Silicon Stimulates Plant Growth and Metabolism in Rice Plants under Conventional and Osmotic Stress Conditions
Abstract
:1. Introduction
2. Results
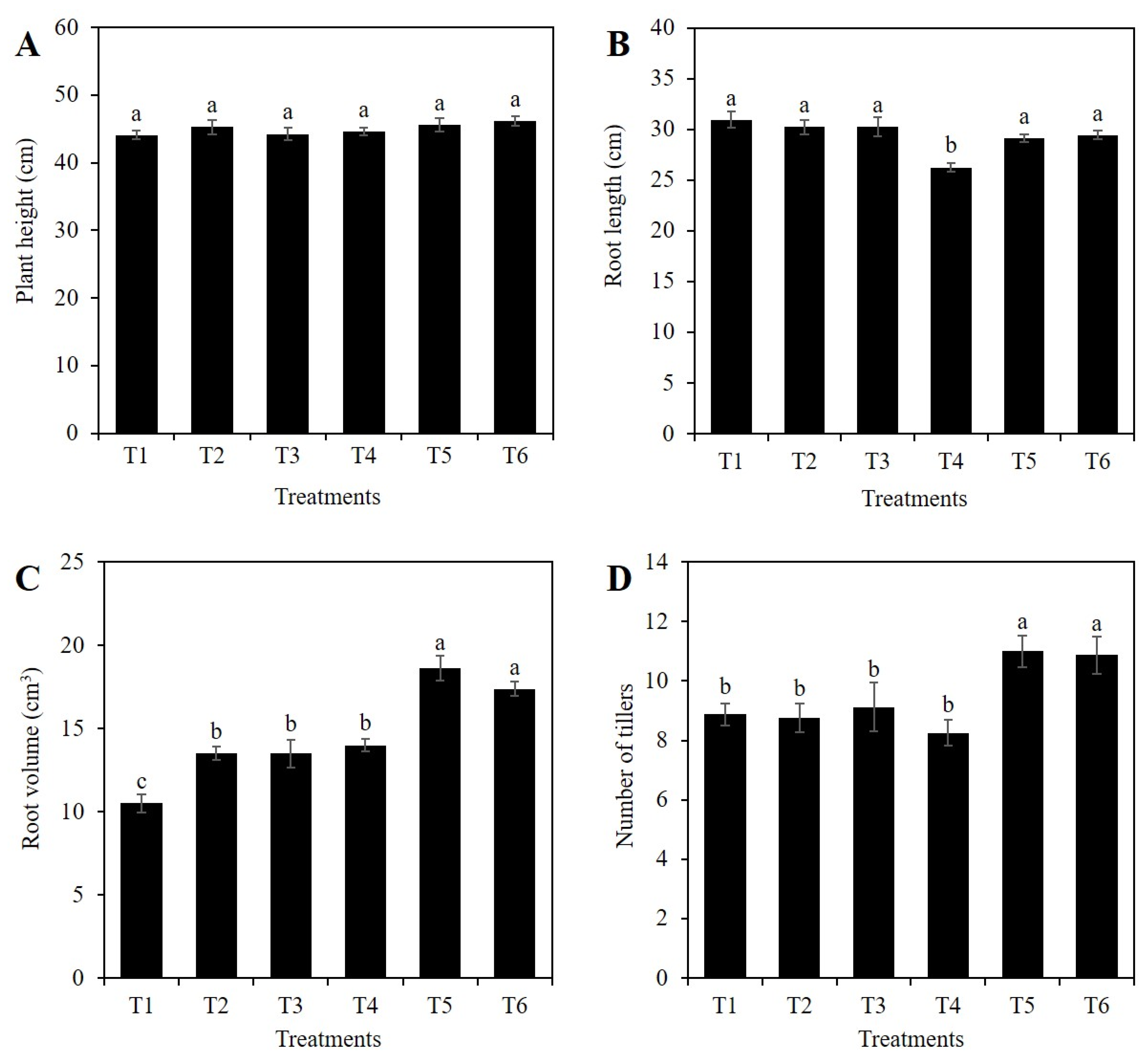
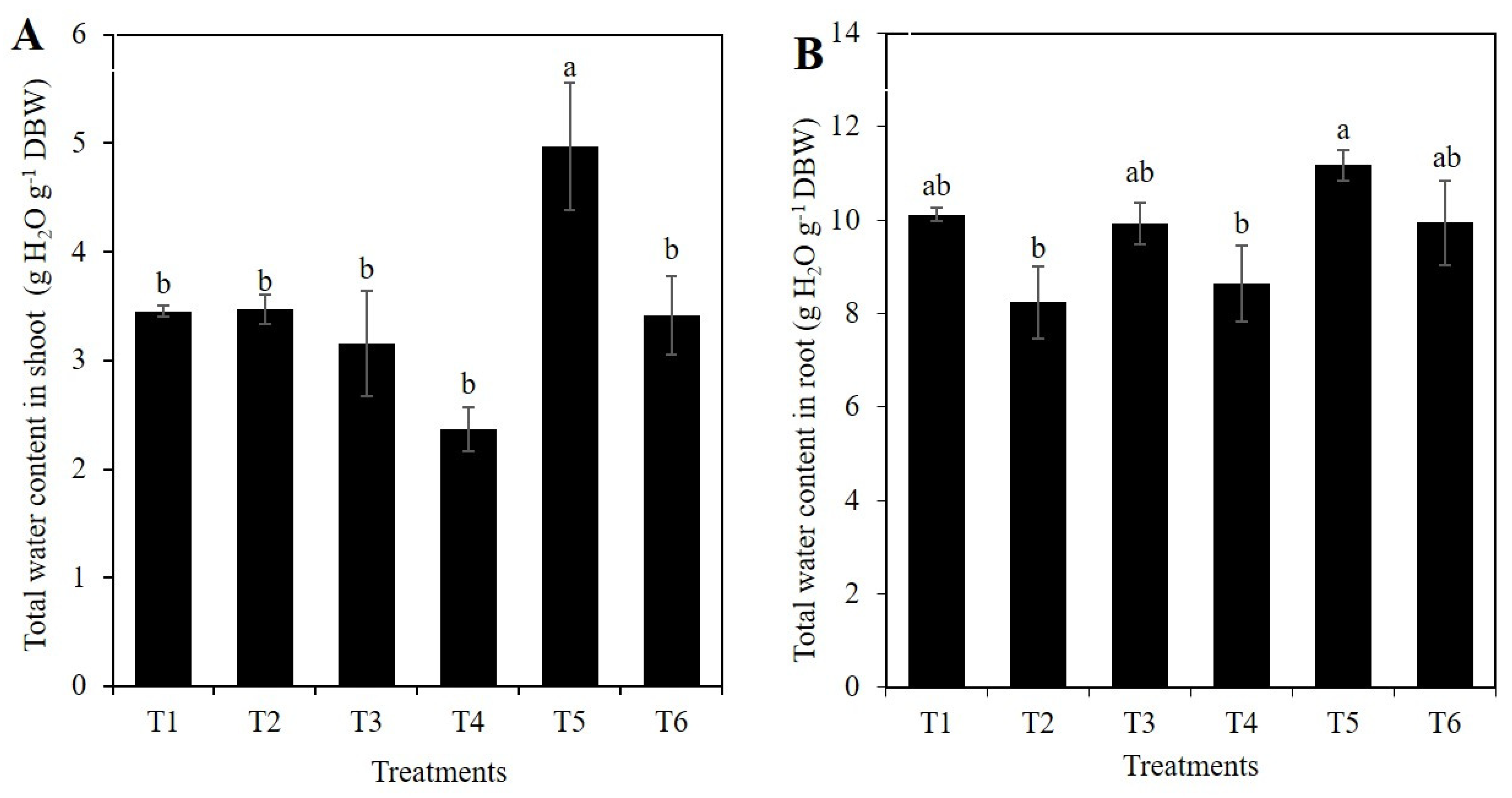
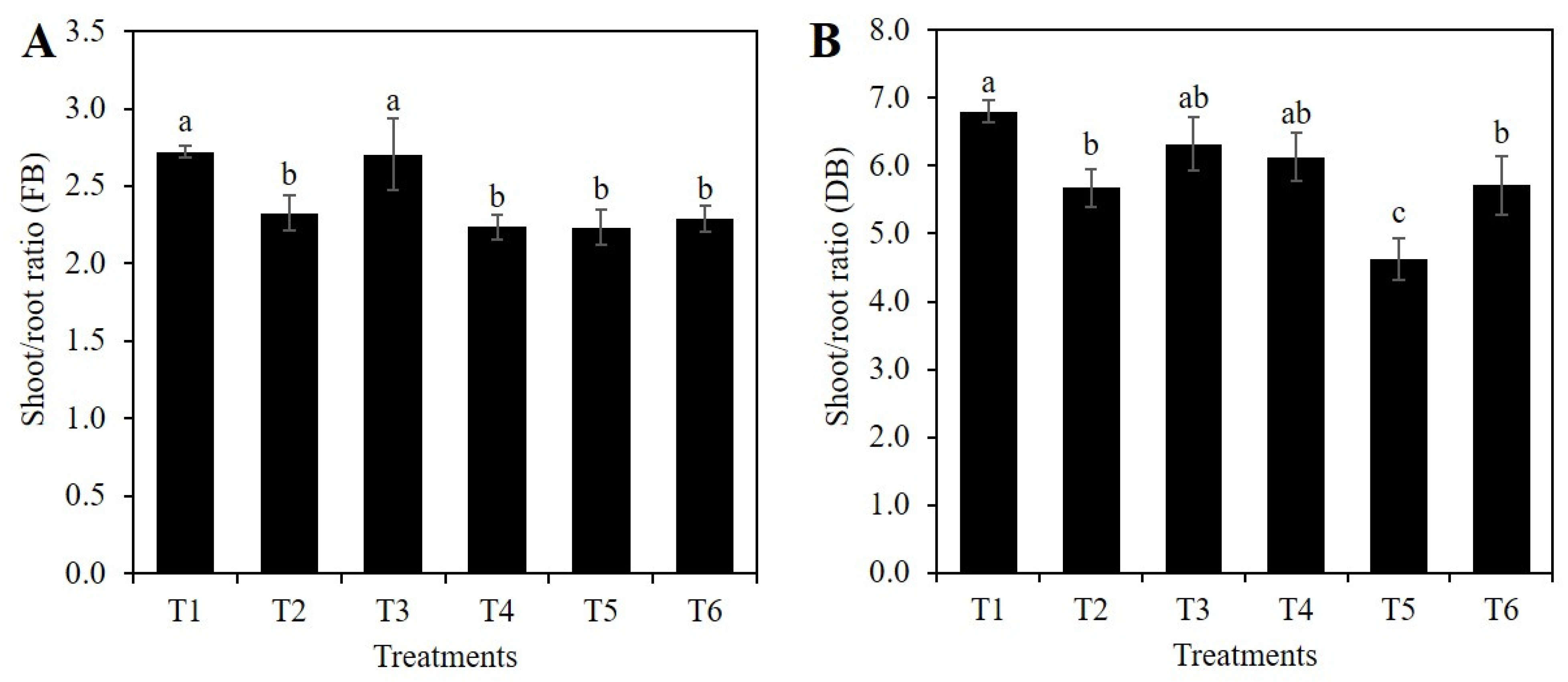
2.1. Si Affects the Vegetative Growth of Rice Depending on the Stress Condition
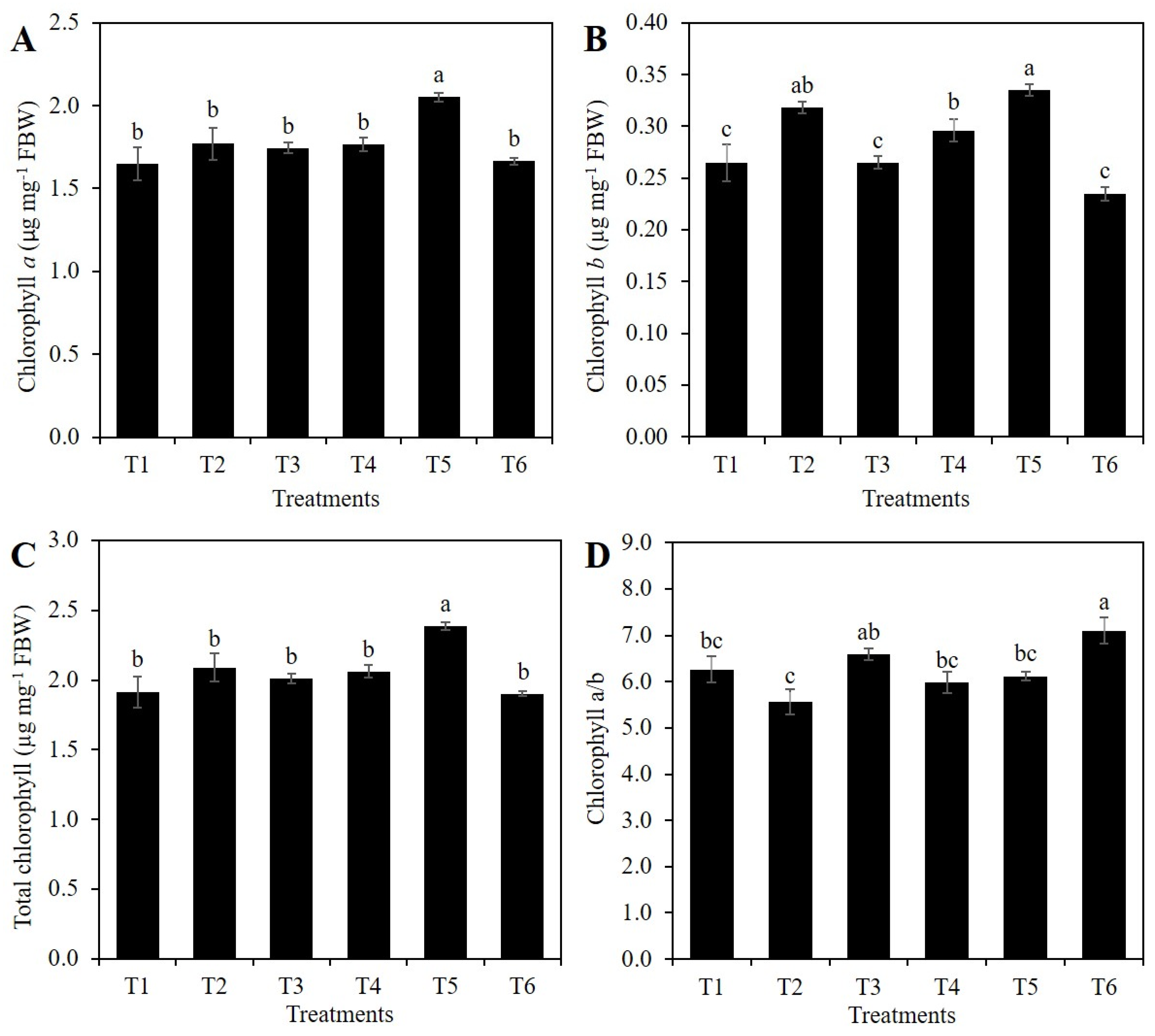
2.2. Chlorophylls Concentration
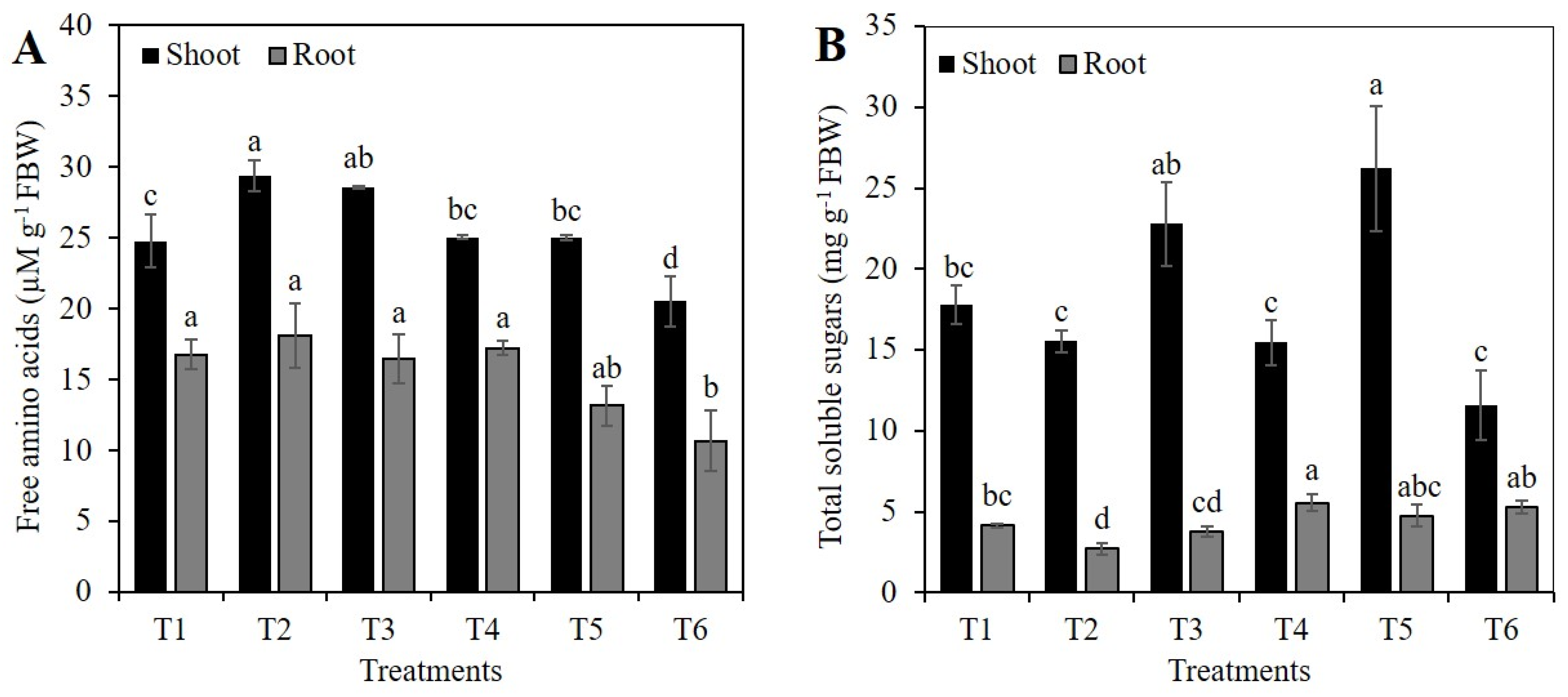
2.3. Concentration of Free Amino Acids and Total Sugars
2.4. Proline Concentration
2.5. Nutrient Concentrations
3. Discussion
4. Materials and Methods
4.1. Plant Material, Seed Disinfection
4.2. Vegetative Growth
4.3. Chlorophylls Concentration
4.4. Concentrations of Amino Acids and Total Sugars
4.5. Proline Concentration
4.6. Nutrient Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seck, P.A.; Diagne, A.; Mohanty, S.; Wopereis, M.C.S. Crops that feed the world 7: Rice. Food Sec. 2012, 4, 7–24. [Google Scholar] [CrossRef]
- FAOSTAT. FAO Statistical Databases. Rice. 2020. Available online: //www.fao.org/faostat/en/#data/QC (accessed on 5 January 2021).
- Pandey, V.; Shukla, A. Acclimation and tolerance strategies of rice under drought stress. Rice Sci. 2015, 22, 147–161. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.R.; Asgher, M.; Fatma, M.; Per, T.S.; Khan, N.A. Drought stress vis a vis plant functions in the era of climate change. Clim. Chang. Environ. Sustain. 2015, 3, 13–25. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Sharoni, A.M.; Kikuchi, S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Front. Microbiol. 2013, 4, 248. [Google Scholar] [CrossRef] [Green Version]
- Akram, R.; Fahad, S.; Masood, N.; Rasool, A.; Ijaz, M.; Ihsan, M.Z.; Kaleem, S. Plant Growth and Morphological Changes in Rice Under Abiotic Stress. In Advances in Rice Research for Abiotic Stress Tolerance, 1st ed.; Hasanuzzaman, M., Fujita, M., Biswaseds, J.K., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 69–85. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, Y.; Gong, H.-J.; Zhao, H.I.; Li, H.I.; Hu, Y.-H.; Wang, Y.C. Beneficial effects of silicon on photosynthesis of tomato seedlings under water stress. J. Integr. Agr. 2018, 17, 2151–2159. [Google Scholar] [CrossRef] [Green Version]
- Rachmawati, D.; Amalia, F. Physiological responses of rice (Oryza sativa L. ‘Situ Bagendit’) to varying water stress and soil type. In Proceedings of the 2nd International Conference on Tropical Agriculture, Yogyakarta, Indonesia, 26–27 October 2017; Sukartiko, A.C., Nuringtyas, T.R., Marliana, S.N., Isnansetyo, A., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Jiang, D.; Chen, G. Silicon improves photosynthetic performance by optimizing thylakoid membrane protein components in rice under drought stress. Environ. Exp. Bot. 2019, 158, 117–124. [Google Scholar] [CrossRef]
- Epstein, E. The anomaly of silicon in plant biology. Proc. Natl. Acad. Sci. USA 1994, 91, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Exley, C. Silicon in life: A bioinorganic solution to bioorganic essentiality. J. Inorg. Biochem. 1998, 69, 139–144. [Google Scholar] [CrossRef]
- Mitani, N.; Ma, J.F. Uptake system of silicon in different plant species. J. Exp. Bot. 2005, 56, 1255–1261. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef]
- Mitani-Ueno, N.; Ma, J.F. Linking transport system of silicon with its accumulation in different plant species. Soil Sci. Plant Nutr. 2020, 67, 10–17. [Google Scholar] [CrossRef]
- Yan, G.; Fan, X.; Peng, M.; Yin, C.; Xiao, Z.; Liang, Y. Silicon improves rice salinity resistance by alleviating ionic toxicity and osmotic constraint in an organ-specific pattern. Front. Plant Sci. 2020, 11, 260. [Google Scholar] [CrossRef]
- Singh, T.; Singh, P.; Singh, A. Silicon significance in crop production: Special consideration to rice: An overview. J. Pharm. Innov. 2021, 10, 223–229. [Google Scholar] [CrossRef]
- Keller, C.; Rizwan, M.; Davidian, J.C.; Pokrovsky, O.S.; Bovet, N.; Chaurand, P.; Meunier, J.D. Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 µM Cu. Planta 2015, 241, 847–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Cao, B.; Qi, L.; Yin, L.; Wang, S.; Deng, X. Silicon-moderated K-deficiency-induced leaf chlorosis by decreasing putrescine accumulation in sorghum. Ann. Bot. 2016, 118, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Rizwan, M.; Ullah, N.; Bharwana, S.A.; Waseem, M.; Farooq, M.A.; Abbasi, G.H.; Farid, M. Physiological and biochemical mechanisms of silicon-induced copper stress tolerance in cotton (Gossypium hirsutum L.). Acta Physiol. Plant. 2016, 38, 262. [Google Scholar] [CrossRef]
- Flora, C.; Khandekar, S.; Boldt, J.; Leisner, S. Silicon alleviates long-term copper toxicity and influences gene expression in Nicotiana tabacum. J. Plant Nutr. 2019, 42, 864–878. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.; Tran, L.S.P. Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front. Plant Sci. 2016, 7, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlovic, J.; Samardzic, J.; Maksimović, V.; Timotijevic, G.; Stevic, N.; Laursen, K.H.; Hansen, T.H.; Husted, S.; Schjoerring, J.K.; Liang, Y.; et al. Silicon alleviates iron deficiency in cucumber by promoting mobilization of iron in the root apoplast. New Phytol. 2013, 198, 1096–1107. [Google Scholar] [CrossRef]
- da Cunha, A.C.M.C.M.; De Oliveira, M.L.; Caballero, E.C.; Martinez, H.E.P.; Fontes, P.C.R.; Pereira, P.R.G. Growth and nutrient uptake of coffee seedlings cultivated in nutrient solution with and without silicon addition. Rev. Ceres 2012, 59, 392–398. [Google Scholar] [CrossRef] [Green Version]
- Detmann, K.C.; Araújo, W.L.; Martins, S.C.; Sanglard, L.M.; Reis, J.V.; Detmann, E.; Rodrigue, F.A.; Nunes-Nesi, A.; Fernie, A.R.; DaMatta, F.M. Silicon nutrition increases grain yield, which, in turn, exerts a feed-forward stimulation of photosynthetic rates via enhanced mesophyll conductance and alters primary metabolism in rice. New Phytol. 2012, 196, 752–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coskun, D.; Britt, D.T.; Huynh, W.Q.; Kronzucker, H.J. The role of silicon in higher plants under salinity and drought stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef]
- Ullah, H.; Luc, P.D.; Gautam, A.; Datta, A. Growth, yield and silicon uptake of rice (Oryza sativa) as influenced by dose and timing of silicon application under water-deficit stress. Arch. Agron. Soil Sci. 2018, 64, 318–330. [Google Scholar] [CrossRef]
- Amin, M.; Ahmad, R.; Ali, A.; Hussain, I.; Mahmood, R.; Aslam, M.; Lee, D.J. Influence of silicon fertilization on maize performance under limited water supply. Silicon 2018, 10, 177–183. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef] [Green Version]
- Sonobe, K.; Hattori, T.; An, P.; Tsuji, W.; Eneji, A.E.; Kobayashi, S.; Kawamura, Y.; Tanaka, K.; Inanaga, S. Effect of silicon application on sorghum root responses to water stress. J. Plant Nutr. 2011, 34, 71–82. [Google Scholar] [CrossRef]
- He, C.; Ma, J.; Wang, L. A hemicellulose-bound form of silicon with potential to improve the mechanical properties and regeneration of the cell wall of rice. New Phytol. 2015, 206, 1051–1062. [Google Scholar] [CrossRef]
- Liu, P.; Yin, L.; Wang, S.; Zhang, M.; Deng, X.; Zhang, S.; Tanaka, K. Enhanced root hydraulic conductance by aquaporin regulation accounts for silicon alleviated salt-induced osmotic stress in Sorghum bicolor L. Environ. Exp. Bot. 2015, 111, 42–51. [Google Scholar] [CrossRef]
- Barrios-Gómez, E.J.; Hernández-Aragón, L.; Tavitas-Fuentes, L.; Hernández-Arenas, M.; Canul-Ku, J. Morelos A-2016: Variety of coarse-grained rice. Rev. Mex. Cien. Agric. 2018, 9, 1547–1552. [Google Scholar] [CrossRef] [Green Version]
- Chávez-Murillo, C.E.; Wang, Y.J.; Quintero-Gutierrez, A.G.; Bello-Pérez, L.A. Physicochemical, textural, and nutritional characterization of Mexican rice cultivars. Cereal Chem. 2011, 88, 245–252. [Google Scholar] [CrossRef]
- Wang, P.; Grimm, B. Connecting Chlorophyll Metabolism with Accumulation of the Photosynthetic Apparatus. Trends Plant Sci. 2020. [Google Scholar] [CrossRef]
- Jinwen, L.; Jingping, Y.; Pinpin, F.; Junlan, S.; Dongsheng, L.; Changshui, G.; Wenyue, C. Responses of rice leaf thickness, SPAD readings and chlorophyll a/b ratios to different nitrogen supply rates in paddy field. Field Crop. Res. 2009, 114, 426–432. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef] [Green Version]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars: Metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [Green Version]
- Mohammadkhani, N.; Heidari, R. Drought-induced accumulation of soluble sugars and proline in two maize varieties. World Appl. Sci. 2008, 3, 448–453. [Google Scholar]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in plants under abiotic stresses: New insights into a classical phenomenon. Planta 2020, 251. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.-L.; Ma, Q.; Zhao, Q.; Wang, L.; Xu, K. Effects of silicon on absorbed light allocation, antioxidant enzymes and ultrastructure of chloroplasts in tomato leaves under simulated drought stress. Sci. Hortic. 2015, 194, 53–62. [Google Scholar] [CrossRef]
- Chen, W.; Yao, X.; Cai, K.; Chen, J. Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol. Trace Elem. Res. 2011, 142, 67–76. [Google Scholar] [CrossRef]
- Haddad, C.; Arkoun, M.; Jamois, F.; Schawarzbenberg, A.; Yvin, J.-C.; Etienne, P.; Laîné, P. Silicon promotes growth of Brassica napus l. and delays leaf senescence induced by nitrogen starvation. Front. Plant Sci. 2018, 9, 516. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Moradtalab, N.; Eshaghi, Z.; Feizy, J. Effect of silicon supplementation on growth and metabolism of strawberry plants at three developmental stages. N. Zeal. J. Crop Hort. 2018, 46, 144–161. [Google Scholar] [CrossRef]
- Chen, D.; Cao, B.; Wang, S.; Liu, P.; Deng, X.; Yin, L.; Zhang, S. Silicon moderated the K deficiency by improving the plant-water status in sorghum. Sci. Rep. 2016, 6, 22882. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Wang, S.; Yin, L.; Deng, X. How does silicon mediate plant water uptake and loss under water deficiency? Front. Plant Sci. 2018, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Vandegeer, R.K.; Zhao, C.; Cibils-Stewart, X.; Wuhrer, R.; Hall, C.R.; Hartley, S.E.; Tissuea, D.T.; Johnson, S.N. Silicon deposition on guard cells increases stomatal sensitivity as mediated by K+ efflux and consequently reduces stomatal conductance. Physiol. Plant. 2020, 171, 358–370. [Google Scholar] [CrossRef]
- Wind, J.; Smeekens, S.; Hanson, J. Sucrose: Metabolite and signaling molecule. Phytochemistry 2010, 71, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Das, P.; Seal, P.; Biswas, A.K. Regulation of growth, antioxidants and sugar metabolism in rice (Oryza sativa L.) seedlings by nacl and its reversal by silicon. Am. J. Plant Sci. 2016, 7, 623–638. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Guo, J.; Feng, R.; Jia, J.; Han, W.; Gong, H. The regulatory role of silicon on carbohydrate metabolism in Cucumis sativus L. under salt stress. Plant Soil 2016, 406, 231–249. [Google Scholar] [CrossRef]
- Muneer, S.; Park, Y.G.; Manivannan, A.; Soundararajan, P.; Jeong, B.R. Physiological and proteomic analysis in chloroplasts of Solanum lycopersicum L. under silicon efficiency and salinity stress. Int. J. Mol. Sci. 2014, 15, 21803–21824. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Niazi, A.; Pessarakli, M.; Arvin, M.J. P5CS expression level and proline accumulation in the sensitive and tolerant wheat cultivars under control and drought stress conditions in the presence/absence of silicon and salicylic acid. J. Plant Interact. 2018, 13, 461–471. [Google Scholar] [CrossRef]
- Song, A.; Li, P.; Fan, F.; Li, Z.; Liang, Y. The effect of Silicon on photosynthesis and expression of its relevant genes in rice (Oryza sativa L.) under high-zinc stress. PLoS ONE 2014, 9, e113782. [Google Scholar] [CrossRef]
- Quick, P.; Siegl, G.; Neuhaus, E.; Feil, R.; Stitt, M. Short-term water stress leads to a stimulation of sucrose synthesis by activating sucrose-phosphate synthase. Planta 1989, 177, 535–546. [Google Scholar] [CrossRef]
- Cao, X.; Zhong, C.; Zhu, C.; Zhu, L.; Zhang, J.; Wu, L.; Jin, Q. Ammonium uptake and metabolism alleviate PEG-induced water stress in rice seedlings. Plant Physiol. Biochem. 2018, 132, 128–137. [Google Scholar] [CrossRef]
- Gonzalez-Dugo, V.; Durand, J.L.; Gastal, F.; Bariac, T.; Poincheval, J. Restricted root-to-shoot translocation and decreased sink size are responsible for limited nitrogen uptake in three grass species under water deficit. Environ. Exp. Bot. 2012, 75, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38, 145. [Google Scholar] [CrossRef]
- Hu, A.Y.; Che, J.; Shao, J.F.; Yokosho, K.; Zhao, X.Q.; Shen, R.F.; Ma, J.F. Silicon accumulated in the shoots results in down-regulation of phosphorus transporter gene expression and decrease of phosphorus uptake in rice. Plant Soil 2018, 423, 317–325. [Google Scholar] [CrossRef]
- Li, P.; Song, A.; Li, Z.; Fan, F.; Liang, Y. Silicon ameliorates manganese toxicity by regulating both physiological processes and expression of genes associated with photosynthesis in rice (Oryza sativa L.). Plant Soil 2015, 397, 289–301. [Google Scholar] [CrossRef]
- Anwaar, S.A.; Ali, S.; Ali, S.; Ishaque, W.; Farid, M.; Farooq, M.A.; Najeeb, U.; Abbas, F.; Sharif, M. Silicon (Si) alleviates cotton (Gossypium hirsutum L.) from zinc (Zn) toxicity stress by limiting Zn uptake and oxidative damage. Environ. Sci. Pollut. Res. 2015, 22, 3441–3450. [Google Scholar] [CrossRef]
- Maillard, A.; Ali, N.; Schwarzenberg, A.; Jamois, F.; Yvin, J.-C.; Hosseini, S.A. Silicon transcriptionally regulates sulfur and ABA metabolism and delays leaf senescence in barley under combined sulfur deficiency and osmotic stress. Environ. Exp. Bot. 2018, 155, 394–410. [Google Scholar] [CrossRef]
- Gottardi, S.; Iacuzzo, F.; Tomasi, N.; Cortella, G.; Manzocco, L.; Pinton, R.; Römheld, V.; Mimmo, T.; Scampicchio, M.; Costa, L.D.; et al. Beneficial effects of silicon on hydroponically grown corn salad (Valerianella locusta (L.) Laterr) plants. Plant Physiol. Biochem. 2012, 56, 14–23. [Google Scholar] [CrossRef]
- Che, J.; Yamaji, N.; Shao, J.F.; Ma, J.F.; Shen, R.F. Silicon decreases both uptake and root-to-shoot translocation of manganese in rice. J. Exp. Bot. 2016, 67, 1535–1544. [Google Scholar] [CrossRef] [Green Version]
- Inal, A.; Pilbeam, D.J.; Gunes, A. Silicon increases tolerance to boron toxicity and reduces oxidative damage in barley. J. Plant Nutr. 2009, 32, 112–128. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier-Academic Press: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Audebert, A. Iron partitioning as a mechanism for iron toxicity tolerance in lowand rice. In Iron Toxicity in Rice-Based Systems in West Africa; Audebert, A., Narteh, L.T., Kiepe, P., Millar, D., Beks, B., Eds.; West Africa Rice Development Association: Cotonou, Benin, 2006; pp. 34–46. [Google Scholar]
- Saikia, T.; Baruah, K.K. Iron toxicity tolerance in rice (Oryza sativa) and its association with anti-oxidative enzyme activity. J. Crop Sci. 2012, 3, 90–94. [Google Scholar] [CrossRef]
- Quintana, J.; Bernal, M.I.; Scholle, M.; Holländer-Czytko, M.; Nga, N.; Piotrowski, M.; Mendoza-Cózatl, D.G.; Haydon, M.J.; Krämer, U. Root-to-shoot iron partitioning in Arabidopsis requires Iron-Regulated Transporter1 (IRT1). bioRxiv 2021. [Google Scholar] [CrossRef]
- von Blanckenburg, F.; von Wirén, N.; Guelke, M.; Weiss, D.J.; Bullen, T.D. Fractionation of metal stable isotopes by higher plants. Elements 2009, 5, 375–380. [Google Scholar] [CrossRef]
- Magnavaca, R.; Gardner, C.O.; Clark, R.B. Evaluation of inbred maize lines for aluminum tolerance in nutrient solution. In Genetic Aspects of Plant Mineral Nutrition, 3rd ed.; Gabelman, W.H., Longhman, B.C., Eds.; Martinus Nijhoff: Dordrecht, The Netherlands, 1987; pp. 255–265. [Google Scholar] [CrossRef] [Green Version]
- Famoso, A.N.; Clark, R.T.; Shaff, J.E.; Craft, E.; McCouch, S.R.; Kochian, L.V. Development of a novel aluminum tolerance phenotyping platform used for comparisons of cereal aluminum tolerance and investigations into rice aluminum tolerance mechanisms. Plant Physiol. 2010, 153, 1678–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, S.; Forno, D.A.; Cock, J.H. Laboratory Manual for Physiological Studies of Rice; International Rice Research Institute: Los Baños, Philippines, 1976; pp. 61–69. [Google Scholar]
- Jones, M.M.; Turner, N.C. Osmotic adjustment in leaves of sorghum in response to water deficits. Plant Physiol. 1978, 61, 122–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ming, D.F.; Pei, Z.F.; Naeem, M.S.; Gong, H.J.; Zhou, W.J. Silicon alleviates PEG-induced water-deficit stress in upland rice seedlings by enhancing osmotic adjustment. J. Agron. Crop Sci. 2012, 198, 14–26. [Google Scholar] [CrossRef]
- Harborne, J.B. Chlorophyll extraction. In Phytochemical Methods, 1st ed.; Harbone, J.B., Ed.; Chapman and Hall: London, UK, 1973; pp. 205–207. [Google Scholar]
- Moore, S.; Stein, W.H. Photometric ninhydrin method for use in the chromatography of amino acids. J. Biol. Chem. 1948, 176, 367–388. [Google Scholar] [CrossRef]
- Morris, D.L. Quantitative determination of carbohydrates with Dreywood’s anthrone reagent. Science 1948, 107, 254–255. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Horneck, D.A.; Miller, R.O. Determination of total nitrogen in plant tissue. In Handbook of Reference Methods for Plant Analysis, 1st ed.; Kalra, Y.P., Ed.; Soil and Plant Analysis Council: Boca Raton, FL, USA, 1998; pp. 75–83. [Google Scholar]



| TRE | Macronutrient Concentration (g kg−1 Dry Biomass Weight) | ||||
|---|---|---|---|---|---|
| N | P | K | Ca | Mg | |
| Shoots | |||||
| T1 | 35.96 ± 0.92 a | 5.46 ± 0.27 ab | 21.10 ± 0.65 a | 1.36 ± 0.02 ab | 3.45 ± 0.11 a |
| T2 | 36.75 ± 1.29 a | 6.02 ± 0.09 a | 22.38 ± 0.93 a | 1.52 ± 0.08 ab | 3.66 ± 0.10 a |
| T3 | 38.59 ± 0.99 a | 5.45 ± 0.39 ab | 21.47 ± 0.85 a | 1.32 ± 0.06 b | 3.17 ± 0.21 a |
| T4 | 27.48 ± 1.79 b | 5.67 ± 0.34 ab | 24.05 ± 2.11 a | 1.55 ± 0.07 a | 3.48 ± 0.27 a |
| T5 | 23.45 ± 3.99 b | 4.90 ± 0.14 b | 22.97 ± 0.95 a | 1.58 ± 0.09 a | 3.16 ± 0.10 a |
| T6 | 28.70 ± 1.59 b | 5.31 ± 0.21 ab | 24.29 ± 0.92 a | 1.53 ± 0.07 ab | 3.39 ± 0.14 a |
| Roots | |||||
| T1 | 20.04 ± 0.92 a | 2.39 ± 0.11 a | 17.71 ± 0.53 a | 0.97 ± 0.03 a | 1.41 ± 0.03 a |
| T2 | 16.45 ± 1.36 ab | 2.52 ± 0.09 a | 15.20 ± 0.95 b | 0.96 ± 0.03 a | 1.35 ± 0.03 ab |
| T3 | 18.90 ± 1.64 ab | 2.58 ± 0.09 a | 17.22 ± 0.46 a | 0.92 ± 0.03 a | 1.42 ± 0.04 a |
| T4 | 18.03 ± 2.67 ab | 2.45 ± 0.13 a | 18.34 ± 0.53 a | 1.03 ± 0.09 a | 1.22 ± 0.02 c |
| T5 | 16.63 ± 1.16 ab | 2.50 ± 0.09 a | 16.57 ± 0.40 ab | 1.02 ± 0.02 a | 1.28 ± 0.01 bc |
| T6 | 14.00 ± 1.48 b | 2.41 ± 0.13 a | 17.91 ± 0.69 a | 1.05 ± 0.04 a | 1.26 ± 0.02 bc |
| TRE | Micronutrient Concentration (mg kg−1 Dry Biomass Weight) | ||||
|---|---|---|---|---|---|
| Fe | Cu | Mn | B | Zn | |
| Shoots | |||||
| T1 | 162.8 ± 13.5 b | 3.4 ± 0.1 a | 179.8 ± 8.6 a | 12.5 ± 1.0 ab | 13.7 ± 0.7 b |
| T2 | 222.8 ± 11.1 a | 3.6 ± 0.2 a | 200.2 ± 8.0 a | 9.4 ± 0.7 b | 22.5 ± 1.3 a |
| T3 | 153.4 ± 15.9 b | 4.1 ± 0.5 a | 179.0 ± 25.2 a | 11.5 ± 1.1 ab | 12.2 ± 2.6 b |
| T4 | 136.5 ± 6.6 b | 3.2 ± 0.3 a | 219.2 ± 30.0 a | 13.4 ± 1.4 ab | 13.5 ± 1.7 b |
| T5 | 142.0 ± 7.9 b | 3.0 ± 0.1 a | 210.8 ± 18.2 a | 14.0 ± 2.2 a | 15.6 ± 2.4 b |
| T6 | 133.2 ± 4.1 b | 3.7 ± 0.7 a | 233 ± 26.4 a | 9.4 ± 0.9 b | 13.4 ± 1.7 b |
| Roots | |||||
| T1 | 717.6 ± 248.0 ab | 3.7 ± 0.5 a | 34.3 ± 2.3 c | 34.3 ± 3.8 a | 28.7 ± 1.5 ab |
| T2 | 1044.5 ± 206.3 ab | 4.3 ± 0.5 a | 25.4 ± 0.8 d | 33.6 ± 2.2 a | 29.7 ± 2.2 a |
| T3 | 570.3 ± 150.6 b | 3.8 ± 0.4 a | 28.4 ± 0.7 cd | 32.7 ± 2.8 a | 27.6 ± 1.2 ab |
| T4 | 909.7 ± 189.4 ab | 3.7 ± 0.4 a | 46.0 ± 3.5 b | 35.0 ± 4.2 a | 22.7 ± 0.9 b |
| T5 | 1533.6 ± 427.8 a | 4.0 ± 0.4 a | 53.8 ± 2.6 a | 30.1 ± 3.7 a | 27.3 ± 2.1 ab |
| T6 | 1089.7 ± 395.4 ab | 4.4 ± 0.4 a | 43.7 ± 2.5 b | 26.4 ± 1.5 a | 27.9 ± 2.9 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Olvera, S.M.; Trejo-Téllez, L.I.; Gómez-Merino, F.C.; Ruíz-Posadas, L.d.M.; Alcántar-González, E.G.; Saucedo-Veloz, C. Silicon Stimulates Plant Growth and Metabolism in Rice Plants under Conventional and Osmotic Stress Conditions. Plants 2021, 10, 777. https://doi.org/10.3390/plants10040777
Ramírez-Olvera SM, Trejo-Téllez LI, Gómez-Merino FC, Ruíz-Posadas LdM, Alcántar-González EG, Saucedo-Veloz C. Silicon Stimulates Plant Growth and Metabolism in Rice Plants under Conventional and Osmotic Stress Conditions. Plants. 2021; 10(4):777. https://doi.org/10.3390/plants10040777
Chicago/Turabian StyleRamírez-Olvera, Sara Monzerrat, Libia Iris Trejo-Téllez, Fernando Carlos Gómez-Merino, Lucero del Mar Ruíz-Posadas, Ernesto Gabriel Alcántar-González, and Crescenciano Saucedo-Veloz. 2021. "Silicon Stimulates Plant Growth and Metabolism in Rice Plants under Conventional and Osmotic Stress Conditions" Plants 10, no. 4: 777. https://doi.org/10.3390/plants10040777






