Information Rate in Humans during Visuomotor Tracking
Abstract
1. Introduction
1.1. Information Processing Rate in Humans
1.2. Pursuit-Tracking Task and Its Feedforward Component
2. Results
2.1. Background
2.2. Definition of Measures
2.3. Validation through Model Simulations
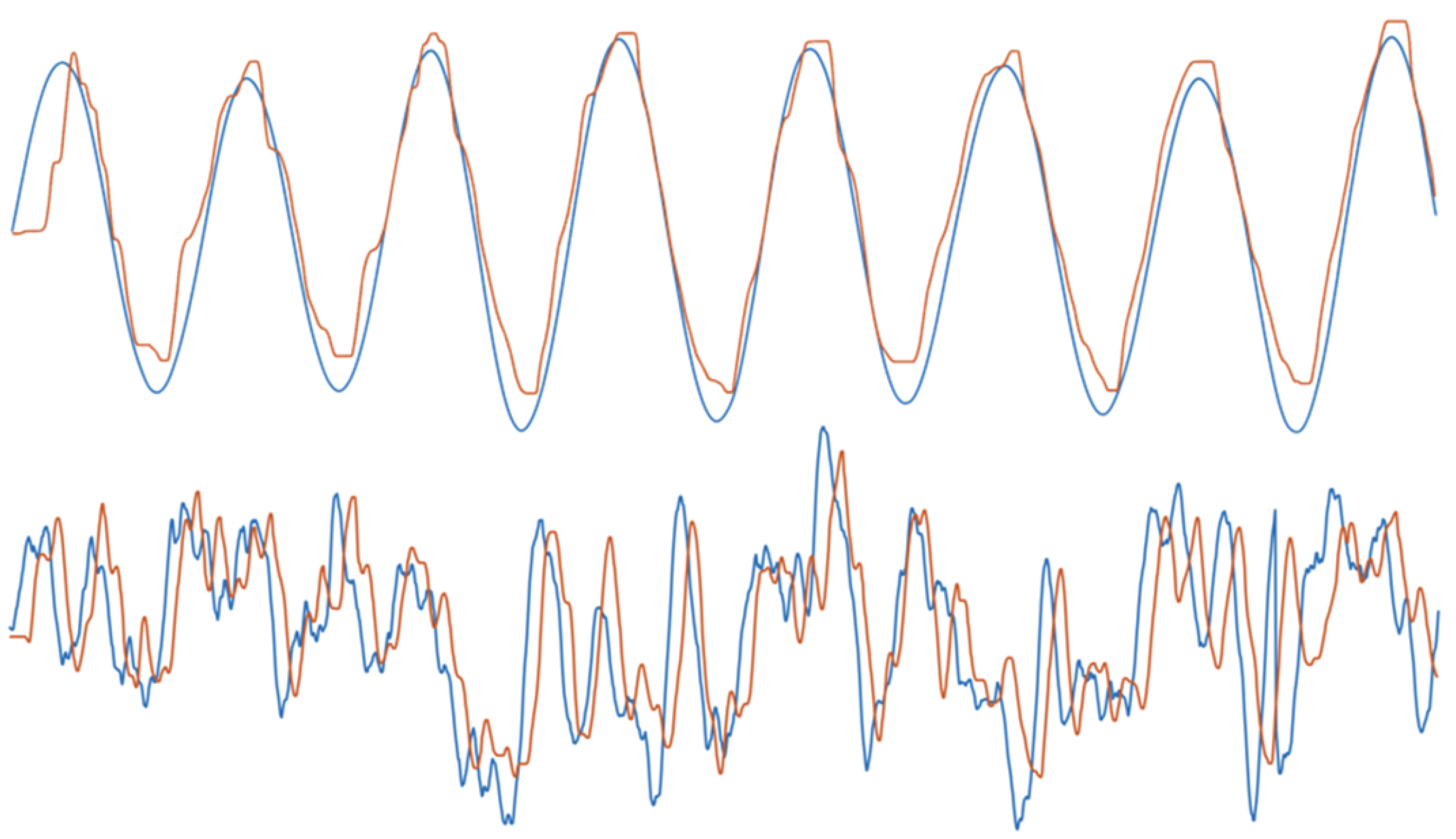
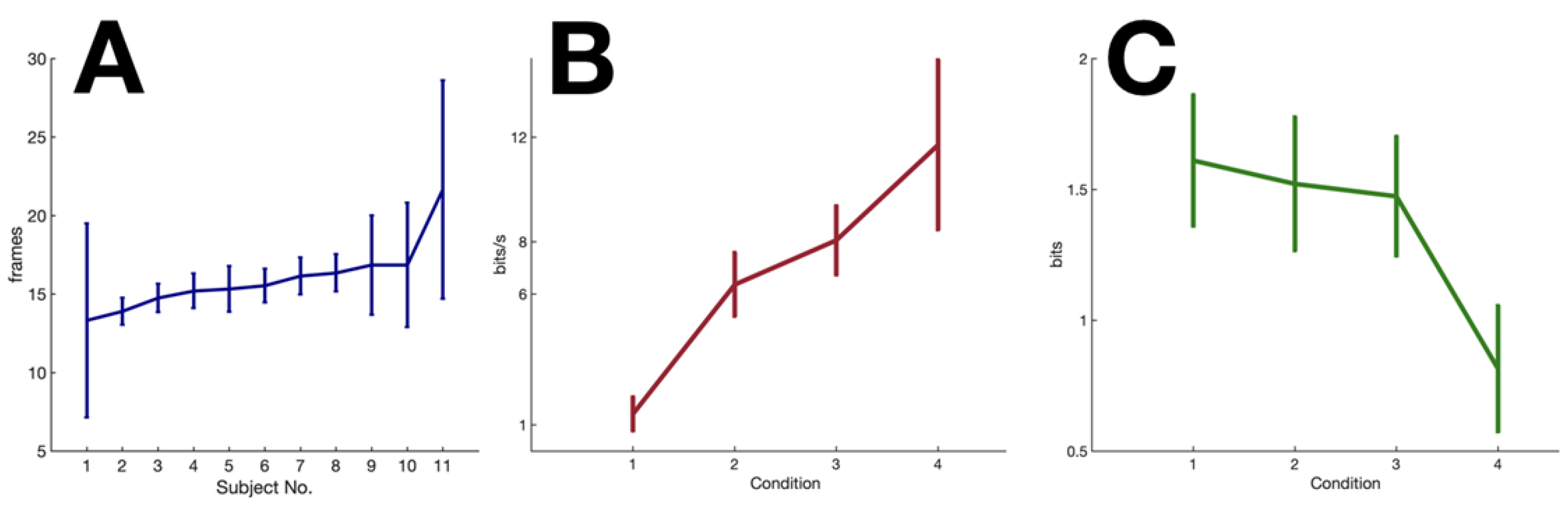
2.4. Experimental Results
3. Discussion
3.1. Information Processing Rate in Humans
3.2. Information-Theoretic Approach to Evaluating Tracking Performance
3.3. Limitations
4. Conclusions
5. Materials and Methods
5.1. Participants
5.2. Experimental Design
5.3. Mutual Information Estimation Using Gaussian Copula
5.4. Linear-Quadratic Regulator Model
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, M.R.; Maunsell, J.H. Attention improves performance primarily by reducing interneuronal correlations. Nat. Neurosci. 2009, 12, 1594. [Google Scholar] [CrossRef]
- Smith, E.C.; Lewicki, M.S. Efficient auditory coding. Nature 2006, 439, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Zenon, A.; Solopchuk, O.; Pezzulo, G. An information-theoretic perspective on the costs of cognition. Neuropsychologia 2019, 123, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Hick, W.E. On the rate of gain of information. Q. J. Exp. Psychol. 1952, 4, 11–26. [Google Scholar] [CrossRef]
- Fitts, P.M. The information capacity of the human motor system in controlling the amplitude of movement. J. Exp. Psychol. 1954, 47, 381. [Google Scholar] [CrossRef]
- Gori, J.; Rioul, O.; Guiard, Y. Speed-accuracy tradeoff: A formal information-theoretic transmission scheme (fitts). ACM Trans. Comput.-Hum. Interact. (TOCHI) 2018, 25, 1–33. [Google Scholar] [CrossRef]
- Grossman, E. The information-capacity of the human motor-system in pursuit tracking. Q. J. Exp. Psychol. 1960, 12, 1–16. [Google Scholar] [CrossRef]
- Poulton, E. On prediction in skilled movements. Psychol. Bull. 1957, 54, 467. [Google Scholar] [CrossRef]
- Yeo, S.H.; Franklin, D.W.; Wolpert, D.M. When optimal feedback control is not enough: Feedforward strategies are required for optimal control with active sensing. PLoS Comput. Biol. 2016, 12, e1005190. [Google Scholar] [CrossRef]
- Maeda, R.S.; Cluff, T.; Gribble, P.L.; Pruszynski, J.A. Feedforward and feedback control share an internal model of the arm’s dynamics. J. Neurosci. 2018, 38, 10505–10514. [Google Scholar] [CrossRef] [PubMed]
- Drop, F.M.; Pool, D.M.; Damveld, H.J.; van Paassen, M.M.; Mulder, M. Identification of the feedforward component in manual control with predictable target signals. IEEE Trans. Cybern. 2013, 43, 1936–1949. [Google Scholar] [CrossRef] [PubMed]
- Drop, F.M.; de Vries, R.; Mulder, M.; Bülthoff, H.H. The predictability of a target signal affects manual feedforward control. IFAC-PapersOnLine 2016, 49, 177–182. [Google Scholar] [CrossRef]
- Trujillo, L.T. Mental Effort and Information-Processing Costs Are Inversely Related to Global Brain Free Energy During Visual Categorization. Front. Neurosci. 2019, 13, 1292. [Google Scholar] [CrossRef]
- Schreiber, T. Measuring information transfer. Phys. Rev. Lett. 2000, 85, 461. [Google Scholar] [CrossRef]
- Mulder, M.; Pool, D.M.; Abbink, D.A.; Boer, E.R.; Zaal, P.M.; Drop, F.M.; van der El, K.; van Paassen, M.M. Manual control cybernetics: State-of-the-art and current trends. IEEE Trans. Hum. Mach. Syst. 2017, 48, 468–485. [Google Scholar] [CrossRef]
- Cover, T.M.; Thomas, J.A. Elements of Information Theory, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2006. [Google Scholar]
- Rao, R.P.; Ballard, D.H. Predictive coding in the visual cortex: A functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 1999, 2, 79–87. [Google Scholar] [CrossRef]
- Kool, W.; McGuire, J.T.; Rosen, Z.B.; Botvinick, M.M. Decision making and the avoidance of cognitive demand. J. Exp. Psychol. Genl. 2010, 139, 665. [Google Scholar] [CrossRef]
- Westbrook, A.; Braver, T.S. Cognitive effort: A neuroeconomic approach. Cogn. Affect. Behav. Neurosci. 2015, 15, 395–415. [Google Scholar] [CrossRef]
- Shenhav, A.; Musslick, S.; Lieder, F.; Kool, W.; Griffiths, T.L.; Cohen, J.D.; Botvinick, M.M. Toward a rational and mechanistic account of mental effort. Annu. Rev. Neurosci. 2017, 40, 99–124. [Google Scholar] [CrossRef]
- Hülsdünker, T.; Ostermann, M.; Mierau, A. The speed of neural visual motion perception and processing determines the visuomotor reaction time of young elite table tennis athletes. Front. Behav. Neurosci. 2019, 13, 165. [Google Scholar] [CrossRef]
- Miall, R.; Weir, D.; Stein, J. Visuomotor tracking with delayed visual feedback. Neuroscience 1985, 16, 511–520. [Google Scholar] [CrossRef]
- Foulkes, A.J.M.; Miall, R.C. Adaptation to visual feedback delays in a human manual tracking task. Exp. Brain Res. 2000, 131, 101–110. [Google Scholar] [CrossRef]
- Ballard, K.J.; Robin, D.A.; Woodworth, G.; Zimba, L.D. Age-related changes in motor control during articulator visuomotor tracking. J. Speech Lang. Hear. Res. 2001, 44, 763–777. [Google Scholar] [CrossRef]
- Bormann, R.; Cabrera, J.L.; Milton, J.G.; Eurich, C.W. Visuomotor tracking on a computer screen—An experimental paradigm to study the dynamics of motor control. Neurocomputing 2004, 58, 517–523. [Google Scholar] [CrossRef]
- Lee, G.; Choi, W.; Jo, H.; Park, W.; Kim, J. Analysis of motor control strategy for frontal and sagittal planes of circular tracking movements using visual feedback noise from velocity change and depth information. PloS ONE 2020, 15, e0241138. [Google Scholar] [CrossRef]
- Takagi, A.; Furuta, R.; Saetia, S.; Yoshimura, N.; Koike, Y.; Minati, L. Behavioral and physiological correlates of kinetically tracking a chaotic target. PLoS ONE 2020, 15, e0239471. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.F.d.C.; Jones, K.E.; Wolpert, D.M. The scaling of motor noise with muscle strength and motor unit number in humans. Exp. Brain Res. 2004, 157, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Ince, R.A.; Giordano, B.L.; Kayser, C.; Rousselet, G.A.; Gross, J.; Schyns, P.G. A statistical framework for neuroimaging data analysis based on mutual information estimated via a gaussian copula. Hum. Brain Mapp. 2017, 38, 1541–1573. [Google Scholar] [CrossRef] [PubMed]
- Kraskov, A.; Stögbauer, H.; Grassberger, P. Estimating mutual information. Phys. Rev. E 2004, 69, 066138. [Google Scholar] [CrossRef]
- Casella, G.; Berger, R.L. Statistical Inference; Duxbury: Pacific Grove, CA, USA, 2002; Volume 2. [Google Scholar]
- Sklar, A. Fonction de répartition dont les marges sont données. Inst. Stat. Univ. Paris 1959, 8, 229–231. [Google Scholar]
- Jenison, R.L.; Reale, R.A. The shape of neural dependence. Neural. Comput. 2004, 16, 665–672. [Google Scholar] [CrossRef]
- Misra, N.; Singh, H.; Demchuk, E. Estimation of the entropy of a multivariate normal distribution. J. Multivar. Anal. 2005, 92, 324–342. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, S.-Y.; Zénon, A. Information Rate in Humans during Visuomotor Tracking. Entropy 2021, 23, 228. https://doi.org/10.3390/e23020228
Lam S-Y, Zénon A. Information Rate in Humans during Visuomotor Tracking. Entropy. 2021; 23(2):228. https://doi.org/10.3390/e23020228
Chicago/Turabian StyleLam, Sze-Ying, and Alexandre Zénon. 2021. "Information Rate in Humans during Visuomotor Tracking" Entropy 23, no. 2: 228. https://doi.org/10.3390/e23020228
APA StyleLam, S.-Y., & Zénon, A. (2021). Information Rate in Humans during Visuomotor Tracking. Entropy, 23(2), 228. https://doi.org/10.3390/e23020228





