Overview of HSS Steel Grades Development and Study of Reheating Condition Effects on Austenite Grain Size Changes
Abstract
1. Introduction
- –
- physical-metallurgical parameters: diameter of austenite and ferrite grain size, recrystallization, precipitation and phase transformations
- –
- processing parameters: reheating temperatures, rolling temperatures and plastic deformations in the spontaneous recrystallization region of austenite, rolling temperatures and plastic deformations in the non-recrystallization austenite region, including the finished rolling temperatures, cooling rate from the finished rolling temperatures and optimization of chemical composition through alloying elements [5].
- (i).
- It had to achieve good weldability by reducing the value of the carbon equivalent (i.e., reduction of carbon content). Manganese replaced carbon for interstitial strengthening [6] because it has a six-times lower influence on the carbon equivalent than carbon [7,8,9]. The result of the research was a chemical composition of C–Mn mild steel with the content of basic elements C ≈0.2 wt.%, Mn ≈1.5 wt.%. This type of steel grade was later referred to as St52 (S355) which was the basis for the development of microalloyed steels containing Nb, V, Ti,
- (ii).
- It had to improve strength and ductility. The author [1] stated that the low-cost alloying element niobium (Nb) with a content of 0.005–0.03% wt.% was used for the first time in 1958 as a microalloying element and had an effect on the formation of Nb carbides and nitrides. Vanadium (V) with a content of 0.08–0.1% wt.% was used before the commercial application of Nb. Both elements are aimed at improving the strength and plastic properties resulting from the precipitating and grain-refining effects. The titanium (Ti) content of 0.1 wt.% has an important effect on grain-size refining [10,11].
- –
- reheating temperature: The level has an effect on the volume of undissolved precipitates, which retard the grain growth of austenite via their pinning effect to the grain boundary motion;
- –
- middle temperature austenite region: This retards structure recovery and the dynamic recrystallization of austenite,
- –
- low-temperature austenite region: The formation of deformation-induced precipitates and precipitates correspond to the thermodynamic conditions. These precipitates are responsible for retarding the recrystallization processes and forming the pancake structure, which is characterized by a high level of ferrite nucleation that affects the resulting diameter of the ferrite grains, and transformation temperature: The effect of precipitation strengthening resulting from precipitation at the phase interface and precipitation itself in ferrite.
- effect on grain size refinement: Nb > Ti > V,
- effect on precipitation strengthening: V > Nb > Ti,
- regarding steelmaking technology, it is necessary to provide deep desulphurization and deoxidation of the melt because the activity of microalloying elements (Ti, V, Nb) defined by standard free enthalpy (∆GT0) to sulfur and oxygen are strong. The following inequalities apply to liquid steel: TiS > VC > NbC > TiC > VN > NbO > NbN > TiN.
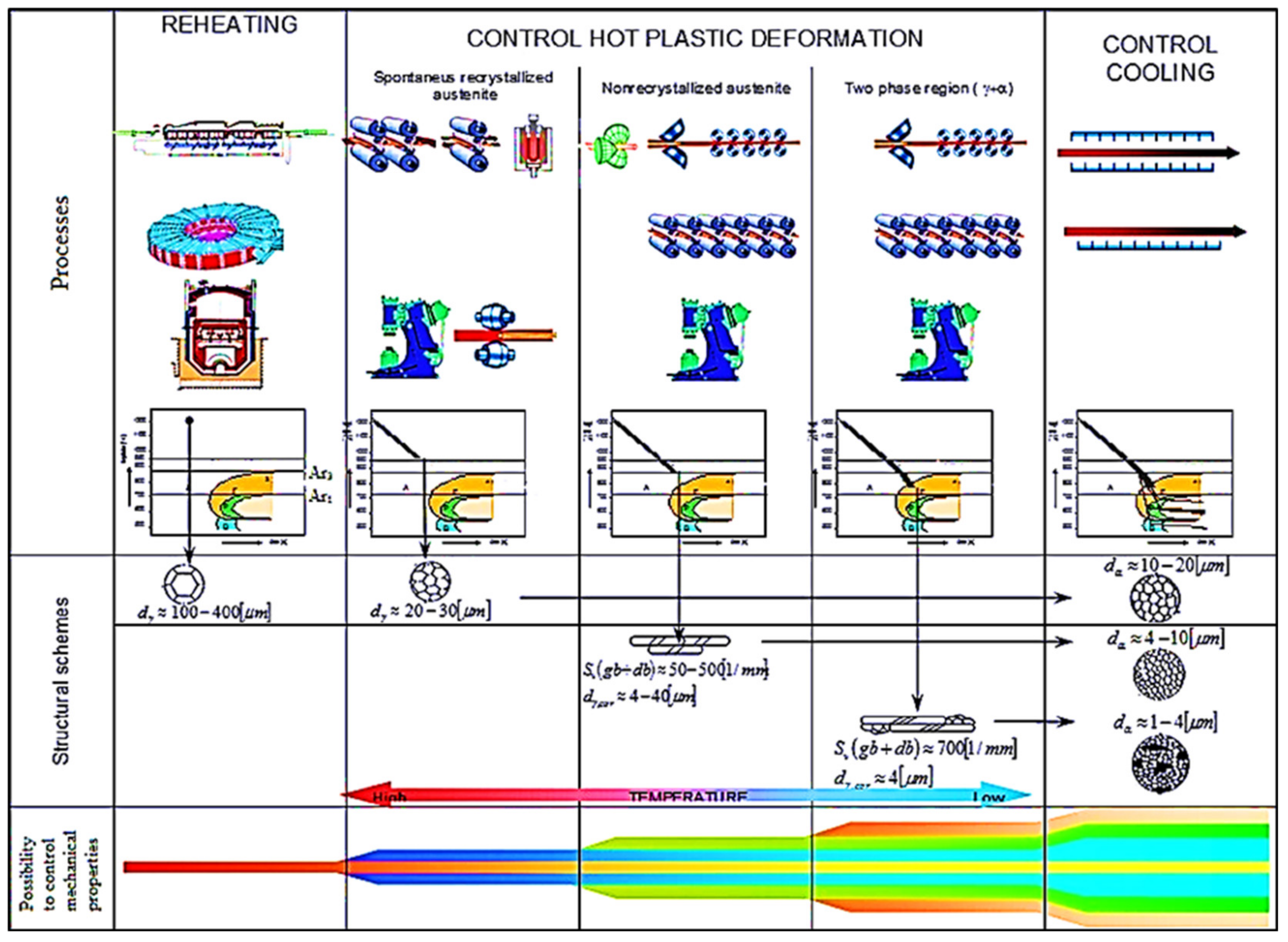
- (a)
- high-temperature austenite:
- –
- control of austenite grain growth by reheating temperature and holding time at this temperature,
- –
- control of the thermo-deformation regime in order to refine the diameter of the austenitic grain size by repeating the cycle" plastic deformation–recrystallization". This technique called "Recrystallization Controlled Rolling" (RCR),
- (b)
- low-temperature austenite:
- –
- control of thermo-deformation regime to form elongated deformed (pancake) austenitic grains with the possibility of continual deformation into the two-phase region of austenite–ferrite,
- (c)
- phase transformation from austenite:
- –
- controlled cooling from finished rolling temperature, i.e., a strategy of controlled transition through the area of phase transformations to obtain the required final structural composition.
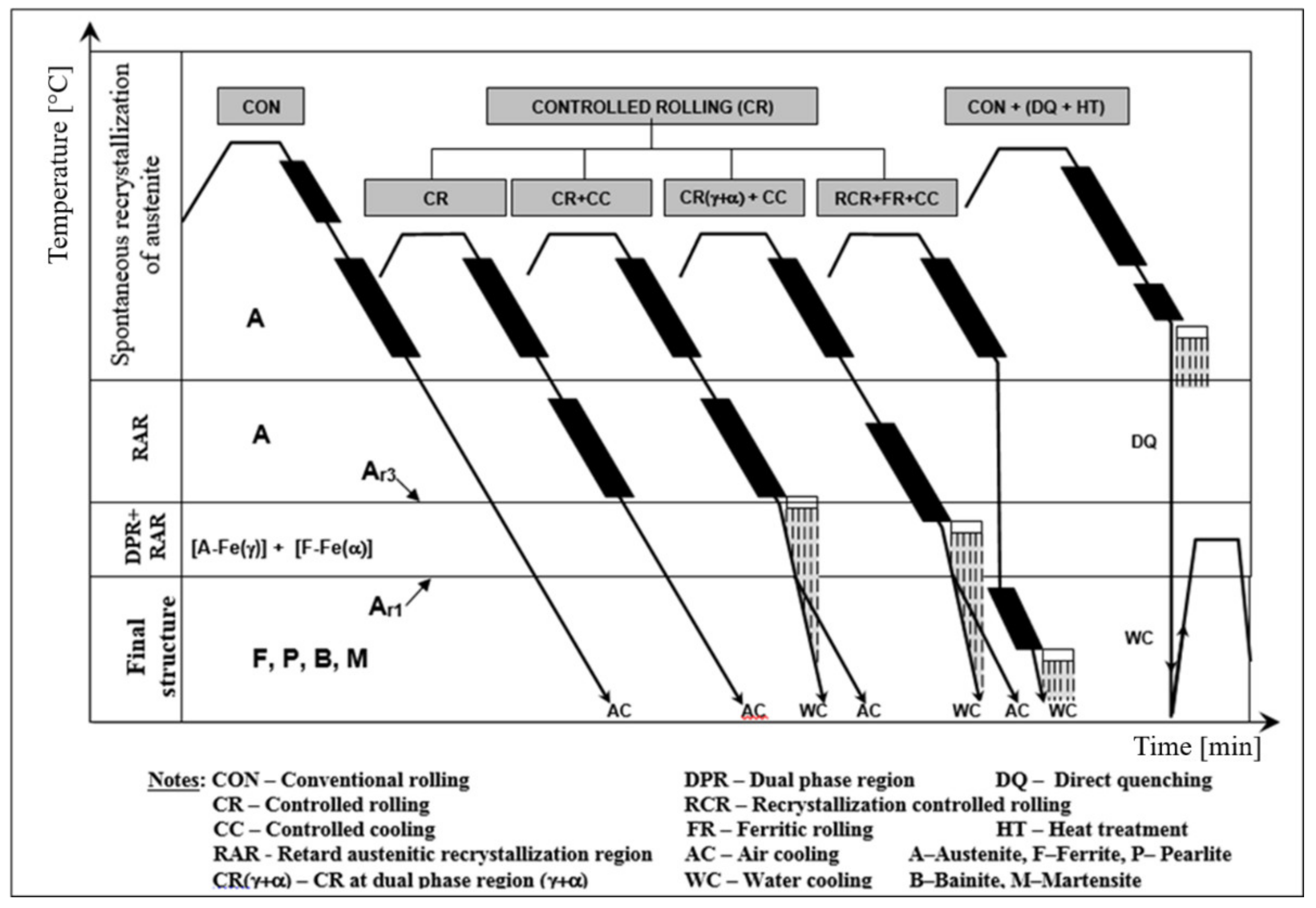
- –
- thermoplastic deformation and cooling named as CR–CC (conventional methods of grain size refinement) with the diameter of the final grain size at the level of microns d ≥ 1 μm
- –
- (i).
- direct phase transformation of austenite from reheating temperature produces very coarse-grained ferrite; the grain size depends on reheating condition.
- (ii).
- plastic deformation conditions realized in
- –
- monophase austenite region at deformation temperatures.
- –
- –
- non-recrystallization austenite region—CR [56,63] (narrowly raised Ar3 temperature) formed deformation-elongated austenitic grains with an effective ferritic nucleation surface calculated from grain boundaries and deformation bands Sv(gb+db) ≈ 25–500 1/mm [56]. This corresponds to the corrected diameter grains of austenite dγ, cor ≈ 4–70 μm) transformed to polycrystalline ferrite with diameter dα ≈ 2–10 μm.
- (iii).
- dual-phase (γ+α) region—CR [55,63] (non-recrystallized austenite–deformed ferrite) bellow Ar3 temperatures with Sv(gb+db) ≈1000 1/mm, corresponding to dγ, cor ≈ 2 μm with subsequent transformation to ferrite (non-recrystallized austenite, to polycrystalline ferrite–deformed ferrite, to ferrite subgrains) with diameter dα ≈ 1–2 μm.
- –
- during grain growth in polycrystalline materials, there is a relatively narrow range of grain sizes and shapes;
- –
- during grain growth, after sufficient time, the distribution of grain sizes becomes scaled to the mean grain size and remains self-similar;
- –
- final grain-size distribution resulting from grain growth is generally insensitive to the initial distribution; and
- –
- during grain growth, the mean grain size (radius) increases with time.
2. Materials and Methods
3. Results and Discussion
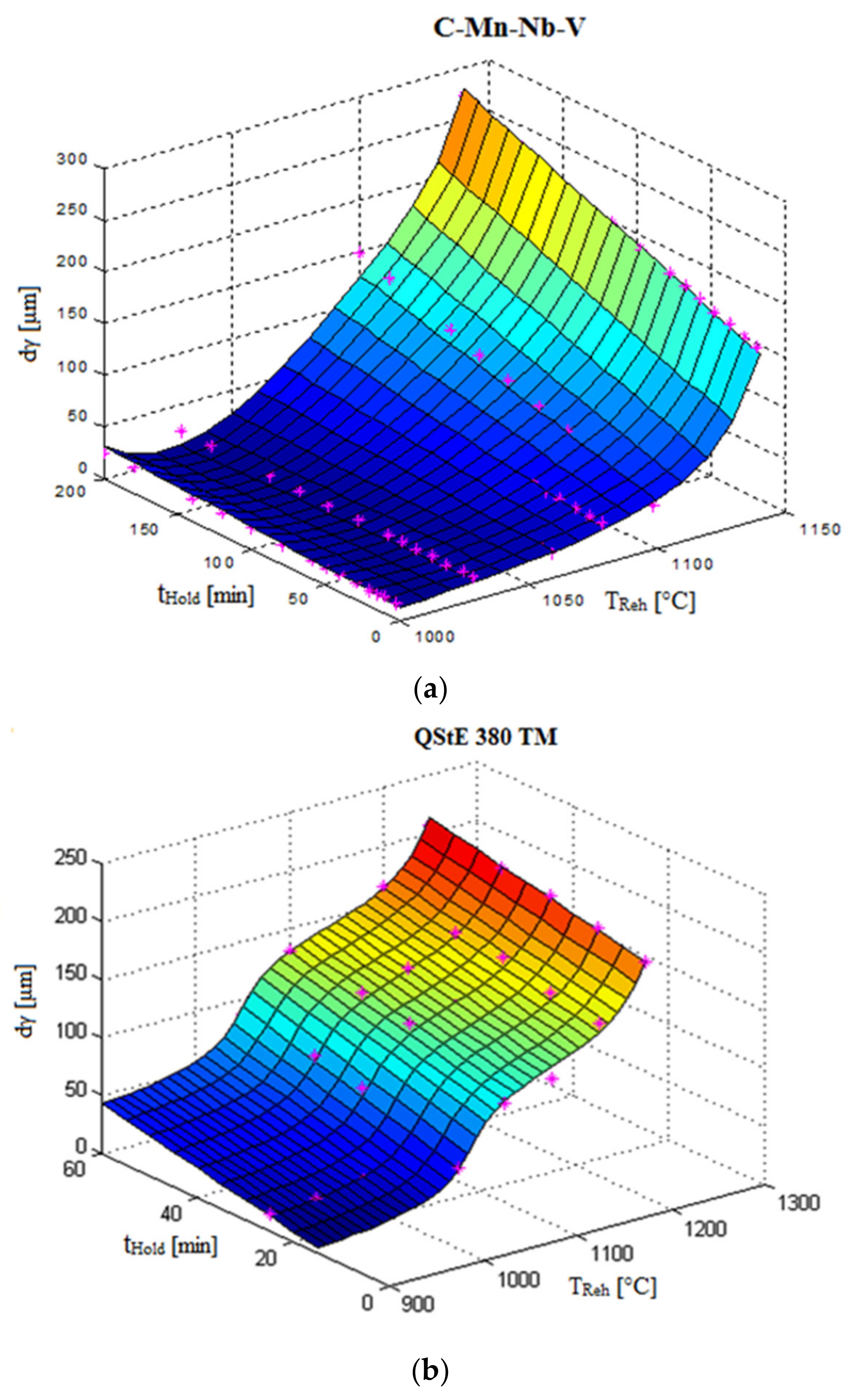
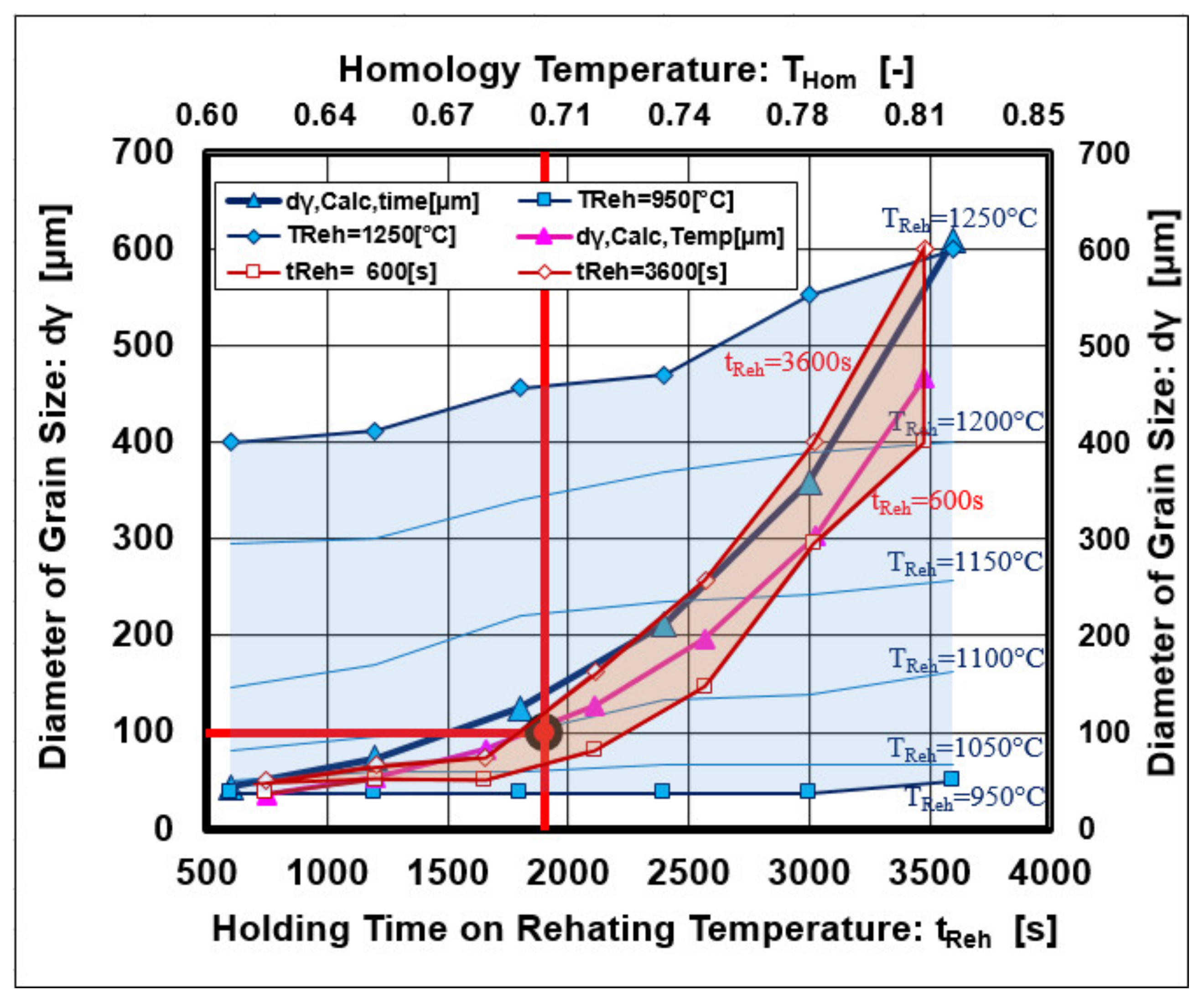
3.1. Physical-Metallurgical Substance of Grain Growth Depending on Reheating Conditions
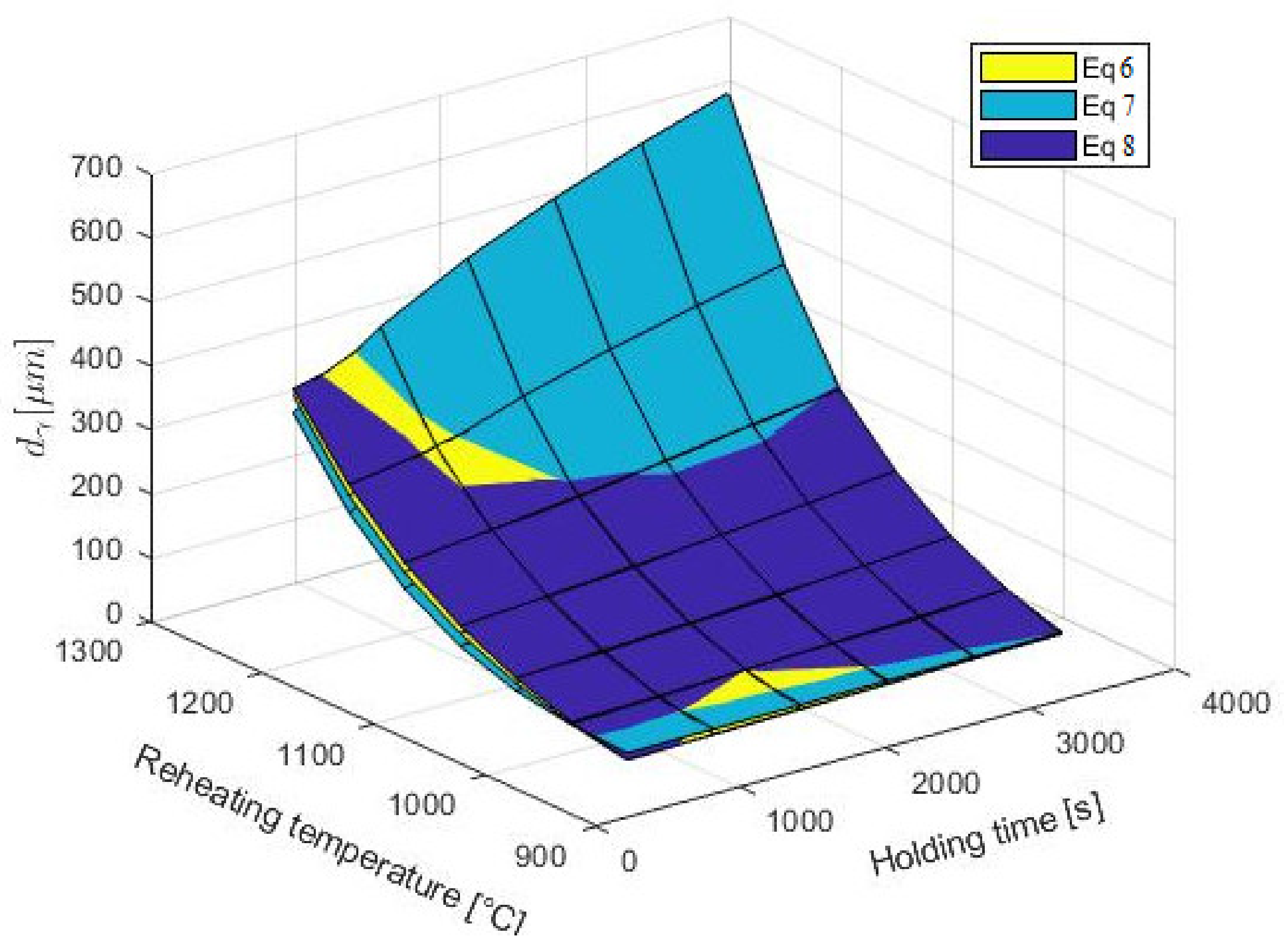
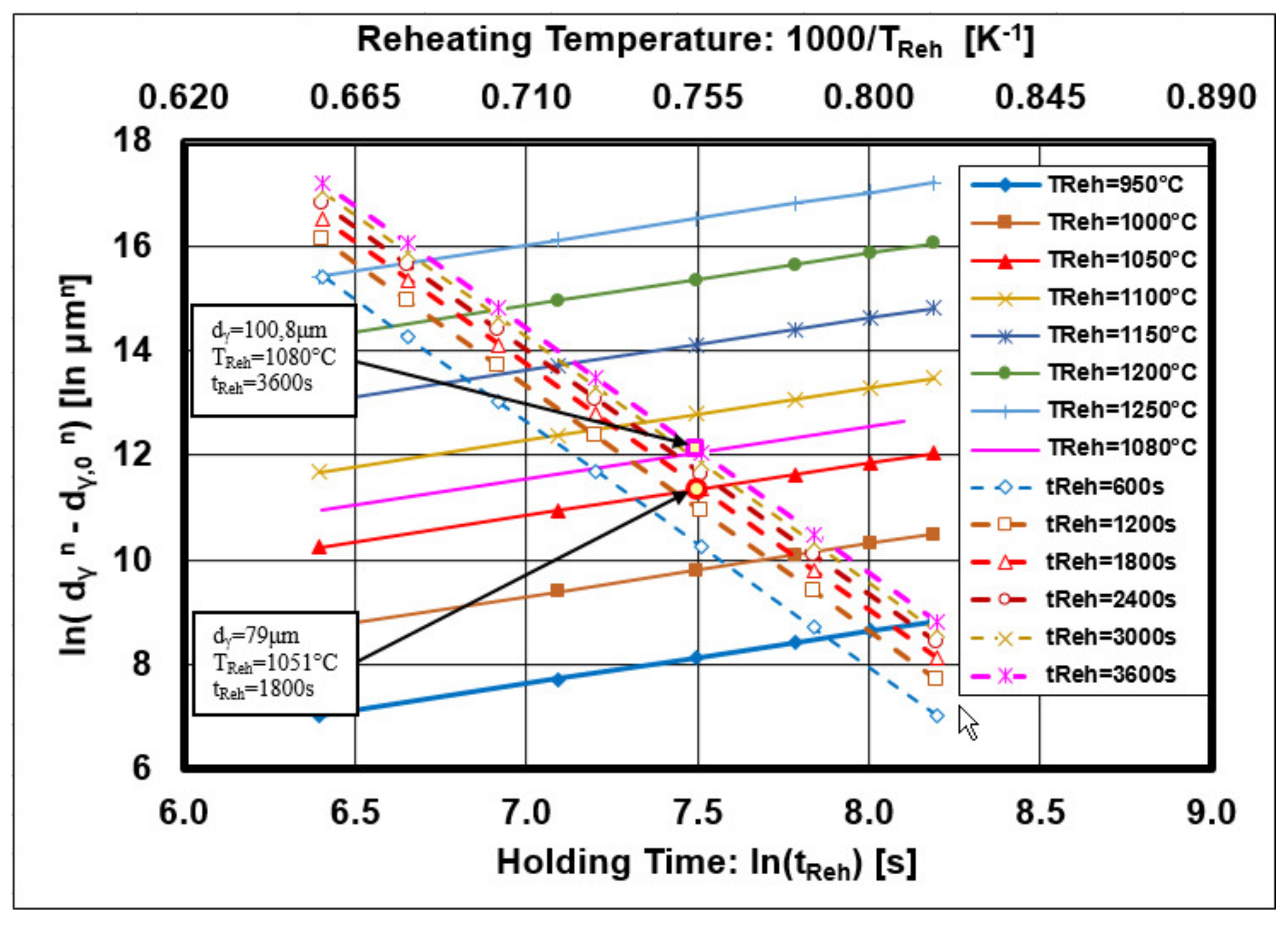
3.2. Mathematical Description of Austenite Grain Growth
3.3. Overview of the Present Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vanderbeck, R.W. European Methods of Rolling at Controlled Temperatures. Weld. J. 1958, 37, 114s–116s. [Google Scholar]
- Meyer, L.; de Boer, H. HSLA plate metallurgy: Alloying, normalizing, controlled rolling. JOM 1977, 29, 17–23. [Google Scholar] [CrossRef]
- Morgan, E.R.; Dancy, T.E.; Korchynsky, M. Improved Steels through Hot–Strip Mill Controlled Cooling. JOM 1965, 17, 829–832. [Google Scholar] [CrossRef]
- Duckworth, W.; Baird, J. Mild Steels. J. Iron Steel Inst. 1969, 207, 854–871. [Google Scholar]
- Irvine, K.J.; Pickering, F.B. Low-Carbon Steels with Ferrite-Pearlite Structures. J. Iron Steel Inst. 1963, 201, 944–960. [Google Scholar]
- Ito, Y.; Bessyo, K. Weld Cracking Formula of High Strength Steels. Tetsu Hagane 1972, 58, 1812–1821. [Google Scholar] [CrossRef][Green Version]
- Yukawa, M. Development of Structural High-Strength Steel and Low-Temperature Steel in Japan. J. Iron Steel Inst. 1968, 206, 11–16. [Google Scholar]
- Le Bon, A. Controlled Rolling of Sheet Products. Rev. Met. 1979, 76, 149–156. [Google Scholar] [CrossRef]
- Nutting, J. Physical Metallurgy of Alloy Steels. J. Iron Steel Inst. 1969, 207, 872–893. [Google Scholar]
- Morrison, W.B. Microalloy Steels—The Beginning. Mater. Sci. Technol. 2009, 25, 1066–1073. [Google Scholar] [CrossRef]
- Megusar, J.; Lavernia, E.; Domalavage, P.; Harling, O.K.; Grant, N.J. Structures and properties of rapidly solidified 9Cr-lMo steel. J. Nucl. Mater. 1984, 122, 789–793. [Google Scholar] [CrossRef]
- Irvine, K.J.; Pickering, F.B.; Gladman, T. Grain Refined C-Mn Steels. J. Iron Steel Inst. 1967, 205, 161–180. [Google Scholar]
- Chaturvedi, M.C.; Honeycombe, R.W.K.; Warrington, D.H. Dislocation Precipitation of Niobium Carbide in Iron-Manganese Austenites. J. Iron Steel Inst. 1968, 206, 1236–1244. [Google Scholar]
- Heisterkamp, F.; Meyer, L. Mechanical Properties of Pearlite Reduced Steels. Thyssen Forsch. 1971, 3, 44–65. [Google Scholar]
- Lauprecht, W.; Imgrund, H.; Coldren, P. High-Strength Structural Steels with a Structure of Low-Carbon Bainite Subjected to Thermomechanical Treatment. Met. Sci. Heat Treat. 1975, 17, 1005–1011. [Google Scholar] [CrossRef]
- Golovanenko, S.A.; Matrosov, J.I. High-Strength Steels for Trunk Gas Lines. Met. Sci. Heat Treat. 1977, 19, 857–866. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Osuka, T.; Taira, T.; Iwasaki, N. Effect of Processing Conditions on the Mechanical Properties of Controlled-Rolled Plate for Large-Diameter Line Pipe. In Microalloying 75, Proceedings of the International Symposium on High Strength, Low-Alloy Steels, Washington DC, USA, 1–3 October 1975; Union Carbide Corp: New York, NY, USA, 1977; pp. 415–419. [Google Scholar]
- Matrosov, Y.I. Mechanisms of the Effect of Vanadium, Niobium, and Titanium Doping on the Structure and Properties of Low-Pearlite Steels. Met. Sci. Heat Treat. 1984, 26, 798–807. [Google Scholar] [CrossRef]
- DeArdo, A.J. Niobium in modern steels. Int. Mater. Rev. 2003, 48, 371–402. [Google Scholar] [CrossRef]
- Garcia, C.I. High Strength Low Alloyed (HSLA) Steels. In Automotive Steels: Design, Metallurgy, Processing and Applications; Radhakanta, R., Shiv Brat, S., Eds.; Woodhead Publishing: Chennai, India, 2017; pp. 145–167. [Google Scholar] [CrossRef]
- Strassburger, C.; Meyer, L. Way of Further Developing the Unalloyed Structural Steels. Thyssen Forsch. 1971, 3, 2–7. [Google Scholar]
- Meyer, L.; Buhle, H.E.; Heisterkamp, F. Metallurgical and Technological Basis for the Development and Production of Pearlite Reduced Structural Steels. Thyssen Forsch. 1971, 3, 8–11. [Google Scholar]
- Lauprecht, W.; Imgrund, H.; Coldren, P. Transformation Controlled Weldable High-Strength Low-Alloy Steels with Low-Carbon Bainitic Microstructure. Stahl Eisen 1973, 93, 1041–1051. [Google Scholar]
- Meyer, L.; Buhler, H.E.; Heisterkamp, F.; Jackel, G.; Ryder, P.L. Metallkundliche Untersuchungen zur Wirkungsweise von Titan in unlegierten Baustählen. Arch. Eisenhuttenwes. 1972, 43, 823–832. [Google Scholar] [CrossRef]
- Meyer, L.; Heisterkamp, F.; Mueschenborn, W. Columbium, Titanium and Vanadium in Normalized, Thermo-Mechanically Treated and Cold-Rolled Steels. In Microalloying 75, Proceedings of the International Symposium on High Strength, Low-Alloy Steels, Washington, DC, USA, 1–3 October 1975; Union Carbide Corp: New York, NY, USA, 1975; Volume 1, pp. 130–144. [Google Scholar]
- Korchynsky, M. New Steels for New Mills. Scand. J. Met. 1999, 28, 40–45. [Google Scholar]
- Amin, R.K.; Korchynsky, M.; Pickering, F.B. Effect of Rolling Variables on Precipitation Strengthening in High-Strength Low-Alloy Steels Containing Vanadium and Nitrogen. Met. Technol. 1981, 8, 250–262. [Google Scholar] [CrossRef]
- Meyer, L.; Heisterkamp, F.; Janke, D. Effect of Low Ti Content on Mechanical Properties of Wire Hot Strip Made from Weldable Structural Steel. Thyssen Forsch. 1970, 2, 86–90. [Google Scholar]
- Meyer, L.; Arncken, G.; Heisterkamp, F.; Pretnar, B.; Schrape, U.; Stich, G. High-strength Hot Strip with Good Cold Formability Made from Ti Alloyed Pearlite Reduced Steel. Thyssen Forsch. 1976, 8, 21–25. [Google Scholar]
- Wang, S.C. The Effect of Titanium and Nitrogen Contents on the Microstructure and Mechanical Properties of Plain Carbon Steels. Mater. Sci. Eng. A 1991, 145, 87–94. [Google Scholar] [CrossRef]
- Ahmadi, E.; Suzuki, R.O.; Kaneko, T.; Kikuchi, T.A. Sustainable Approach for Producing Ti and TiS2 from TiC. Met. Mater. Trans. B 2021, 52, 77–87. [Google Scholar] [CrossRef]
- Kvackaj, M.; Kral, M.; Kvackaj, T.; Bidulska, J.; Bacso, J.; Nemethova, L. Influence of Coiling Conditions on IF Steel Properties. Hut. Wiad. Hut. 2009, 76, 613–616. [Google Scholar]
- Yeo, R.B.G.; Melville, A.G.; Repas, P.E.; Gray, J.M. Properties and Control of Hot-Rolled Steels. JOM 1968, 20, 33–43. [Google Scholar] [CrossRef]
- Baker, T.N. Microalloyed Steels. Ironmak. Steelmak. 2016, 43, 264–307. [Google Scholar] [CrossRef]
- Villalobos, J.; Del-Pozo, A.; Campillo, B.; Mayen, J.; Serna, S. Microalloyed Steels through History until 2018: Review of Chemical Composition, Processing and Hydrogen Service. Metals 2018, 8, 351. [Google Scholar] [CrossRef]
- Le Bon, A.B.; de Saint-Martin, L.N. Using Laboratory Simulations to Improve Rolling Schedules and Equipment. In Microalloying 75, Proceedings of the International Symposium on High Strength, Low-Alloy Steels, Washington DC, USA, 1–3 October 1975; Union Carbide Corp: New York, NY, USA; pp. 90–99.
- Civallero, M.A.; Parrini, C.; Pizzimenti, N. Production of Large Diameter High Strength Low-Alloy Pipe in Italy. In Microalloying 75: Proceedings of the International Symposium on High Strength, Low-Alloy Steels, Washington DC, USA, 1–3 October 1975; Union Carbide Corp: New York, NY, USA; pp. 451–469.
- Yamamoto, S.; Ouchi, C.; Osuka, T. The Effect of Microalloying Elements on the Recovery and Recrystallization in Deformed Austenite. In Proceedings of the International Symposium on Thermomechanical Processing of Microalloyed Austenite, Pittsburgh, PA, USA, 17–19 August 1981; TMS-AIME: Pittsburgh, PA, USA, 1981; pp. 613–639. [Google Scholar]
- Pickering, F.B. The Spectrum of Microalloyed High Strength Low Alloy Steels. In Proceedings of the HSLA Steels: Technology and Applications, Philadelphia, PA, USA, 3–6 October 1984; ASM: Metals Park, OH, USA, 1984; pp. 1–31. [Google Scholar]
- Kimura, T.; Ohmori, A.; Kawabata, F.; Amano, K. Ferrite Grain Refinement Through Intra-Granular Ferrite Transformation on VN Precipitation in TMCP of HSLA Steel. In Proceedings of the International Symposium THERMEC 97, Wollongong, Australia, 7–11 July 1997; TMS: Pittsburgh, PA, USA, 1997; pp. 645–651. [Google Scholar]
- DeArdo, A.J. Microalloyed Steel: Past, Present and Future. In Proceedings of the International Symposium on HSLA Steels 2015, Microalloying 2015 and Offshore Engineering Steels 2015, Hanghou, China, 11–13 November 2015; pp. 17–32. [Google Scholar]
- Bhattacharya, D. Modern Niobium Microalloyed Steels for the Automotive Industry. In Proceedings of the International Symposium on HSLA Steels 2015, Microalloying 2015 and Offshore Engineering Steels 2015, Hanghou, China, 11–13 November 2015; pp. 71–83. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, C.; Shang, C. New Development of HSLA Steels in China. In Proceedings of the International Symposium on HSLA Steels 2015, Microalloying 2015 and Offshore Engineering Steels 2015, Hanghou, China, 11–13 November 2015; pp. 1–15. [Google Scholar] [CrossRef]
- Tamura, I.; Sekine, H.; Tanaka, T.; Ouchi, C. Thermomechanical Processing of HSLA Steels; Butterworth-Heinemann: Oxford, UK, 1988. [Google Scholar] [CrossRef]
- Tanaka, T. Controlled Rolling of Steel Plate and Strip. Int. Met. Rev. 1981, 26, 185–212. [Google Scholar] [CrossRef]
- Kovac, F.; Siwecki, T.; Hutchinson, W.; Zajac, S. Finishing Conditions Appropriate for Recrystallization-Controlled Rolling of Ti-V-N-Steel. Met. Mater. Trans. A 1992, 23, 373–375. [Google Scholar] [CrossRef]
- Gladman, T. The Physical Metallurgy of Microalloyed Steels, 1st ed.; Institute of Materials: London, UK, 1997. [Google Scholar]
- Litvinenko, D.A.; Matrosov, J.I. Influence of Controlled Rolling on the Properties Steels. Stal 1974, 10, 931–936. [Google Scholar]
- Le Bon, A.; Rofes-Vernis, J.; Rossard, C. Recrystallization and Precipitation During Hot Working of Nb-Bearing HSLA Steel. Met. Sci. 1975, 9, 36–40. [Google Scholar] [CrossRef]
- Roberts, W. The evolution of microstructure during controlled rolling of microalloyed steels. Scand. J. Met. 1980, 9, 13–20. [Google Scholar]
- Akben, M.G.; Weiss, I.; Jonas, J.J. Dynamic Precipitation and Solute Hardening in V Microalloyed Steel and Two Nb Steels Containing High Levels of Mn. Acta Met. 1981, 29, 111–121. [Google Scholar] [CrossRef]
- Kvackaj, T.; Mamuzic, I. Mathematic description of austenite dynamic recrystallization in C-Mn base low carbon steel. Metalurgija 1995, 34, 139–143. [Google Scholar]
- Kvačkaj, T.; Voronov, A.N. Controlled Rolling of Microalloyed Steel. Stal 1990, 74–76. [Google Scholar]
- Voronov, A.N.; Kvačkaj, T.; Žadan, V.T.; Pavluš, M. Modelling of Austenite Transformation During Steel Cooling. Metally 1991, 2, 81–89. [Google Scholar]
- Kvačkaj, T. Mathematical Description of the Influence of the Nature of Austenite Before Recrystallization on Transformation Kinetics. Metalurgija 1994, 33, 87–91. [Google Scholar]
- Kvačkaj, T.; Mamuzič, I. A Quantitative Characterization of Austenite Microstructure after Deformation in Nonrecrystallization Region and its Influence on Ferrite Microstructure after Transformation. ISIJ Int. 1998, 38, 1270–1276. [Google Scholar] [CrossRef]
- Kvačkaj, T.; Zrník, J.; Vrchovinský, V.; Wangyao, P. Controlled Rolling of Hastelloy-N. High Temp. Mater. Process. 2002, 21, 351–359. [Google Scholar] [CrossRef]
- Kvackaj, T.; Kočiško, R.; Tiža, J.; Bidulská, J.; Kovácová, A.; Bidulský, R.; Bacsó, J.; Vlado, M. Application of workability test to SPD processing. Arch. Met. Mater. 2013, 58, 407–412. [Google Scholar] [CrossRef]
- Kvackaj, T.; Pokorny, I.; Vrchovinsky, V.; Vlado, M. Properties of ARMCO-Fe after ECAP Processes. J. Met. Mater. Min. 2004, 14, 21–25. [Google Scholar]
- Čížek, J.; Janeček, M.; Krajňák, T.; Stráská, J.; Hruška, P.; Gubicza, J.; Kim, H.S. Structural characterization of ultrafine-grained interstitial-free steel prepared by severe plastic deformation. Acta Mater. 2016, 105, 258–272. [Google Scholar] [CrossRef]
- Máthis, K.; Krajňák, T.; Kužel, R.; Gubicza, J. Structure and mechanical behaviour of interstitial-free steel processed by equal-channel angular pressing. J. Alloys Compd. 2011, 509, 3522–3525. [Google Scholar] [CrossRef]
- Kvackaj, T. Thermo-deformation Regime at the First Phase of Controlled Rolling. Hutník 1985, 35, 50–53. [Google Scholar]
- Sas, J.; Kvačkaj, T.; Milkovič, O.; Zemko, M. Influence of Hot Plastic Deformation in and (γ+α) Area on the Structure and Mechanical Properties of HSLA Steel. Materials 2016, 9, 971. [Google Scholar] [CrossRef]
- Kvackaj, T.; Hala, K.; Cerveny, E.; Jurca, M. Contribution to Technology of Heavy Plate Production from Microalloyed Steel with Nb+Ti. Hutnik 1982, 32, 21–25. [Google Scholar]
- Haensch, W.; Klinkenberg, C. Low Carbon Niobium Alloyed High Strength Steel for Automotive Hot Strip. Ironmak. Steelmak. 2005, 32, 342–346. [Google Scholar] [CrossRef]
- Mohrbacher, H. Reverse Metallurgical Engineering Towards Sustainable Manufacturing of Vehicles Using Nb and Mo Alloyed High Performance Steels. Adv. Manuf. 2013, 1, 28–41. [Google Scholar] [CrossRef][Green Version]
- Kvačkaj, T. The review of steel and steel products flat-rolled production. Metalurgija 2000, 39, 185–189. [Google Scholar]
- Kvackaj, T. Research of Steel Materials for Ultralight Steel Autobody. Acta Metall. Slovaca 2005, 11, 389–403. [Google Scholar]
- Le, H.; Nguyen, C.-S.; Bui, A. Experimental processing of ultra-low carbon steel using vacuum treatment. Acta Metall. Slovaca 2018, 24, 4–12. [Google Scholar] [CrossRef]
- Bublíková, D.; Jirková, H.; Rubešová, K.; Peković, M.; Volkmannová, J.; Graf, M. Effects of cooling rate on the volume fraction of retained austenite in forgings from high-strength Mn-Si steels. Acta Metall. Slovaca 2019, 25, 93–100. [Google Scholar] [CrossRef]
- Jirková, H.; Opatová, K.; Jeníček, Š.; Vrtáček, J.; Kučerová, L.; Kurka, P. Use of multi-phase trip steel for press-hardening technology. Acta Metall. Slovaca 2019, 25, 101–106. [Google Scholar] [CrossRef]
- Krbata, M.; Eckert, M.; Majerik, J.; Barenyi, I. Wear Behavior of High Strength Tool Steel 90MnCrV8 in Contact with Si3N4. Metals 2020, 10, 756. [Google Scholar] [CrossRef]
- ASM Handbook Committee. Properties and Selection: Irons, Steels, and High-Performance Alloys. In High-Strength Structural and High-Strength Low-Alloy Steels; ASM International: Materials Park, OH, USA, 1990; Volume 1. [Google Scholar] [CrossRef]
- Nemethova, L.; Kvackaj, T.; Fujda, M.; Misicko, R.; Tiza, J.; Kvackaj, M. Austenite Grain Size Change of Nb-V Steel in Dependence on Controlled Rolling Conditions. Acta Metall. Slovaca 2010, 16, 102–108. [Google Scholar]
- Hung, L.T.; Dinh, V.T.; Phuong, D.T.; Kien, L.T. Effect of springback in DP980 advanced high strength steel on product precision in bending process. Acta Metall. Slovaca 2019, 25, 150–157. [Google Scholar] [CrossRef]
- Arcelor Mittal Home Page. Available online: https://automotive.arcelormittal.com/home (accessed on 12 February 2021).
- Tata Steel Europe Home Page. Available online: https://www.tatasteeleurope.com (accessed on 12 February 2021).
- Efremenko, V.; Kussa, R.; Petryshynets, I.; Shimizu, K.; Kromka, F.; Zurnadzhy, V.; Gavrilova, V. Element partitioning in low-carbon Si2Mn2CrMoVNb trip-assisted steel in intercritical temperature range. Acta Met. Slovaca 2020, 26, 93–100. [Google Scholar] [CrossRef]
- Atkinson, H.V. Overview no. 65: Theories of normal grain growth in pure single phase systems. Acta Met. 1988, 36, 469–491. [Google Scholar] [CrossRef]
- Thompson, C.V. Grain Growth and Evolution of other Cellular Structures. Solid State Phys. 2001, 55, 269–314. [Google Scholar] [CrossRef]
- Koch, C.C.; Ovidko, I.A.; Seal, S.; Veprek, S. Structural Nanocrystalline Materials: Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar] [CrossRef]
- Novikov, V.Y. Computer Simulation of Normal Grain Growth. Acta Met. 1978, 26, 1739–1744. [Google Scholar] [CrossRef]
- Kurtz, S.K.; Carpay, F.M.A. Microstructure and Normal Grain Growth in Metals and Ceramics. Part I. Theory. J. Appl. Phys. 1980, 51, 5725. [Google Scholar] [CrossRef]
- Gusak, A.M.; Tu, K.N. Theory of Normal Grain Growth in Normalized Size Space. Acta Mater. 2003, 51, 3895–3904. [Google Scholar] [CrossRef]
- Burke, J.E.; Turnbull, D. Recrystallization and Grain Growth. Progr. Met. Phys. 1952, 3, 220–244. [Google Scholar] [CrossRef]
- Rollett, A.D.; Brahme, A.P.; Roberts, C.G. An Overview of Accomplishments and Challenges in Recrystallization and Grain Growth. Mater. Sci. Forum 2007, 558–559, 33–42. [Google Scholar] [CrossRef]
- Kobayashi, S.; Zaefferer, S. Microstructure Control Using Recrystallization in Particle-Containing Fe3Al Alloys. Mater. Sci. Forum 2007, 558–559, 235–240. [Google Scholar] [CrossRef]
- Rofman, O.V.; Bate, P.S. Dynamic Grain and Particle Growth in a Non-Superplastic Al-4Cu Alloy. Mater. Sci. Forum 2007, 558–559, 797–802. [Google Scholar] [CrossRef]
- Kinoshita, T.; Ohno, M. Phase-Field Simulation of Abnormal Grain Growth During Carburization in Nb-Added Steel. Comput. Mater. Sci. 2020, 177, 109558. [Google Scholar] [CrossRef]
- Pogorzhelskyj, V.J.; Matrosov, Y.J.; Nasibov, A.G. Controlled Rolling of Microalloyed Steel. In Microalloying 75, Proceedings of an Int. Symposium on High Strength, Low- Alloy Steels, Washington DC, USA, 1–3 October 1975; Korchynsky, M., Ed.; Union Carbide Corp: New York, NY, USA; pp. 100–106.
- Rios, P. Abnormal Grain Growth Development from Uniform Grain Size Distributions Due to a Mobility Advantage. Scr. Mater. 1998, 38, 1359–1364. [Google Scholar] [CrossRef]
- Rios, P.R. Abnormal Grain Growth Development from Uniform Grain Size Distributions in the Presence of Stable Particles. Scr. Mater. 1998, 39, 1725–1730. [Google Scholar] [CrossRef]
- Harase, J.; Shimizu, R. Texture Evolution by Grain Growth in the Presence of MnS and AIN Precipitates in Fe-3% Si Alloy. Acta Met. Mater. 1990, 38, 1395–1403. [Google Scholar] [CrossRef]
- Wörner, C.H.; Romero, S.; Hazzledine, P.M. Extension of Gladman’s Model for Abnormal Grain Growth. J. Mater. Res. 1991, 6, 1773–1778. [Google Scholar] [CrossRef]
- Fortes, M.A.; Deus, A.M. Effects of Triple Grain Junctions on Equilibrium Boundary Angles and Grain Growth Kinetics. Mater. Sci. Forum 2004, 455–456, 648–652. [Google Scholar] [CrossRef]
- Sellars, C.M.; Whiteman, J.A. Recrystallization and Grain Growth in Hot Rolling. Met. Sci. 1979, 13, 187–194. [Google Scholar] [CrossRef]
- Anderson, M.P.; Srolovitz, D.J.; Grest, G.A.; Sahni, P.S. Computer Simulation of Grain Growth—I. Kinetics. Acta Met. 1984, 32, 783–791. [Google Scholar] [CrossRef]
- Kvackaj, T.; Nemethova, L.; Misicko, R.; Pokorny, I.; Molnarova, M. Influence of Reheating Conditions on Austenite Grain Growth. High Temp. Mater. Process. 2011, 30, 535–538. [Google Scholar] [CrossRef]
- Kvačkaj, T.; Bacsó, J.; Bidulská, J.; Lupták, M.; Pokorný, I.; Kvačkaj, M.; Vlado, M. Influence of cryo and classic rolling conditions on processing parameters of C-Si steel. Acta Metall. Slovaca 2010, 16, 268–276. [Google Scholar]
- Napoli, G.; Fabrizi, G.; Rufini, R.; Mengaroni, S.; Di Schino, A. Effect of quenching and tempering process on a medium c steel with low chromium and molybenum addition for forged components. Acta Metall. Slovaca 2018, 24, 112–118. [Google Scholar] [CrossRef]
- Bublíková, D.; Jeníček, Š.; Peković, M.; Jirková, H. New treatment route for closed-die forgings of steels with 2.5% manganese. Acta Metall. Slovaca 2018, 24, 119–125. [Google Scholar] [CrossRef]
- García-Aguirre, K.-A.; Felguera-Jiménez, J.-L.; Herranz, G.; Calvo-Muñoz, J.; Benito-Páramo, J.-A.; Cabrera-Marrero, J.-M. Metal injection moulding (MIM) as an alternative fabrication process for the production of TWIP steel. Powder Met. 2019, 62, 205–211. [Google Scholar] [CrossRef]
- Benito, J.A.; García, K.; Herranz Sánchez-Cosgalla, G.; Calvo, J.; Cabrera, J.M. Development of sintered steels with TWIP effect. In Proceedings of the Euro PM 2017: International Powder Metallurgy Congress and Exhibition 2017, Euro PM 2017, Milano Congressi (MiCo), Milan, Italy, 1–4 October 2017; EPMA: Shrewsbury, UK, 2017; p. 140833. [Google Scholar]
- Vicenzi, B.; Boz, K.; Aboussouan, L. Powder metallurgy in aerospace—Fundamentals of PM processes and examples of applications. Acta Met. Slovaca 2020, 26, 144–160. [Google Scholar] [CrossRef]
- Jeon, J.; Nam, S.; Kang, S.; Shin, J.; Choi, H. Mechanical behavior of ultrafine-grained high-Mn steels containing nanoscale oxides produced by powder technology. Mater. Des. 2016, 92, 73–78. [Google Scholar] [CrossRef]
- Kvackaj, T.; Rozsypalova, A.; Kocisko, R.; Bidulska, J.; Petrousek, P.; Vlado, M.; Simcak, D. Influence of Processing Conditions on Properties of AISI 316LN Steel Grade. J. Mater. Eng. Perform. 2020, 29, 1509–1514. [Google Scholar] [CrossRef]
- Petrousek, P.; Kvackaj, T.; Kocisko, R.; Bidulska, J.; Luptak, M.; Manfredi, D.; Grande, M.A.; Bidulsky, R. Influence of Cryorolling on Properties of L-PBF 316L Stainless Steel Tested at 298K and 77K. Acta Metall. Slovaca 2019, 25, 283–290. [Google Scholar] [CrossRef]
- Souckova-Siegolová, J. Treatment and usage of iron in the Hittite empire in the 2nd millennium BC. Mediterr. Archaeol. 2001, 14, 189–193. [Google Scholar]
- Wertime, T.A. The Coming of the Age of Steel; University of Chicago Press: Chicago, OH, USA, 1962. [Google Scholar]
- Zhang, Y.; Li, X.; Liu, Y.; Liu, C.; Dong, J.; Yu, L.; Li, H. Study of the Kinetics of Austenite Grain Growth by Dynamic Ti-rich and Nb-rich Carbonitride Dissolution in HSLA Steel: In-situ Observation and Modeling. Mater. Charact. 2020, 169, 110612. [Google Scholar] [CrossRef]
- Bidulsky, R.; Bidulska, J.; Gobber, F.S.; Kvackaj, T.; Petrousek, P.; Actis-Grande, M.; Weiss, K.P.; Manfredi, D. Case Study of the Tensile Fracture Investigation of Additive Manufactured Austenitic Stainless Steels Treated at Cryogenic Conditions. Materials 2020, 13, 3328. [Google Scholar] [CrossRef] [PubMed]
- Ridolfi, M.R.; Folgarait, P.; Di Schino, A. Modelling of laser powder bed fusion process for different type materials. Acta Metall. Slovaca 2020, 26, 7–10. [Google Scholar] [CrossRef]
- Di Schino, A.; Fogarait, P.; Corapi, D.; Di Pietro, O.; Zitelli, C. Austenitic stainless steels manufacturing by laser powder bed fusion technique. Acta Metall. Slovaca 2020, 26, 24–26. [Google Scholar] [CrossRef]
- Calignano, F.; Manfredi, D.; Ambrosio, E.P.; Biamino, S.; Lombmbardi, M.; Atzeni, E.; Salmi, A.; Minetola, P.; Iuliano, L.; Fino, P. Overview on Additive Manufacturing Technologies. Proc. IEEE 2017, 105, 593–612. [Google Scholar] [CrossRef]






| Metal | Al | Fe | Pb | Sn |
|---|---|---|---|---|
| Exponent “n” | 4 | 2.5 | 2.4–2.5 | 2.0–2.3 |
| Steel Grades | C | Mn | Si | Al | P | S | Nb | V | Ti | |
|---|---|---|---|---|---|---|---|---|---|---|
| HSLA | QStE380TM (S380MC) | 0.080 | 0.800 | 0.030 | 0.040 | 0.011 | 0.008 | 0.020 | 0.050 | 0.020 |
| QStE460TM (S460MC) | 0.090 | 1.120 | 0.020 | 0.050 | 0.013 | 0.009 | 0.040 | 0.030 | 0.070 | |
| X70 (C–Mn–Nb–V) | 0.090 | 1.600 | 0.200 | 0.040 | 0.013 | 0.007 | 0.040 | 0.060 | 0.008 | |
| 38MnSiVS35 | 0.340 | 1.200 | 0.350 | - | 0.034 | 0.035 | - | 0.120 | 0.050 | |
| HSS | St52 (S355) | 0.200 | 1.500 | 0.500 | - | 0.020 | 0.020 | - | - | - |
| IF | 0.030 | 0.170 | 0.020 | 0.040 | 0.010 | 0.007 | - | - | 0.070 | |
| C–2.3Si | 0.015 | 0.250 | 2.300 | 0.464 | 0.018 | 0.004 | - | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kvackaj, T.; Bidulská, J.; Bidulský, R. Overview of HSS Steel Grades Development and Study of Reheating Condition Effects on Austenite Grain Size Changes. Materials 2021, 14, 1988. https://doi.org/10.3390/ma14081988
Kvackaj T, Bidulská J, Bidulský R. Overview of HSS Steel Grades Development and Study of Reheating Condition Effects on Austenite Grain Size Changes. Materials. 2021; 14(8):1988. https://doi.org/10.3390/ma14081988
Chicago/Turabian StyleKvackaj, Tibor, Jana Bidulská, and Róbert Bidulský. 2021. "Overview of HSS Steel Grades Development and Study of Reheating Condition Effects on Austenite Grain Size Changes" Materials 14, no. 8: 1988. https://doi.org/10.3390/ma14081988
APA StyleKvackaj, T., Bidulská, J., & Bidulský, R. (2021). Overview of HSS Steel Grades Development and Study of Reheating Condition Effects on Austenite Grain Size Changes. Materials, 14(8), 1988. https://doi.org/10.3390/ma14081988








