1. Introduction
The electrocardiogram (ECG) is one of the most widely analyzed and clinically utilized physiological signals among others such as electroencephalograms (EEGs), electromyograms (EMGs), electrooculograms (EOGs), and respiratory signals. It reflects the electrical signature of the cardiac cycles and thus can be used as the starting point for the quantification of heart rate variability (HRV), which represents the variation of the heart rate and is based on normal-to-normal interbeat intervals. HRV reflects the physiological process of many factors regulating the normal rhythm of the heart on a beat-by-beat basis in order to achieve an—ideally—optimal nourishment of the body by providing cardiac output [
1].
More than five decades ago, it was already discovered that HRV could be used as a marker for cardiovascular health status, when a reduction of fetal HRV during labor was associated with distressed babies [
2]. Since then, the usefulness of HRV for risk stratification has become more and more evident. In recent years, its predictive value has been demonstrated in several populations, ranging from post-myocardial infarction [
3] to chronic kidney disease and end-stage renal disease (ESRD) patients undergoing hemodialysis [
4,
5], by the use of traditional measures in the time and/or frequency domain to newer nonlinear measures.
A vast number of measures for the quantification of HRV have been introduced in recent decades. Traditional measures originate mainly from the time and/or frequency domain [
6,
7]. Newer methods (e.g., long-range correlation and fractal analysis, short-term complexity, entropy or detrended fluctuation analysis) can contribute essentially to the understanding of the underlying mechanisms of HRV and to the quantification of the complex regulation mechanisms, which cannot be described by traditional measures accurately [
8]. Furthermore, Sassi et al. [
8] highlighted in their critical review the so-far rather limited success of these advanced HRV approaches in clinical applications.
Entropy measures are part of this new family quantifying the variability of the heart rate [
9]. Their information theory principles have clear potential to better understand the intrinsic dynamics and mechanisms underlying HRV and its physiological origins. This is underlined by their ability to discover certain patterns and shifts in the “apparent ensemble amount of randomness” of a stochastic process [
10]. They can measure randomness as well as the predictability of processes [
11]. The family of entropy measures has grown immensely in recent years, and these are applied in various domains (e.g., [
9]). Particularly for HRV analysis, the following measures can be highlighted: approximate entropy (
) [
12], sample entropy (
) [
13], fuzzy entropy (
) [
14], and their further developments fuzzy measure entropy (
) [
15] and corrected
(
) [
16].
One aspect that all entropy measures have in common is their dependency on the choice of parameters, as these determine their behavior and thus are crucial for the results. In recent years, a large amount of research has been done to define parameter sets for various application fields [
13,
17,
18,
19]. These studies were, to our knowledge, mainly based on cross-sectional data. Hence, longitudinal evidence is still scarce. Nevertheless, agreement has been reached on some parameters, and studies’ results for parameter selection are converging for others.
We therefore aim to use recently published parameter sets for different entropy measures for risk prediction in a cohort of ESRD patients undergoing hemodialysis. Questions raised by Holzinger et al. [
9] on the clinical applicability of entropy measures and their dependency on the choice of parameters are addressed. Therefore, parameters are not optimized for the data at hand, but they are challenged for risk prediction as reported in a very special and different cohort, that is, on high-risk patients with a five-year survival range between 40% and 50% [
20]. As a result, the focus is on
,
,
,
and
, using the parameter sets recently determined by Mayer et al. [
1,
19].
3. Results
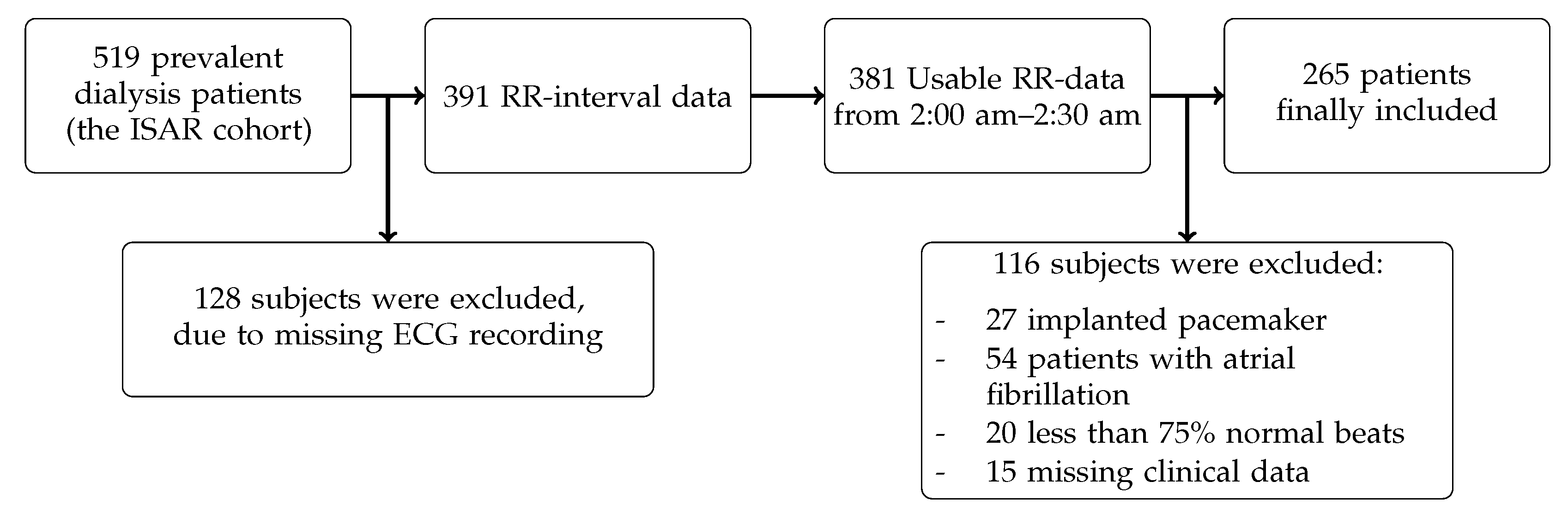
During the median follow-up time of 43 months, 70 out of the 265 patients with ESRD undergoing hemodialysis died (26%). The patient baseline data are shown in
Table 2. The mean age of the patients was 62 years, and 175 were male (66%). Of 265 patients, 250 suffered from hypertension, which corresponded to 94%. The median duration per dialysis session was 4.23 h.
Table 3 shows the traditional HRV measures, as well as entropy measures at baseline.
Univariate Cox models identified the following independent risk factors: age, height, dialysis duration per session, serum albumin, high-sensitivity C-reactive protein (hsCRP), anticoagulant, and adapted CCI (see
Table 4). Adapted CCI and hsCRP showed the highest HRs with values of 1.24 (1.16, 1.33) and 1.26 (1.02, 1.56), respectively. Hence, an increase of one unit in one of either variable increased the risk of death by about 25%. The HR of 0.52 (0.30, 0.89) producing an increase of 1 h per dialysis session indicated that the chance of survival is doubled if the dialysis duration is extended by 1 h. These predictors were used in Model B to adjust the HRV measures for mortality risk. Another set for adjustment is found in the literature and is defined as Model A with the following variables: age, serum albumin, hsCRP, and calcium × phosphate [
5].
Table 5 shows unadjusted and adjusted hazard ratios of HRV and entropy measures. Of the standard HRV parameters, SDNN, HRVTI, total P, LF, and LF/HF predicted mortality significantly. The HR of 0.82 (0.69, 0.96) for LF showed that an increase in LF power is associated with an increased chance of survival. After adjustment (with either model), only LF was an independent predictor. LF/HF had a significant predictive value if Model B was used for adjustment and a borderline significance for Model A.
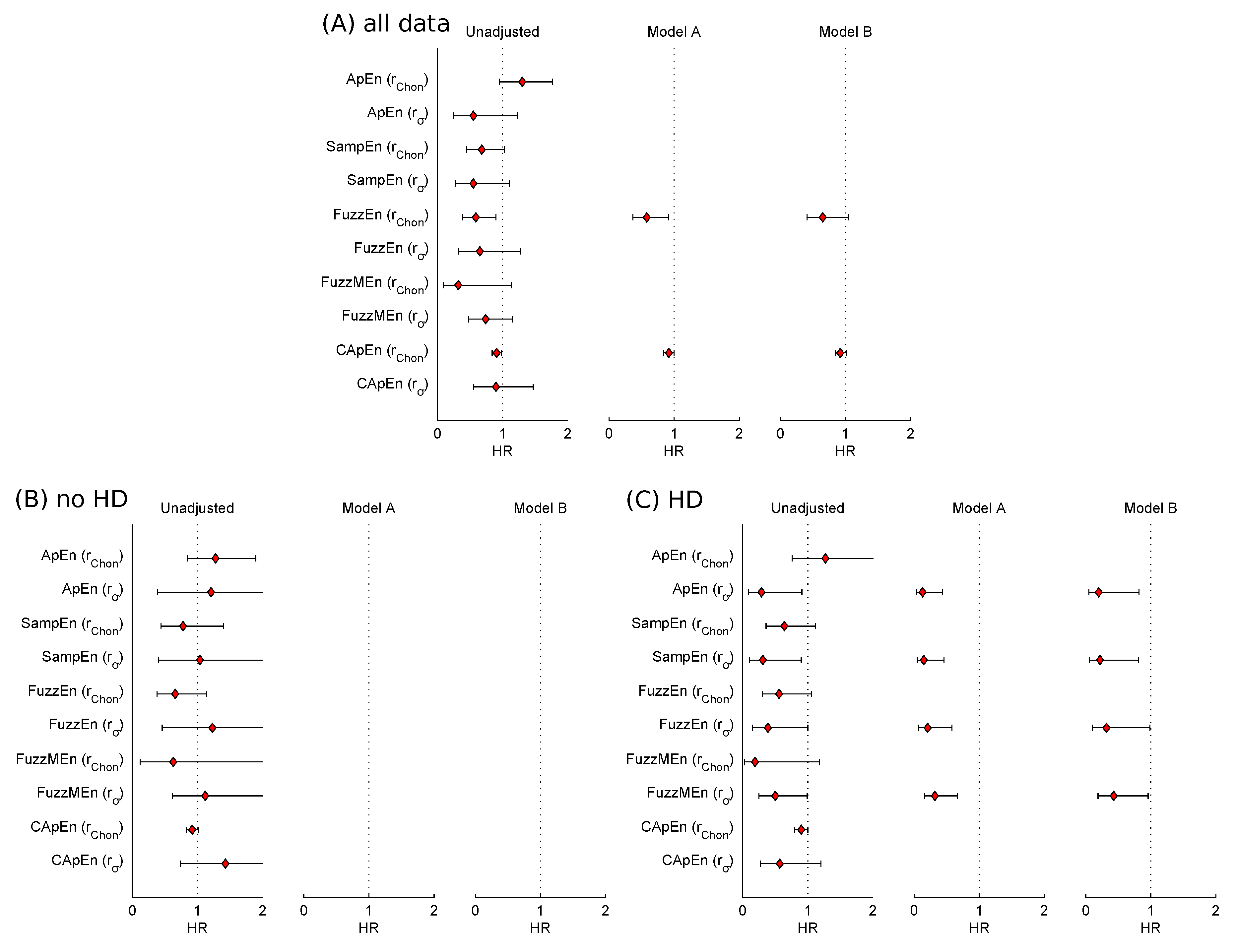
FuzzyEn and CApEn significantly predicted mortality out of 10 entropy measures in univariate analysis. An increased chance of survival for higher entropy values was manifested by a HR of 0.59 (0.39, 0.90) with an increase of one unit for FuzzyEn. After adjusting with Model A, both entropy measures retained significant risk predictors. None of the entropies was a significant predictor of mortality after adjustment with Model B.
Interaction terms testing the interaction between the risk predictors (i.e., entropy measures) and HD status were borderline significant if
was chosen (
). Hence, a subgroup analysis was performed, dividing the cohort into patients with HD (
, 38 events) and those without HD (
, 32 events). Baseline differences between these groups are provided in the online
Supplementary Tables S1 and S2.
The results shown in
Table 6 and
Table 7 confirm the differences between the two subgroups, that is, patients with HD and patients without HD, regarding entropy measures as risk predictors, as already indicated by interaction analysis. For the patients without HD, none of the entropies was a significant predictor of mortality. Considering the subgroup of patients with HD, the results are completely different, as all entropies with
except CApEn
predicted mortality significantly (see
Table 7). After adjustment (with either model), all entropies retained their significant predictive values.
Additionally,
Figure 2 visualizes HRs of all entropy measures before adjustment and after adjustment if univariate analysis was significant.
4. Discussion
In this work, we used recently published parameter sets for different entropy measures for risk prediction in a cohort of ESRD patients undergoing hemodialysis. Prior studies have noted the importance of parameter selection for the different entropy measures [
14,
15,
18,
31]. Thus, we challenged recently published parameter sets [
1,
19] in their clinical applicability by testing their prediction value in a very special and different cohort, that is, including high-risk patients with five year survival ranges of between 40% and 50% [
20], without optimization of the parameters for the present data.
In general, a higher HRV indicates that the adaption of the organism to internal and external influences is faster and more flexible, as a result of the optimization of the interplay of the sympathetic and parasympathetic nervous system [
6]. Furthermore, this complex optimization tends to exhibit chaotic properties [
34,
35]. Because entropy is a measure of unpredictability and thus quantifies these chaotic properties, it can be concluded that a reduced HRV entropy reflects an impairment of the heart rate regulation. This conclusion is in accordance with the results of this work, as all entropy measures with significant predictive power showed a HR below 1. Hence, an increased HRV entropy is associated with an increased chance of survival.
Baseline clinical, traditional time and frequency domain HRV data were in agreement with a similar HRV study in ESRD patients, although this study used 24 h data as opposed to 30 min segments at night that was used for the present study, and the study was based on Japanese as opposed to Caucasian/German patients [
5]. Univariate Cox regression analysis revealed known clinical- and dialysis-related risk factors in ESRD patients as possible confounders, which were used for adjustment (i.e., serum albumin, dialysis duration per session, hsCRP, and the use of anticoagulants).
In univariate Cox regression analysis, the following HRV measures, SDNN, HRVTI, LF, and LF/HF, were identified as significant risk predictors for all-cause mortality, and after adjustment only LF and LF/HF remained (borderline) significant. These results are in agreement with those obtained by Suzuki et al. [
5], except that for LF. These findings are again contrary to previous studies with post-myocardial infarction (MI) patients, which have suggested the independent predictive power of traditional HRV measures [
36,
37,
38,
39]. Suzuki et al. [
5] provided a possible explanation by highlighting the fact that post-MI studies did not adjust for serum albumin and (hs)CRP levels, whose predictive power and their association to cardiac vagal dysfunction might mask effects [
7]. Suzuki et al. [
5] identified a decreased scaling exponent
, a representative of nonlinear HRV measures, as an independent risk predictor for mortality in chronic hemodialysis patients, improving risk stratification.
One further observation concerns the parameter choice for the entropy calculation. In the analysis of the entire cohort, only entropy values calculated using
showed significant predictive power, while in the subgroup analysis of HD patients, only entropy values with
achieved these results. Upon closer inspection of the data, it was found that using
captured effects in the entire cohort, but only resulted in a small change of the respective entropy values. In this case, statistical significance was reached only in the entire cohort as a result of the larger sample size. In contrast, entropy values calculated using
captured effects specific to the HD group. Further investigations are required to clarify these differences. A note of caution is due here, as the number of covariates used for adjustment in Model B was borderline in relation to the number of fatal events in the HD and no HD subgroups, respectively [
21].
Additionally, the interpretation of HRs of Box-Cox-transformed predictors is not straightforward. If the predictor is not transformed, HRs quantify the risk of additive changes of the predictor: one absolute unit increase in the predictor, holding all other variables constant, is associated with the hazard being multiplied by the respective HR value. Furthermore, if the predictor is
-transformed, HRs quantify the risk of multiplicative changes of the predictor; for example, an
-fold relative increase in the predictor is associated with the hazard being multiplied by the respective HR value [
40,
41]. However, there is no such intuitive interpretation relating HR and predictor values after a Box-Cox transformation [
33,
42]. Therefore, while the HRs certainly contain the same quality of information as for the untransformed or log-transformed cases, the relation of absolute
values and mortality needs to be interpreted carefully. In any case, a Box-Cox transformation for
using
was necessary because of its right-skewed distribution, in order to fulfil assumptions for Cox regression models.
Finally, a number of important limitations of this study need to be considered. First, the generalizability of these results is subject to certain restrictions, as all participants were from dialysis centers in Munich and the suburban area and were mainly Caucasians. Thus, extrapolation of findings to other countries/populations is limited. Second, subgroup analysis based on HD raises some sample-size limitations, as the number of fatal events was rather low for each group and thus calls for full adjustment. Finally, this study suggests that entropy measures may be useful for risk prediction of ESRD patients undergoing hemodialysis with HD, but it is unclear whether reduced entropy is treatable and modifiable by treatment to reduce risk.