReview
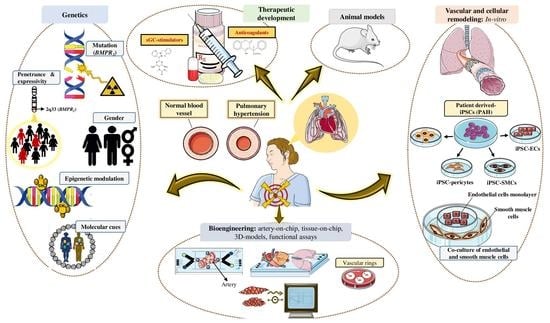
The Role of Bone Morphogenetic Protein Receptor Type 2 (BMPR2) and the Prospects of Utilizing Induced Pluripotent Stem Cells (iPSCs) in Pulmonary Arterial Hypertension Disease Modeling
by
Anichavezhi Devendran, Sumanta Kar, Rasheed Bailey and Maria Giovanna Trivieri
Cited by 7 | Viewed by 10295
Abstract
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by increased pulmonary vascular resistance (PVR), causing right ventricular hypertrophy and ultimately death from right heart failure. Heterozygous mutations in the bone morphogenetic protein receptor type 2 (
BMPR2) are linked to approximately
[...] Read more.
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by increased pulmonary vascular resistance (PVR), causing right ventricular hypertrophy and ultimately death from right heart failure. Heterozygous mutations in the bone morphogenetic protein receptor type 2 (
BMPR2) are linked to approximately 80% of hereditary, and 20% of idiopathic PAH cases, respectively. While patients carrying a
BMPR2 gene mutation are more prone to develop PAH than non-carriers, only 20% will develop the disease, whereas the majority will remain asymptomatic. PAH is characterized by extreme vascular remodeling that causes pulmonary arterial endothelial cell (PAEC) dysfunction, impaired apoptosis, and uncontrolled proliferation of the pulmonary arterial smooth muscle cells (PASMCs). To date, progress in understanding the pathophysiology of PAH has been hampered by limited access to human tissue samples and inadequacy of animal models to accurately mimic the pathogenesis of human disease. Along with the advent of induced pluripotent stem cell (iPSC) technology, there has been an increasing interest in using this tool to develop patient-specific cellular models that precisely replicate the pathogenesis of PAH. In this review, we summarize the currently available approaches in iPSC-based PAH disease modeling and explore how this technology could be harnessed for drug discovery and to widen our understanding of the pathophysiology of PAH.
Full article
►▼
Show Figures
Open AccessArticle
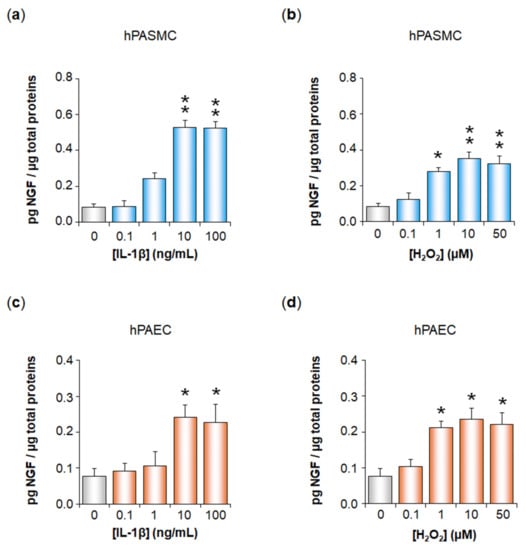
Inflammation and Oxidative Stress Induce NGF Secretion by Pulmonary Arterial Cells through a TGF-β1-Dependent Mechanism
by
Clément Bouchet, Guillaume Cardouat, Matthieu Douard, Florence Coste, Paul Robillard, Frédéric Delcambre, Thomas Ducret, Jean-François Quignard, Pierre Vacher, Isabelle Baudrimont, Roger Marthan, Patrick Berger, Christelle Guibert and Véronique Freund-Michel
Cited by 4 | Viewed by 1981
Abstract
Expression of the nerve growth factor NGF is increased in pulmonary hypertension (PH). We have here studied whether oxidative stress and inflammation, two pathological conditions associated with transforming growth factor-β1 (TGF-β1) in PH, may trigger NGF secretion by pulmonary arterial (PA) cells. Effects
[...] Read more.
Expression of the nerve growth factor NGF is increased in pulmonary hypertension (PH). We have here studied whether oxidative stress and inflammation, two pathological conditions associated with transforming growth factor-β1 (TGF-β1) in PH, may trigger NGF secretion by pulmonary arterial (PA) cells. Effects of hydrogen peroxide (H
2O
2) and interleukin-1β (IL-1β) were investigated ex vivo on rat pulmonary arteries, as well as in vitro on human PA smooth muscle (hPASMC) or endothelial cells (hPAEC). TβRI expression was assessed by Western blotting. NGF PA secretion was assessed by ELISA after TGF-β1 blockade (anti-TGF-β1 siRNA, TGF-β1 blocking antibodies, TβRI kinase, p38 or Smad3 inhibitors). TβRI PA expression was evidenced by Western blotting both ex vivo and in vitro. H
2O
2 or IL-1β significantly increased NGF secretion by hPASMC and hPAEC, and this effect was significantly reduced when blocking TGF-β1 expression, binding to TβRI, TβRI activity, or signaling pathways. In conclusion, oxidative stress and inflammation may trigger TGF-β1 secretion by hPASMC and hPAEC. TGF-β1 may then act as an autocrine factor on these cells, increasing NGF secretion via TβRI activation. Since NGF and TGF-β1 are relevant growth factors involved in PA remodeling, such mechanisms may therefore be relevant to PH pathophysiology.
Full article
►▼
Show Figures
Open AccessEditor’s ChoiceReview
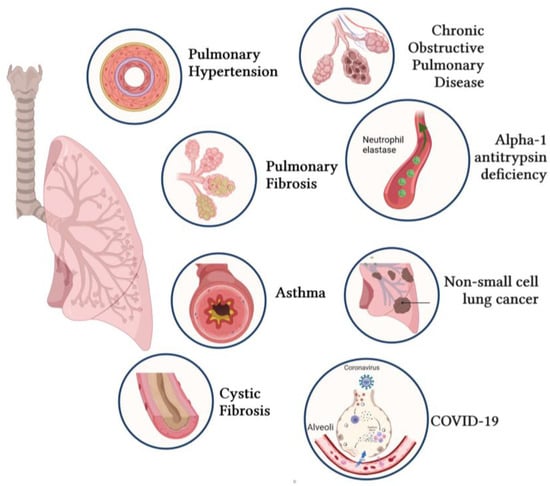
Novel Insights into the Therapeutic Potential of Lung-Targeted Gene Transfer in the Most Common Respiratory Diseases
by
Malik Bisserier, Xiao-Qing Sun, Shahood Fazal, Irene C. Turnbull, Sébastien Bonnet and Lahouaria Hadri
Cited by 13 | Viewed by 9110
Abstract
Over the past decades, a better understanding of the genetic and molecular alterations underlying several respiratory diseases has encouraged the development of new therapeutic strategies. Gene therapy offers new therapeutic alternatives for inherited and acquired diseases by delivering exogenous genetic materials into cells
[...] Read more.
Over the past decades, a better understanding of the genetic and molecular alterations underlying several respiratory diseases has encouraged the development of new therapeutic strategies. Gene therapy offers new therapeutic alternatives for inherited and acquired diseases by delivering exogenous genetic materials into cells or tissues to restore physiological protein expression and/or activity. In this review, we review (1) different types of viral and non-viral vectors as well as gene-editing techniques; and (2) the application of gene therapy for the treatment of respiratory diseases and disorders, including pulmonary arterial hypertension, idiopathic pulmonary fibrosis, cystic fibrosis, asthma, alpha-1 antitrypsin deficiency, chronic obstructive pulmonary disease, non-small-cell lung cancer, and COVID-19. Further, we also provide specific examples of lung-targeted therapies and discuss the major limitations of gene therapy.
Full article
►▼
Show Figures
Open AccessArticle
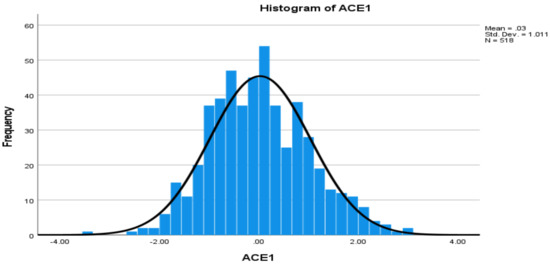
Levels of Angiotensin-Converting Enzyme and Apolipoproteins Are Associated with Alzheimer’s Disease and Cardiovascular Diseases
by
Chun Xu, Debra Garcia, Yongke Lu, Kaysie Ozuna, Donald A. Adjeroh, Kesheng Wang and on behalf of the Alzheimer’s Disease Neuroimaging Initiative
Cited by 16 | Viewed by 3041
Abstract
Angiotensin-converting enzyme-1 (ACE1) and apolipoproteins (APOs) may play important roles in the development of Alzheimer’s disease (AD) and cardiovascular diseases (CVDs). This study aimed to examine the associations of AD, CVD, and endocrine-metabolic diseases (EMDs) with the levels of ACE1 and 9 APO
[...] Read more.
Angiotensin-converting enzyme-1 (ACE1) and apolipoproteins (APOs) may play important roles in the development of Alzheimer’s disease (AD) and cardiovascular diseases (CVDs). This study aimed to examine the associations of AD, CVD, and endocrine-metabolic diseases (EMDs) with the levels of ACE1 and 9 APO proteins (ApoAI, ApoAII, ApoAIV, ApoB, ApoCI, ApoCIII, ApoD, ApoE, and ApoH). Non-Hispanic white individuals including 109 patients with AD, 356 mild cognitive impairment (MCI), 373 CVD, 198 EMD and controls were selected from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) dataset. Multivariable general linear model (GLM) was used to examine the associations.
ApoE ε4 allele was associated with AD, as well as ApoAIV, ApoB and ApoE proteins, but not associated with CVD and EMD. Both AD and CVD were associated with levels of ACE1, ApoB, and ApoH proteins. AD, MCI and EMD were associated with levels of ACE1, ApoAII, and ApoE proteins. This is the first study to report associations of ACE1 and several APO proteins with AD, MCI, CVD and EMD, respectively, including upregulated and downregulated protein levels. In conclusion, as specific or shared biomarkers, the levels of ACE1 and APO proteins are implicated for AD, CVD, EMD and
ApoE ε4 allele. Further studies are required for validation to establish reliable biomarkers for these health conditions.
Full article
►▼
Show Figures
Open AccessReview
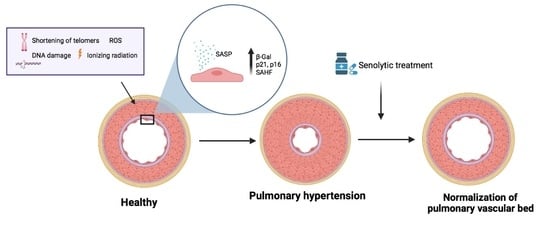
Senescence Alterations in Pulmonary Hypertension
by
Inés Roger, Javier Milara, Nada Belhadj and Julio Cortijo
Cited by 13 | Viewed by 5609
Abstract
Cellular senescence is the arrest of normal cell division and is commonly associated with aging. The interest in the role of cellular senescence in lung diseases derives from the observation of markers of senescence in chronic obstructive pulmonary disease (COPD), pulmonary fibrosis (IPF),
[...] Read more.
Cellular senescence is the arrest of normal cell division and is commonly associated with aging. The interest in the role of cellular senescence in lung diseases derives from the observation of markers of senescence in chronic obstructive pulmonary disease (COPD), pulmonary fibrosis (IPF), and pulmonary hypertension (PH). Accumulation of senescent cells and the senescence-associated secretory phenotype in the lung of aged patients may lead to mild persistent inflammation, which results in tissue damage. Oxidative stress due to environmental exposures such as cigarette smoke also promotes cellular senescence, together with additional forms of cellular stress such as mitochondrial dysfunction and endoplasmic reticulum stress. Growing recent evidence indicate that senescent cell phenotypes are observed in pulmonary artery smooth muscle cells and endothelial cells of patients with PH, contributing to pulmonary artery remodeling and PH development. In this review, we analyze the role of different senescence cell phenotypes contributing to the pulmonary artery remodeling process in different PH clinical entities. Different molecular pathway activation and cellular functions derived from senescence activation will be analyzed and discussed as promising targets to develop future senotherapies as promising treatments to attenuate pulmonary artery remodeling in PH.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
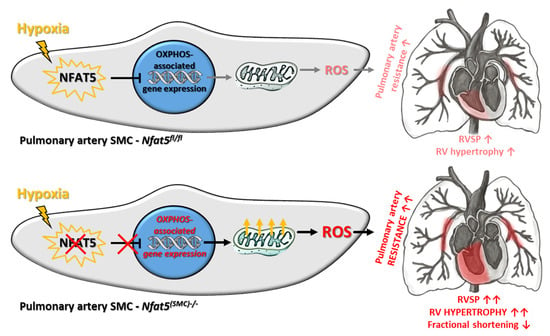
NFAT5/TonEBP Limits Pulmonary Vascular Resistance in the Hypoxic Lung by Controlling Mitochondrial Reactive Oxygen Species Generation in Arterial Smooth Muscle Cells
by
Hebatullah Laban, Sophia Siegmund, Maren Zappe, Felix A. Trogisch, Jörg Heineke, Carolina De La Torre, Beate Fisslthaler, Caroline Arnold, Jonathan Lauryn, Michael Büttner, Carolin Mogler, Katsuhiro Kato, Ralf H. Adams, Hanna Kuk, Andreas Fischer, Markus Hecker, Wolfgang M. Kuebler and Thomas Korff
Cited by 6 | Viewed by 3280
Abstract
Chronic hypoxia increases the resistance of pulmonary arteries by stimulating their contraction and augmenting their coverage by smooth muscle cells (SMCs). While these responses require adjustment of the vascular SMC transcriptome, regulatory elements are not well defined in this context. Here, we explored
[...] Read more.
Chronic hypoxia increases the resistance of pulmonary arteries by stimulating their contraction and augmenting their coverage by smooth muscle cells (SMCs). While these responses require adjustment of the vascular SMC transcriptome, regulatory elements are not well defined in this context. Here, we explored the functional role of the transcription factor nuclear factor of activated T-cells 5 (NFAT5/TonEBP) in the hypoxic lung. Regulatory functions of NFAT5 were investigated in cultured artery SMCs and lungs from control (
Nfat5fl/fl) and SMC-specific
Nfat5-deficient (
Nfat5(SMC)−/−) mice. Exposure to hypoxia promoted the expression of genes associated with metabolism and mitochondrial oxidative phosphorylation (OXPHOS) in
Nfat5(SMC)−/− versus
Nfat5fl/fl lungs. In vitro, hypoxia-exposed
Nfat5-deficient pulmonary artery SMCs elevated the level of OXPHOS-related transcripts, mitochondrial respiration, and production of reactive oxygen species (ROS). Right ventricular functions were impaired while pulmonary right ventricular systolic pressure (RVSP) was amplified in hypoxia-exposed
Nfat5(SMC)−/− versus
Nfat5fl/fl mice. Scavenging of mitochondrial ROS normalized the raise in RVSP. Our findings suggest a critical role for NFAT5 as a suppressor of OXPHOS-associated gene expression, mitochondrial respiration, and ROS production in pulmonary artery SMCs that is vital to limit ROS-dependent arterial resistance in a hypoxic environment.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
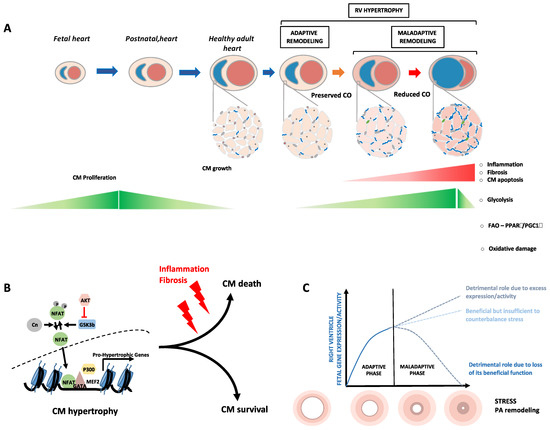
Fetal Gene Reactivation in Pulmonary Arterial Hypertension: GOOD, BAD, or BOTH?
by
Sarah-Eve Lemay, Charifa Awada, Tsukasa Shimauchi, Wen-Hui Wu, Sébastien Bonnet, Steeve Provencher and Olivier Boucherat
Cited by 10 | Viewed by 3721
Abstract
Pulmonary arterial hypertension is a debilitating chronic disorder marked by the progressive obliteration of the pre-capillary arterioles. This imposes a pressure overload on the right ventricle (RV) pushing the latter to undergo structural and mechanical adaptations that inexorably culminate in RV failure and
[...] Read more.
Pulmonary arterial hypertension is a debilitating chronic disorder marked by the progressive obliteration of the pre-capillary arterioles. This imposes a pressure overload on the right ventricle (RV) pushing the latter to undergo structural and mechanical adaptations that inexorably culminate in RV failure and death. Thanks to the advances in molecular biology, it has been proposed that some aspects of the RV and pulmonary vascular remodeling processes are orchestrated by a subversion of developmental regulatory mechanisms with an upregulation of a suite of genes responsible for the embryo’s early growth and normally repressed in adults. In this review, we present relevant background regarding the close relationship between overactivation of fetal genes and cardiopulmonary remodeling, exploring whether the reawakening of developmental factors plays a causative role or constitutes a protective mechanism in the setting of PAH.
Full article
►▼
Show Figures
Planned Papers
The below list represents only planned manuscripts. Some of these
manuscripts have not been received by the Editorial Office yet. Papers
submitted to MDPI journals are subject to peer-review.
Title: The Mechanosensitive and Nobel Prize Winner Piezo1 in Pulmonary Hypertension
Authors: Mazen Shihan; Tatyana Novoyatleva; Monika Brosien; Thilo Lehmeyer; Akylbek Sydykov; Ralph T. Schermuly
Affiliation: Excellence Cluster Cardio-Pulmonary Institute (CPI), Universities of Giessen and Marburg Lung Center (UGMLC), German Center for Lung Research (DZL), Justus Liebig University of Giessen, Aulweg 130, 35392 Giessen, Germany
Abstract: Pulmonary arterial hypertension (PAH), group 1 of pulmonary hypertension (PH), is a progressive and so far not curable disease that is characterized by increased pulmonary arterial pressure (PAP) >20 mm Hg, with pulmonary arterial wedge pressure (PWP) 3 Wood units at rest. The increased PAP is a result of dysfunction and hyper-proliferation of the pulmonary arterial cells that, consequently, leads to thickening of the pulmonary arterial wall, narrowing the pulmonary arteries lumen, and resistance to vasorelaxation. Piezo1, a mechanosensitive channel that is discovered by the end of 2010 and is the winner of the Nobel Prize for the year 2021, is getting much more interest. Disorders of the Piezo1 gene were associated with a few diseases such as; Dehydrated Hereditary Stomatocytosis, Hemolytic Anemia, and Immune Hydrops Fetalis. Recently, Piezo1 was shown to play important role in the transduction of mechanical forces e.g. fluid shear stress to more endothelial release of nitric oxide (NO) and maintenance of the systemic blood pressure. Similarly, a recent study showed endothelial Piezo1 to relax intrapulmonary arteries in chronically hypoxic mice, a model of pulmonary hypertension disease (PH). In this review, we will approach any possible role of Piezo1 in the cellular and molecular mechanism, development, and treatment of PH.