Evaluation of an Innovative Method for Calculating Energy Intake of Hospitalized Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Staff Training
2.2. Reliability and Accuracy of the Calorie Counts
2.3. Dietary Staff Time Savings
2.4. Statistical Methods
3. Results
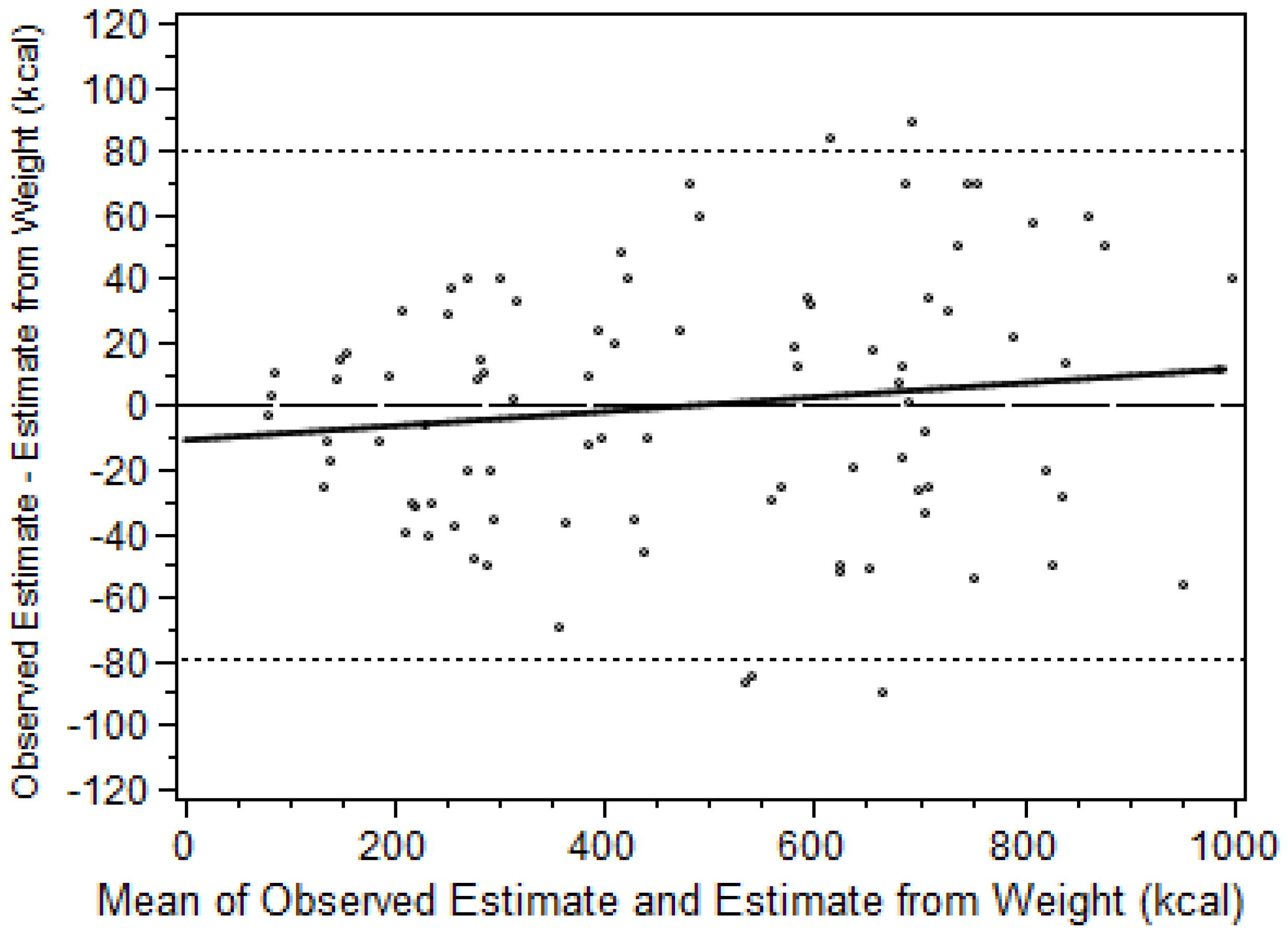
3.1. Reliability and Accuracy of the Calorie Counts
3.2. Staff Time Savings
4. Discussion
4.1. Overview
4.2. Strengths
4.3. Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Souza, T.T.; Sturion, C.J.; Faintuch, J. Is the Skeleton Still in the Hospital Closet? A Review of Hospital Malnutrition Emphasizing Health Economic Aspects. Clin. Nutr. 2015, 34, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Stratton, R.J.; Hackston, A.; Longmore, D.; Dixon, R.; Price, S.; Stroud, M.; King, C.; Elia, M. Malnutrition in Hospital Outpatients and Inpatients: Prevalence, Concurrent Validity and Ease of Use of the ‘Malnutrition Universal Screening Tool’ (‘MUST’) for Adults. Br. J. Nutr. 2004, 92, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Mudge, A.M.; Ross, L.J.; Young, A.M.; Isenring, E.A.; Banks, M.D. Helping Understand Nutritional Gaps in the Elderly (HUNGER): A Prospective Study of Patient Factors Associated With Inadequate Nutritional Intake in Older Medical Inpatients. Clin. Nutr. 2011, 30, 320–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, D.H.; Patch, G.A.; Walls, R.C.; Lipschitz, D.A. Impact of Nutrition Status on Morbidity and Mortality in a Select Population of Geriatric Rehabilitation Patients. Am. J. Clin. Nutr. 1990, 51, 749–758. [Google Scholar] [PubMed]
- Sullivan, D.H.; Walls, R.C.; Lipschitz, D.A. Protein-Energy Undernutrition and the Risk of Mortality within 1 y of Hospital Discharge in a Select Population of Geriatric Rehabilitation Patients. Am. J. Clin. Nutr. 1991, 53, 599–605. [Google Scholar] [PubMed]
- Mudge, A.M.; O’Rourke, P.; Denaro, C.P. Timing and Risk Factors for Functional Changes Associated with Medical Hospitalization in Older Patients. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.H.; Walls, R.C. Impact of Nutritional Status on Morbidity in a Population of Geriatric Rehabilitation Patients. J. Am. Geriatr. Soc. 1994, 42, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.H.; Johnson, L.E.; Dennis, R.A.; Roberson, P.K.; Garner, K.K.; Padala, P.R.; Padala, K.P.; Bopp, M.M. Nutrient Intake, Peripheral Edema, and Weight Change in Elderly Recuperative Care Patients. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.H.; Sun, S.; Walls, R.C. Protein-Energy Undernutrition Among Elderly Hospitalized Patients: A Prospective Study. JAMA 1999, 281, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Holyday, M.; Daniells, S.; Bare, M.; Caplan, G.A.; Petocz, P.; Bolin, T. Malnutrition Screening and Early Nutrition Intervention in Hospitalised Patients in Acute Aged Care: A Randomised Controlled Trial. J. Nutr. Health Aging 2012, 16, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.L.; Compher, C.; Sullivan, D.H.; Mullin, G.E. Recognizing Malnutrition in Adults: Definitions and Characteristics, Screening, Assessment, and Team Approach. JPEN J. Parenter. Enter. Nutr. 2013, 37, 802–807. [Google Scholar] [CrossRef] [PubMed]
- White, J.V.; Stotts, N.; Jones, S.W.; Granieri, E. Managing Postacute Malnutrition (Undernutrition) Risk. JPEN J. Parenter. Enter. Nutr. 2013, 37, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Tappenden, K.A.; Quatrara, B.; Parkhurst, M.L.; Malone, A.M.; Fanjiang, G.; Ziegler, T.R. Critical Role of Nutrition in Improving Quality of Care: An Interdisciplinary Call to Action to Address Adult Hospital Malnutrition. J. Acad. Nutr. Diet. 2013, 113, 1219–1237. [Google Scholar] [CrossRef] [PubMed]
- Gaskill, D.; Pearson, A. The Nutritionally Vulnerable Patient: A Pilot Study to Compare Nurses’ Assessment of Intake With Actual Intake. J. Clin. Nurs. 1992, 1, 101–106. [Google Scholar] [CrossRef]
- Palmer, M.; Miller, K.; Noble, S. The Accuracy of Food Intake Charts Completed by Nursing Staff As Part of Usual Care When No Additional Training in Completing Intake Tools Is Provided. Clin. Nutr. 2015, 34, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Dubois, S. Accuracy of Visual Estimates of Plate Waste in the Determination of Food Consumption. J. Am. Diet. Assoc. 1990, 90, 382–387. [Google Scholar] [PubMed]
- Dietscher, J.E.; Preece, C.K.; Lewis, R.D.J.; Foulks, C.J.; Bass, M. Comparison of Patients’ Meal Intake Estimates: The Eye Is Bigger Than the Plate. J. Ren. Nutr. 1997, 7, 199–203. [Google Scholar] [CrossRef]
- Levine, J.A.; Madden, A.M.; Morgan, M.Y. Validation of a Computer Based System for Assessing Dietary Intake. Br. Med. J. (Clin. Res. Ed.) 1987, 295, 369–372. [Google Scholar] [CrossRef] [Green Version]
- Lorefalt, B.; Unosson, M. Estimation of Energy Intake in Clinical Practice: A Comparison Between a Food Record Protocol and a Precoded Food Record Book. J. Clin. Nurs. 2002, 11, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Bjornsdottir, R.; Oskarsdottir, E.S.; Thordardottir, F.R.; Ramel, A.; Thorsdottir, I.; Gunnarsdottir, I. Validation of a Plate Diagram Sheet for Estimation of Energy and Protein Intake in Hospitalized Patients. Clin. Nutr. 2013, 32, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Forli, L.; Oppedal, B.; Skjelle, K.; Vatn, M. Validation of a Self-Administered Form for Recording Food Intake in Hospital Patients. Eur. J. Clin. Nutr. 1998, 52, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.K.; Gines, D.J. Dietary Recall Method Comparison for Hospitalized Elderly Subjects. J. Am. Diet. Assoc. 1985, 85, 202–205. [Google Scholar] [PubMed]
- Sullivan, S.C.; Bopp, M.; Weaver, D.L.; Sullivan, D.H. Innovations in Calculating Precise Nutrient Intake of Hospitalized Patients. Nutrients 2016, 8, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.H.; Johnson, L.E.; Dennis, R.A.; Roberson, P.K.; Heif, M.; Garner, K.K.; Bopp, M.M. The Interrelationships Among Albumin, Nutrient Intake, and Inflammation in Elderly Recuperative Care Patients. J. Nutr. Health Aging 2011, 15, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Dennis, R.A.; Johnson, L.E.; Roberson, P.K.; Heif, M.; Bopp, M.M.; Garner, K.K.; Padala, K.P.; Padala, P.R.; Dubbert, P.M.; Sullivan, D.H. Changes in Activities of Daily Living, Nutrient Intake, and Systemic Inflammation in Elderly Adults Receiving Recuperative Care. J. Am. Geriatr. Soc. 2012, 60, 2246–2253. [Google Scholar] [CrossRef] [PubMed]
- Bird, G.; Elwood, P.C. The Dietary Intakes of Subjects Estimated From Photographs Compared with a Weighed Record. Hum. Nutr. Appl. Nutr. 1983, 37, 470–473. [Google Scholar] [PubMed]
- Williamson, D.A.; Allen, H.R.; Martin, P.D.; Alfonso, A.J.; Gerald, B.; Hunt, A. Comparison of Digital Photography to Weighed and Visual Estimation of Portion Sizes. J. Am. Diet. Assoc. 2003, 103, 1139–1145. [Google Scholar] [CrossRef]
- Martin, C.K.; Nicklas, T.; Gunturk, B.; Correa, J.B.; Allen, H.R.; Champagne, C. Measuring Food Intake with Digital Photography. J. Hum. Nutr. Diet. 2014, 27 (Suppl. 1), 72–81. [Google Scholar] [CrossRef] [PubMed]
- Simmons, S.F.; Reuben, D. Nutritional Intake Monitoring for Nursing Home Residents: A Comparison of Staff Documentation, Direct Observation, and Photography Methods. J. Am. Geriatr. Soc. 2000, 48, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Dennis, R.A.; Johnson, L.E.; Roberson, P.K.; Heif, M.; Bopp, M.M.; Cook, J.; Sullivan, D.H. Changes in Prealbumin, Nutrient Intake, and Systemic Inflammation in Elderly Recuperative Care Patients. J. Am. Geriatr. Soc. 2008, 56, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. USDA National Nutrient Database for Standard Reference; United States Department of Agriculture: Beltsville, MD, USA, 2000.
- Bland, J.M.; Altman, D.G. Measuring Agreement in Method Comparison Studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Mulsant, B.H.; Kastango, K.B.; Rosen, J.; Stone, R.A.; Mazumdar, S.; Pollock, B.G. Interrater Reliability in Clinical Trials of Depressive Disorders. Am. J. Psychiatry 2002, 159, 1598–1600. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.F. Nutritive Value of American Foods in Common Units, 1st ed.; Agricultural Research Service United States Department of Agriculture: Washington, DC, USA, 1975. [Google Scholar]
- United States Department of Agriculture. Composition of Foods Raw, Processed, Prepared USDA National Nutrient Database for Standard Reference; United States Department of Agriculture: Beltsville, MD, USA, 1992.
- Thompson, F.E.; Kirkpatrick, S.I.; Subar, A.F.; Reedy, J.; Schap, T.E.; Wilson, M.M.; Krebs-Smith, S.M. The National Cancer Institute’s Dietary Assessment Primer: A Resource for Diet Research. J. Acad. Nutr. Diet. 2015, 115, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, A.; Sudo, N.; Amitani, Y.; Caballero, Y.; Sekiyama, M.; Mukamugema, C.; Matsuoka, T.; Imanishi, H.; Sasaki, T.; Matsuda, H. Development and Validation of a Data-Based Food Frequency Questionnaire for Adults in Eastern Rural Area of Rwanda. Nutr. Metab. Insights 2016, 9, 31–42. [Google Scholar] [PubMed]
- Yokoyama, Y.; Takachi, R.; Ishihara, J.; Ishii, Y.; Sasazuki, S.; Sawada, N.; Shinozawa, Y.; Tanaka, J.; Kato, E.; Kitamura, K.; et al. Validity of Short and Long Self-Administered Food Frequency Questionnaires in Ranking Dietary Intake in Middle-Aged and Elderly Japanese in the Japan Public Health Center-Based Prospective Study for the Next Generation (JPHC-NEXT) Protocol Area. J. Epidemiol. 2016, 26, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.M.; Perez-Rodrigo, C.; Lopez-Sobaler, A.M. Dietary Assessment Methods: Dietary Records. Nutr. Hosp. 2015, 31 (Suppl. 3), 38–45. [Google Scholar] [PubMed]
- Eysteinsdottir, T.; Thorsdottir, I.; Gunnarsdottir, I.; Steingrimsdottir, L. Assessing Validity of a Short Food Frequency Questionnaire on Present Dietary Intake of Elderly Icelanders. Nutr. J. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
| Strategy | Location | Sample Details | Method |
|---|---|---|---|
| A: Initial Training: in some sessions REs * were blinded to weight. Results not recorded | Clinical Laboratory | Variable number of trays; All REs | Trainer simulated meals by removing food and weighed remaining items; raters observed and photographed trays pre- and post then estimated consumption; Trainer compared RE estimates of energy consumption to estimates based on weight change |
| B: Initial Accuracy and Reliability testing | Clinical Laboratory | Three blinded RE raters; same 30 trays evaluated by each over five days (Total 90 assessments) | Same as for training (Strategy A). Compare RE estimates to estimates based on weights (Accuracy) and matched RE estimates to each other (Reliability) |
| C: Clinical Reliability assessment | Actual Clinical Setting | Each of 6 blinded REs assessed 5 breakfast, 5 lunch, and 5 dinner trays independent from but at the same time as at least 2 other REs for a total of 90 assessments. REs initially grouped randomly | Intra-class Correlation Coefficient using a Mixed Model Analysis of Covariance; fixed effects for meal and day, random effect for each individual food tray |
| D: Time Savings: Compare time to complete calorie counts using the automated Multi-Component Method and traditional hospital methods | Clinical Laboratory Setting | Four REs assigned to new or traditional method in alternating manner; recorded total time to complete calorie count for each RE for each session. See Table 2 for Assignment Matrix | Mixed Model Analysis of Covariance with fixed effects for method and for each covariate; random effect included for each RE |
| Rater (RE) | Method | Day | Session | Meal | Time (in Seconds) | ||
|---|---|---|---|---|---|---|---|
| Task 1 Pre-Meal | Task 2 Post-Meal | Task 3 Data Entry | |||||
| 1 | MCM | 1 | 1 | Dinner | 156 | 129 | 198 |
| 2 | TM | 1 | 1 | Dinner | 436 | 154 | 946 |
| 3 | TM | 1 | 1 | Dinner | 517 | 72 | 966 |
| 4 | MCM | 1 | 1 | Dinner | 228 | 101 | 209 |
| 1 | TM | 1 | 2 | Lunch | 498 | 89 | 906 |
| 2 | MCM | 1 | 2 | Lunch | 174 | 142 | 160 |
| 3 | MCM | 1 | 2 | Lunch | 186 | 184 | 179 |
| 4 | MCM | 1 | 2 | Lunch | 411 | 274 | 828 |
| 1 | MCM | 2 | 1 | Breakfast | 120 | 120 | 180 |
| 2 | MCM | 2 | 1 | Breakfast | 84 | 101 | 178 |
| 3 | TM | 2 | 1 | Breakfast | 420 | 180 | 740 |
| 4 | TM | 2 | 1 | Breakfast | 312 | 108 | 780 |
| 1 | TM | 2 | 2 | Lunch | 396 | 174 | 792 |
| 2 | TM | 2 | 2 | Lunch | 468 | 234 | 852 |
| 3 | MCM | 2 | 2 | Lunch | 138 | 108 | 246 |
| 4 | MCM | 2 | 2 | Lunch | 150 | 174 | 162 |
| 1 | TM | 3 | 1 | Breakfast | 96 | 132 | 186 |
| 2 | MCM | 3 | 1 | Breakfast | 420 | 159 | 660 |
| 3 | TM | 3 | 1 | Breakfast | 91 | 112 | 203 |
| 4 | MCM | 3 | 1 | Breakfast | 374 | 122 | 726 |
| 1 | TM | 3 | 2 | Dinner | 547 | 172 | 1049 |
| 2 | MCM | 3 | 2 | Dinner | 208 | 121 | 259 |
| 3 | TM | 3 | 2 | Dinner | 547 | 142 | 968 |
| 4 | MCM | 3 | 2 | Dinner | 189 | 101 | 199 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cox Sullivan, S.; Bopp, M.M.; Roberson, P.K.; Lensing, S.; Sullivan, D.H. Evaluation of an Innovative Method for Calculating Energy Intake of Hospitalized Patients. Nutrients 2016, 8, 557. https://doi.org/10.3390/nu8090557
Cox Sullivan S, Bopp MM, Roberson PK, Lensing S, Sullivan DH. Evaluation of an Innovative Method for Calculating Energy Intake of Hospitalized Patients. Nutrients. 2016; 8(9):557. https://doi.org/10.3390/nu8090557
Chicago/Turabian StyleCox Sullivan, Sheila, Melinda M. Bopp, Paula K. Roberson, Shelly Lensing, and Dennis H. Sullivan. 2016. "Evaluation of an Innovative Method for Calculating Energy Intake of Hospitalized Patients" Nutrients 8, no. 9: 557. https://doi.org/10.3390/nu8090557
APA StyleCox Sullivan, S., Bopp, M. M., Roberson, P. K., Lensing, S., & Sullivan, D. H. (2016). Evaluation of an Innovative Method for Calculating Energy Intake of Hospitalized Patients. Nutrients, 8(9), 557. https://doi.org/10.3390/nu8090557




