Carbide Formation in Refractory Mo15Nb20Re15Ta30W20 Alloy under a Combined High-Pressure and High-Temperature Condition
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Phase and Microstructure at Low-Pressure
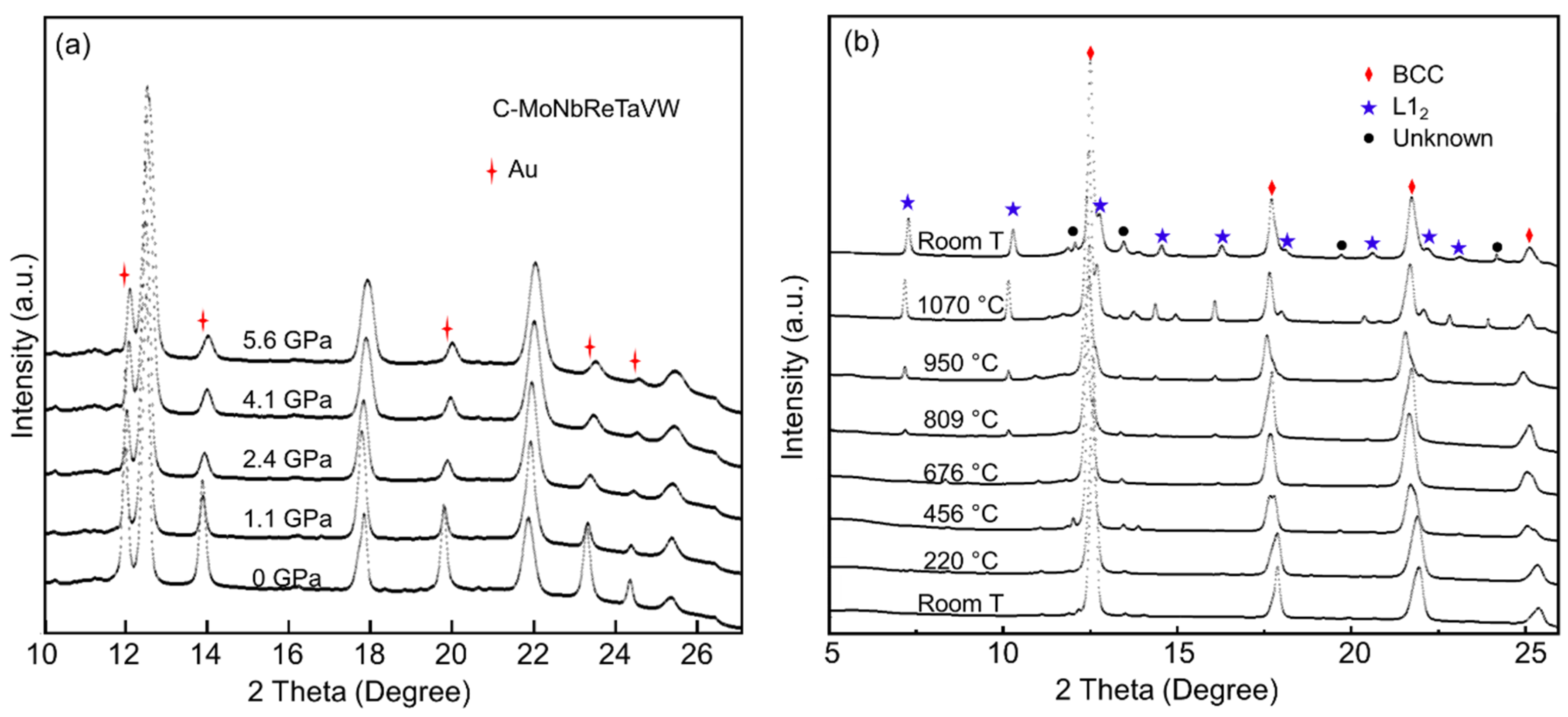
3.2. C0.1-Alloy under High P/low T and High P/high T
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Kang, B.; Lee, J.; Ryu, H.J.; Hong, S.H. Ultra-high strength WNbMoTaV high-entropy alloys with fine grain structure fabricated by powder metallurgical process. Mater. Sci. Eng. A 2018, 712, 616–624. [Google Scholar] [CrossRef]
- Yao, M.J.; Pradeep, K.G.; Tasan, C.C.; Raabe, D. A novel, single phase, non-equiatomic FeMnNiCoCr high-entropy alloy with exceptional phase stability and tensile ductility. Scr. Mater. 2014, 72–73, 5–8. [Google Scholar] [CrossRef]
- Patriarca, L.; Ojha, A.; Sehitoglu, H.; Chumlyakov, Y.I. Slip nucleation in single crystal FeNiCoCrMn high entropy alloy. Scr. Mater. 2016, 112, 54–57. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Tong, C.-J.; Chen, Y.-L.; Yeh, J.-W.; Lin, S.-J.; Chen, S.-K.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Microstructure characterization of AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 881–893. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Ma, Z.L.; Wang, M.; Chen, Y.W.; Tan, Y.D.; Cheng, X.W. Design of novel low-density refractory high entropy alloys for high-temperature applications. Mater. Sci. Eng. A 2019, 755, 318–322. [Google Scholar] [CrossRef]
- Kang, M.; Lim, K.R.; Won, J.W.; Lee, K.S.; Na, Y.S. Al-Ti-Containing lightweight high-entropy alloys for intermediate temperature applications. Entropy 2018, 20, 355. [Google Scholar] [CrossRef]
- Youssef, K.M.; Zaddach, A.J.; Niu, C.; Irving, D.L.; Koch, C.C. A Novel Low-Density, High-hardness, high-entropy alloy with close-packed single-phase nanocrystalline structures. Mater. Res. Lett. 2015, 3, 95–99. [Google Scholar] [CrossRef]
- Bhandari, U.; Zhang, C.; Yang, S. Mechanical and thermal properties of low-density Al20+xCr20−xMo20−yTi20V20+y alloys. Crystals 2020, 10, 278. [Google Scholar] [CrossRef]
- Bei, H. Multi-Component Solid Solution Alloys Having High Mixing Entropy. U.S. Patent No 9,150,945, 6 October 2015. [Google Scholar]
- Klimova, M.V.; Semenyuk, A.O.; Shaysultanov, D.G.; Salishchev, G.A.; Zherebtsov, S.V.; Stepanov, N.D. Effect of carbon on cryogenic tensile behavior of CoCrFeMnNi-type high entropy alloys. J. Alloy. Compd. 2019, 811, 152000. [Google Scholar] [CrossRef]
- Joo, S.H.; Kato, H.; Jang, M.J.; Moon, J.; Kim, E.B.; Hong, S.J.; Kim, H.S. Structure and properties of ultrafine-grained CoCrFeMnNi high-entropy alloys produced by mechanical alloying and spark plasma sintering. J. Alloy. Compd. 2017, 698, 591–604. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, H.Y.; Xie, Y.C.; Tang, Q.H.; Dai, P.Q. Controllable fabrication of a carbide-containing FeCoCrNiMn high-entropy alloy: Microstructure and mechanical properties. Mater. Sci. Technol. 2017, 33, 2032–2039. [Google Scholar] [CrossRef]
- Shun, T.-T.; Du, Y.-C. Age hardening of the Al0.3CoCrFeNiC0.1 high entropy alloy. J. Alloy. Compd. 2009, 478, 269–272. [Google Scholar] [CrossRef]
- Velo, I.L.; Gotor, F.J.; Alcalá, M.D.; Real, C.; Córdoba, J.M. Fabrication and characterization of WC-HEA cemented carbide based on the CoCrFeNiMn high entropy alloy. J. Alloy. Compd. 2018, 746, 1–8. [Google Scholar] [CrossRef]
- Guo, N.N.; Wang, L.; Luo, L.S.; Li, X.Z.; Chen, R.R.; Su, Y.Q.; Guo, J.J.; Fu, H.Z. Microstructure and mechanical properties of in-situ MC-carbide particulates-reinforced refractory high-entropy Mo0.5NbHf0.5ZrTi matrix alloy composite. Intermetallics 2016, 69, 74–77. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, C.; Zhang, Y.; Feng, X.; Gu, Y.; Li, Z.; Jiao, H.; Tan, X.; Xu, H. The AC Soft Magnetic Properties of FeCoNixCuAl (1.0 ≤ x ≤ 1.75) High-Entropy Alloys. Mater. Res. Lett. 2019, 12, 4222. [Google Scholar] [CrossRef]
- Li, J.; Gao, B.; Wang, Y.; Chen, X.; Xin, Y.; Tang, S.; Liu, B.; Liu, Y.; Song, M. Microstructures and mechanical properties of nano carbides reinforced CoCrFeMnNi high entropy alloys. J. Alloy. Compd. 2019, 792, 170–179. [Google Scholar] [CrossRef]
- Yan, J.; Knight, J.; Kunz, M.; Vennila Raju, S.; Chen, B.; Gleason, A.E.; Godwal, B.K.; Geballe, Z.; Jeanloz, R.; Clark, S.M. The resistive-heating characterization of laser heating system and LaB6 characterization of X-ray diffraction of beamline 12.2.2 at advanced light source. J. Phys. Chem. Solids 2010, 71, 1179–1182. [Google Scholar] [CrossRef]
- Bassett, W.A. Diamond anvil cell, 50th birthday. High Press. Res. 2009, 29, 163–186. [Google Scholar] [CrossRef]
- Kunz, M.; Yan, J.; Cornell, E.; Domning, E.E.; Yen, C.E.; Doran, A.; Beavers, C.M.; Treger, A.; Williams, Q.; MacDowell, A.A. Implementation and application of the peak scaling method for temperature measurement in the laser heated diamond anvil cell. Rev. Sci. Instrum. 2018, 89, 083903. [Google Scholar] [CrossRef]
- Raju, S.V.; Zaug, J.M.; Chen, B.; Yan, J.; Knight, J.W.; Jeanloz, R.; Clark, S.M. Determination of the variation of the fluorescence line positions of ruby, strontium tetraborate, alexandrite, and samarium-doped yttrium aluminum garnet with pressure and temperature. J. Appl. Phys. 2011, 110, 023521. [Google Scholar] [CrossRef]
- Pasternak, S.; Aquilanti, G.; Pascarelli, S.; Poloni, R.; Canny, B.; Coulet, M.-V.; Zhang, L. A diamond anvil cell with resistive heating for high pressure and high temperature X-ray diffraction and absorption studies. Rev. Sci. Instrum. 2008, 79, 085103. [Google Scholar] [CrossRef]
- Bhandari, U.; Zhang, C.; Zeng, C.; Guo, S.; Yang, S. Computational and experimental investigation of refractory high entropy alloy Mo15Nb20Re15Ta30W20. J. Mater. Res. Technol. 2020, 9, 8929–8936. [Google Scholar] [CrossRef]
- Yan, J.; Yang, S. An Alternative Thermal Equation of State for Solids at High Temperature High Pressure. Submitted to Minerals, under revision.
- Prescher, C.; Prakapenka, V.B. DIOPTAS: A program for reduction of two-dimensional X-ray diffraction data and data exploration. High Press. Res. 2015, 35, 223–230. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar]
- Bragg, W.H.; Bragg, W.L. The reflection of X-rays by crystals. Proc. R. Soc. Lond. Ser. A Contain. Pap. Math. Phys. Character 1913, 88, 428–438. [Google Scholar] [CrossRef]
- Yao, H.W.; Qiao, J.W.; Hawk, J.A.; Zhou, H.F.; Chen, M.W.; Gao, M.C. Mechanical properties of refractory high-entropy alloys: Experiments and modeling. J. Alloy. Compd. 2017, 696, 1139–1150. [Google Scholar] [CrossRef]
- Altomare, A.; Corriero, N.; Cuocci, C.; Falcicchio, A.; Moliterni, A.; Rizzi, R. Main features of QUALX2.0 software for qualitative phase analysis. Powder Diffr. 2017, 32, S129–S134. [Google Scholar] [CrossRef]
- Shivam, V.; Basu, J.; Pandey, V.K.; Shadangi, Y.; Mukhopadhyay, N.K. Alloying behaviour, thermal stability and phase evolution in quinary AlCoCrFeNi high entropy alloy. Adv. Powder Technol. 2018, 29, 2221–2230. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Rubinovich, L.; Polak, M. Site-specific segregation and compositional ordering in Ni-based ternary alloy nanoclusters computed by the free-energy concentration expansion method. Phys. Rev. B 2004, 69, 155405. [Google Scholar] [CrossRef]
- Polak, M.; Rubinovich, L. Stabilization and transformation of asymmetric configurations in small-mismatch alloy nanoparticles: The role of coordination dependent energetics. Phys. Chem. Chem. Phys. 2014, 16, 1569–1575. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Brady, M.P.; Lu, Z.P.; Maziasz, P.J.; Liu, C.T.; Pint, B.A.; More, K.L.; Meyer, H.M.; Payzant, E.A. Creep-resistant, Al2O3-forming austenitic stainless steels. Science 2007, 316, 433. [Google Scholar] [CrossRef]
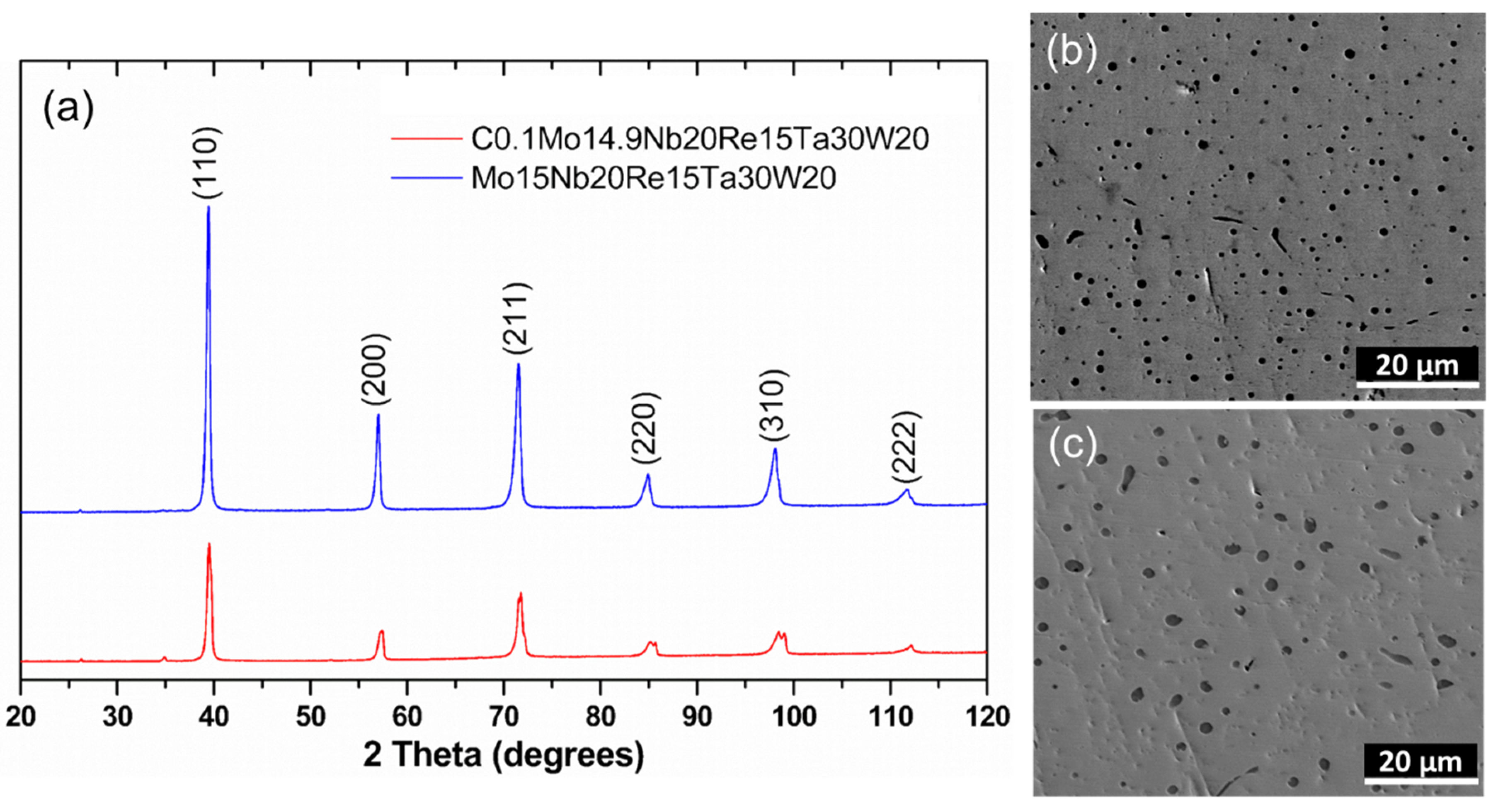


| Sample | C | Mo | Nb | Re | Ta | W | Hardness | ||
|---|---|---|---|---|---|---|---|---|---|
| 100 gf | 500 gf | 2000 gf | |||||||
| alloy | - | 14.43 ± 0.17 | 22.74 ± 0.38 | 15.29 ± 0.09 | 26.66 ± 0.19 | 20.90 ± 0.19 | 6.451 ± 0.140 | 6.035 ± 0.303 | 5.400 ± 0.213 |
| C-doped | 0.06 | 14.32 ± 0.41 | 22.63 ± 0.54 | 18.12 ± 0.28 | 26.44 ± 0.32 | 18.43 ± 0.26 | 5.826 ± 0.104 | 5.905 ± 0.086 | 5.749 ± 0.203 |
| (a) | C | Mo | Nb | Re | Ta | W | Elements | ||
|---|---|---|---|---|---|---|---|---|---|
| (b) | |||||||||
| C | - | −67 | −102 | −101 | −60 | −42 | C | ||
| Mo | 0.39 | - | −6 | −7 | −5 | 0 | Mo | ||
| Nb | 0.95 | 0.56 | - | −26 | 0 | −8 | Nb | ||
| Re | 0.65 | 0.26 | 0.3 | - | −24 | −4 | Re | ||
| Ta | 1.05 | 0.66 | 0.1 | 0.4 | - | −7 | Ta | ||
| W | 0.19 | 0.2 | 0.76 | 0.46 | 0.86 | - | W | ||
| Elements | C | Mo | Nb | Re | Ta | W | (a) | ||
| (b) | |||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Bhandari, U.; Zeng, C.; Ding, H.; Guo, S.; Yan, J.; Yang, S. Carbide Formation in Refractory Mo15Nb20Re15Ta30W20 Alloy under a Combined High-Pressure and High-Temperature Condition. Entropy 2020, 22, 718. https://doi.org/10.3390/e22070718
Zhang C, Bhandari U, Zeng C, Ding H, Guo S, Yan J, Yang S. Carbide Formation in Refractory Mo15Nb20Re15Ta30W20 Alloy under a Combined High-Pressure and High-Temperature Condition. Entropy. 2020; 22(7):718. https://doi.org/10.3390/e22070718
Chicago/Turabian StyleZhang, Congyan, Uttam Bhandari, Congyuan Zeng, Huan Ding, Shengmin Guo, Jinyuan Yan, and Shizhong Yang. 2020. "Carbide Formation in Refractory Mo15Nb20Re15Ta30W20 Alloy under a Combined High-Pressure and High-Temperature Condition" Entropy 22, no. 7: 718. https://doi.org/10.3390/e22070718
APA StyleZhang, C., Bhandari, U., Zeng, C., Ding, H., Guo, S., Yan, J., & Yang, S. (2020). Carbide Formation in Refractory Mo15Nb20Re15Ta30W20 Alloy under a Combined High-Pressure and High-Temperature Condition. Entropy, 22(7), 718. https://doi.org/10.3390/e22070718








