Spike Timing-Dependent Plasticity with Enhanced Long-Term Depression Leads to an Increase of Statistical Complexity
Abstract
1. Introduction
2. Materials and Methods
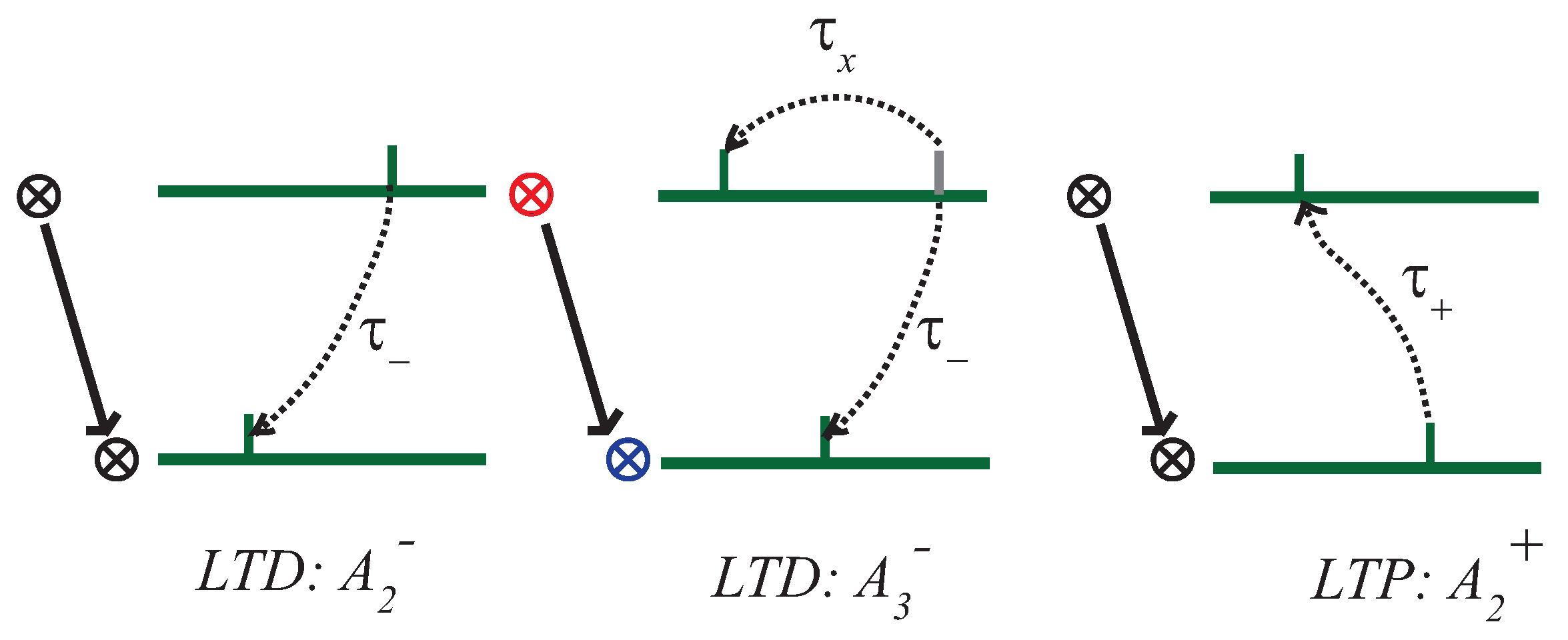
2.1. Computational Modelling
2.2. Information Theory Quantifiers
2.2.1. Bandt and Pompe Methodology
2.2.2. Shannon Entropy
2.2.3. Metrics
2.2.4. Fisher Information
2.2.5. Statistical Complexity
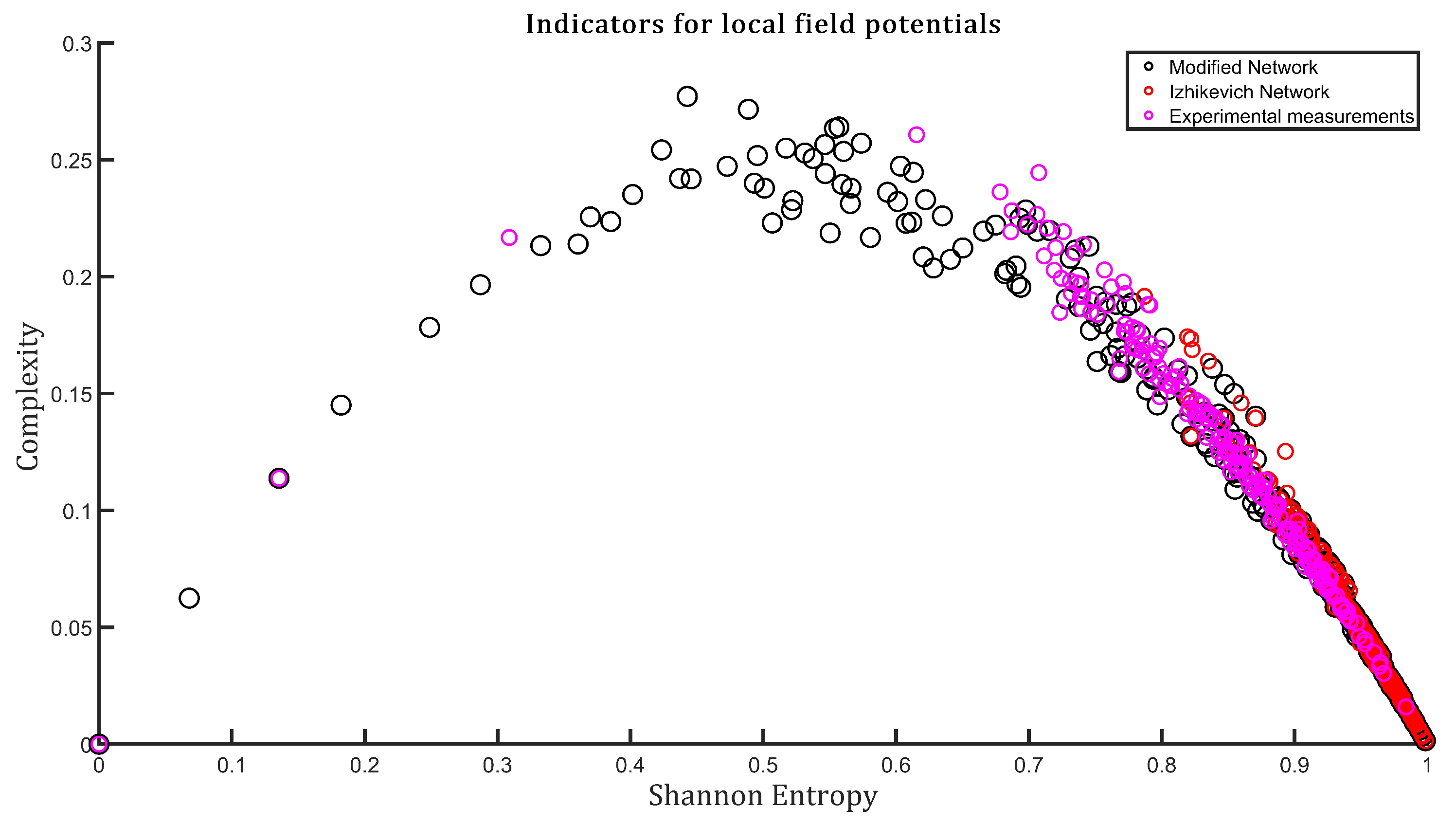
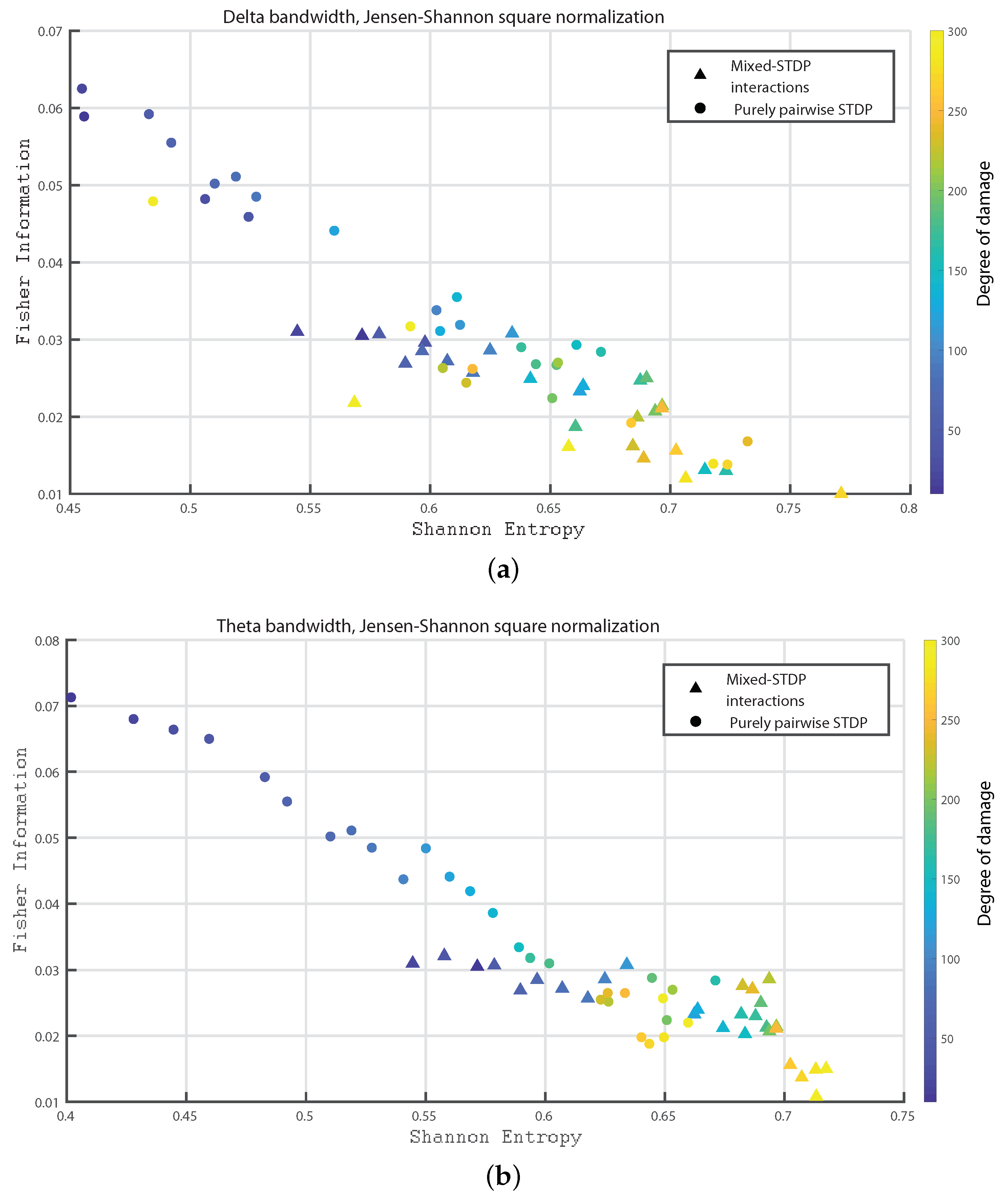
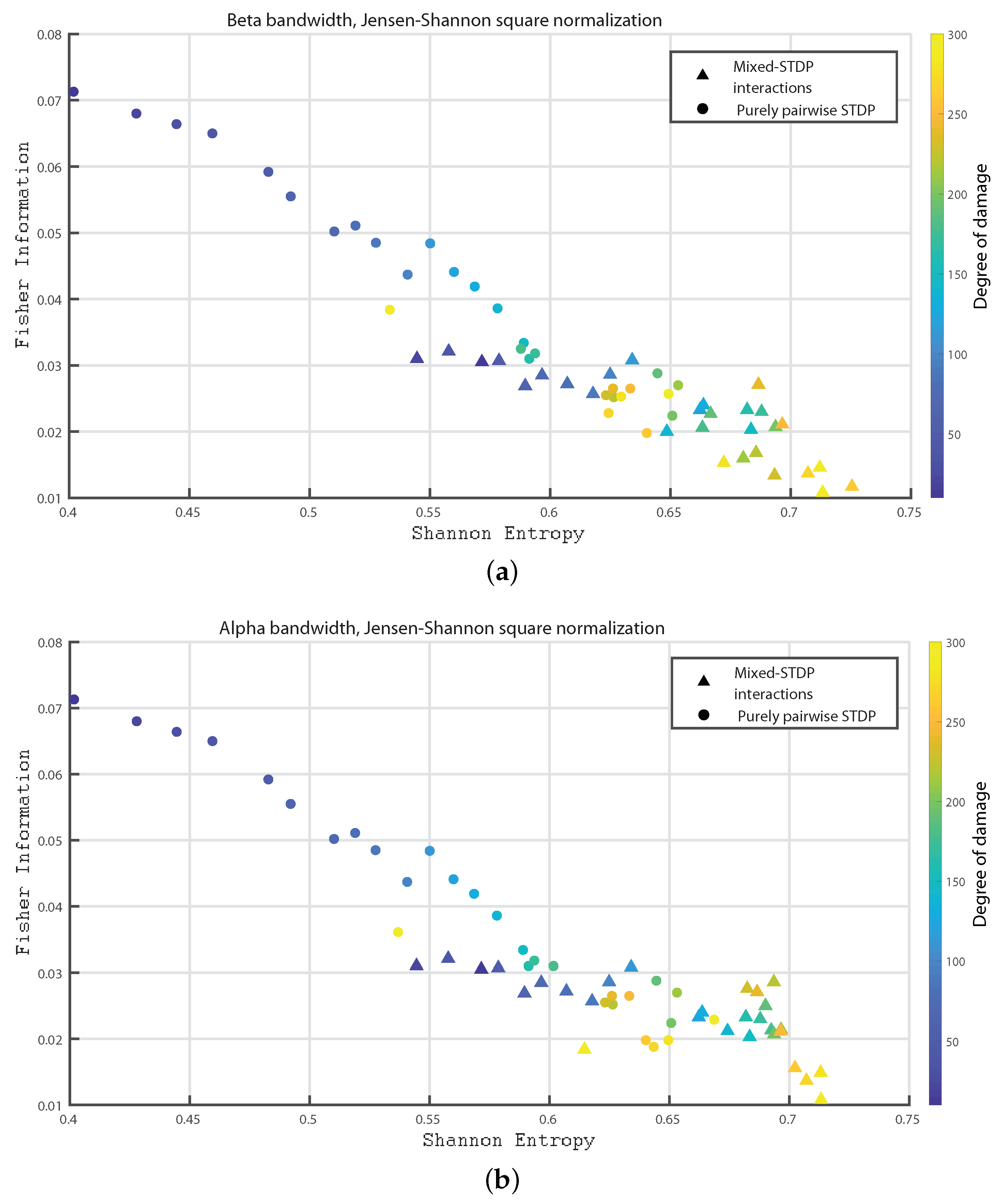
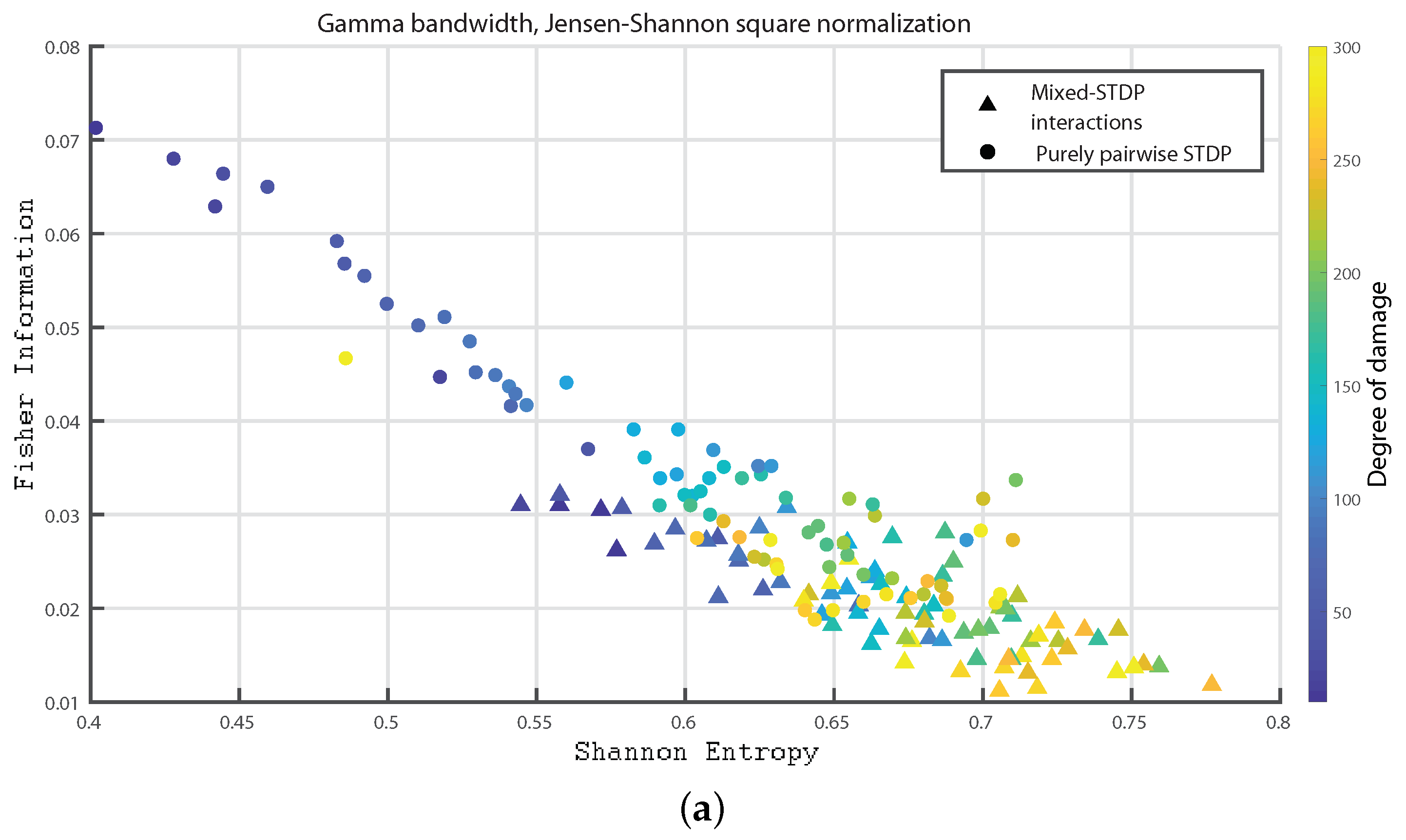
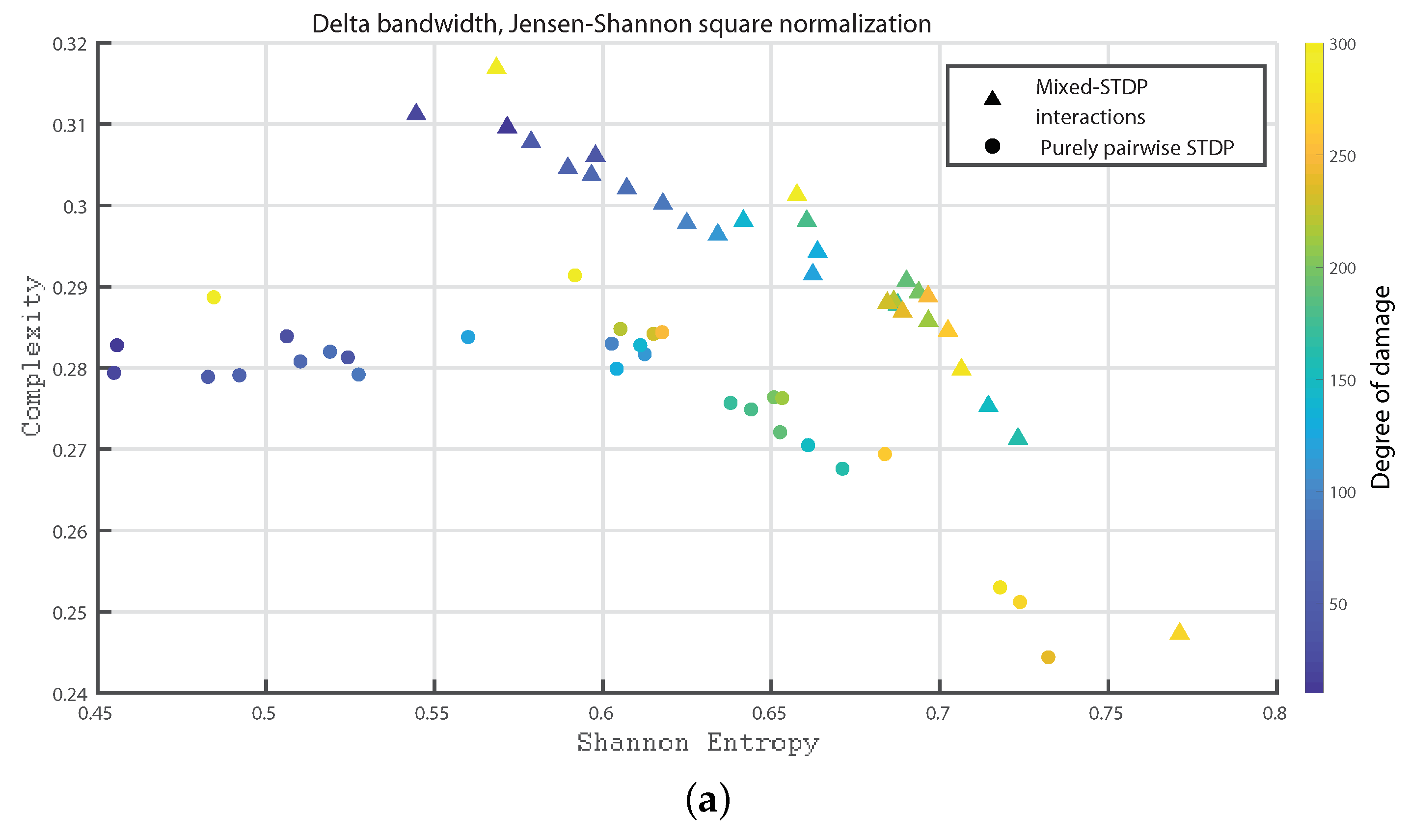
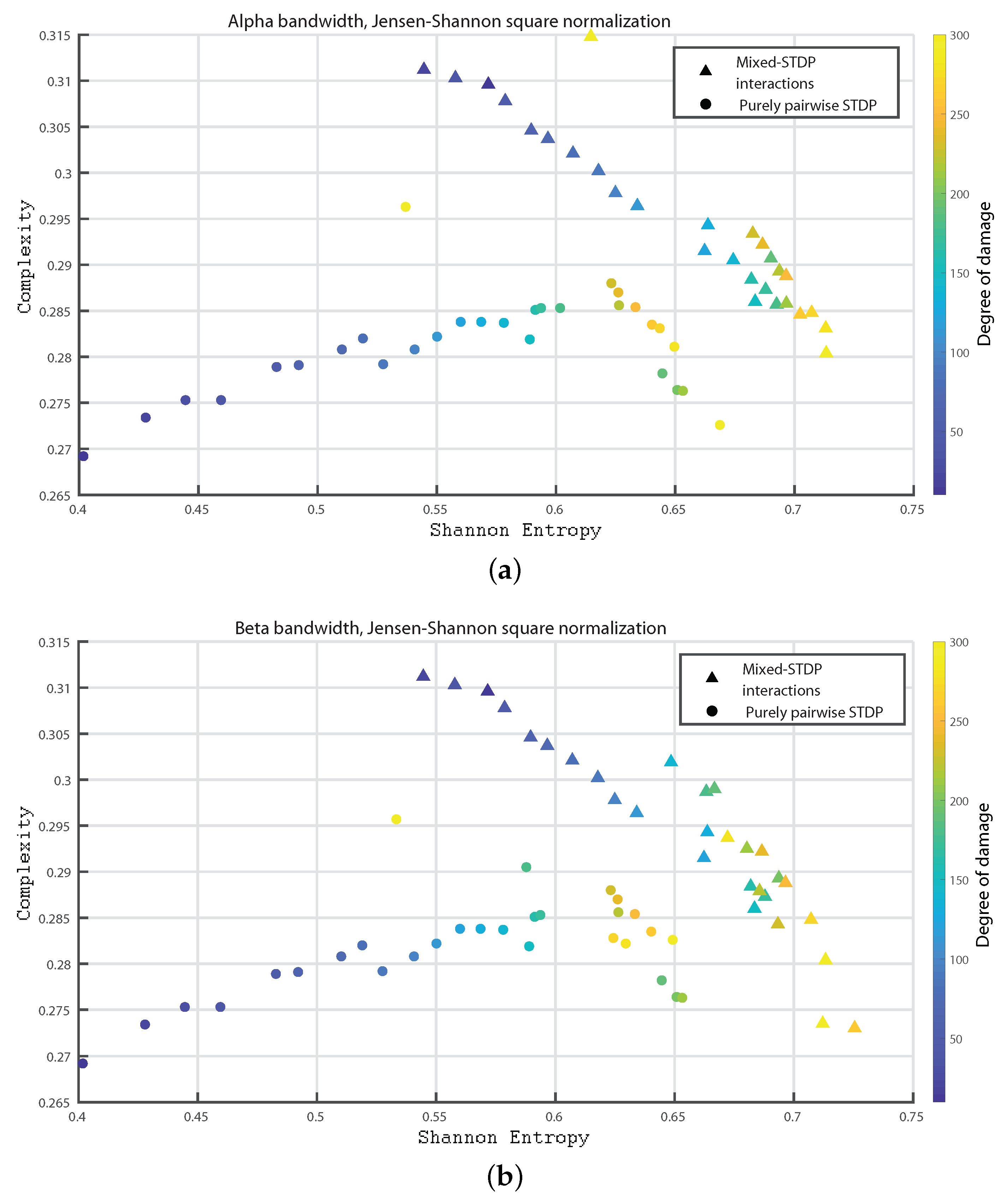
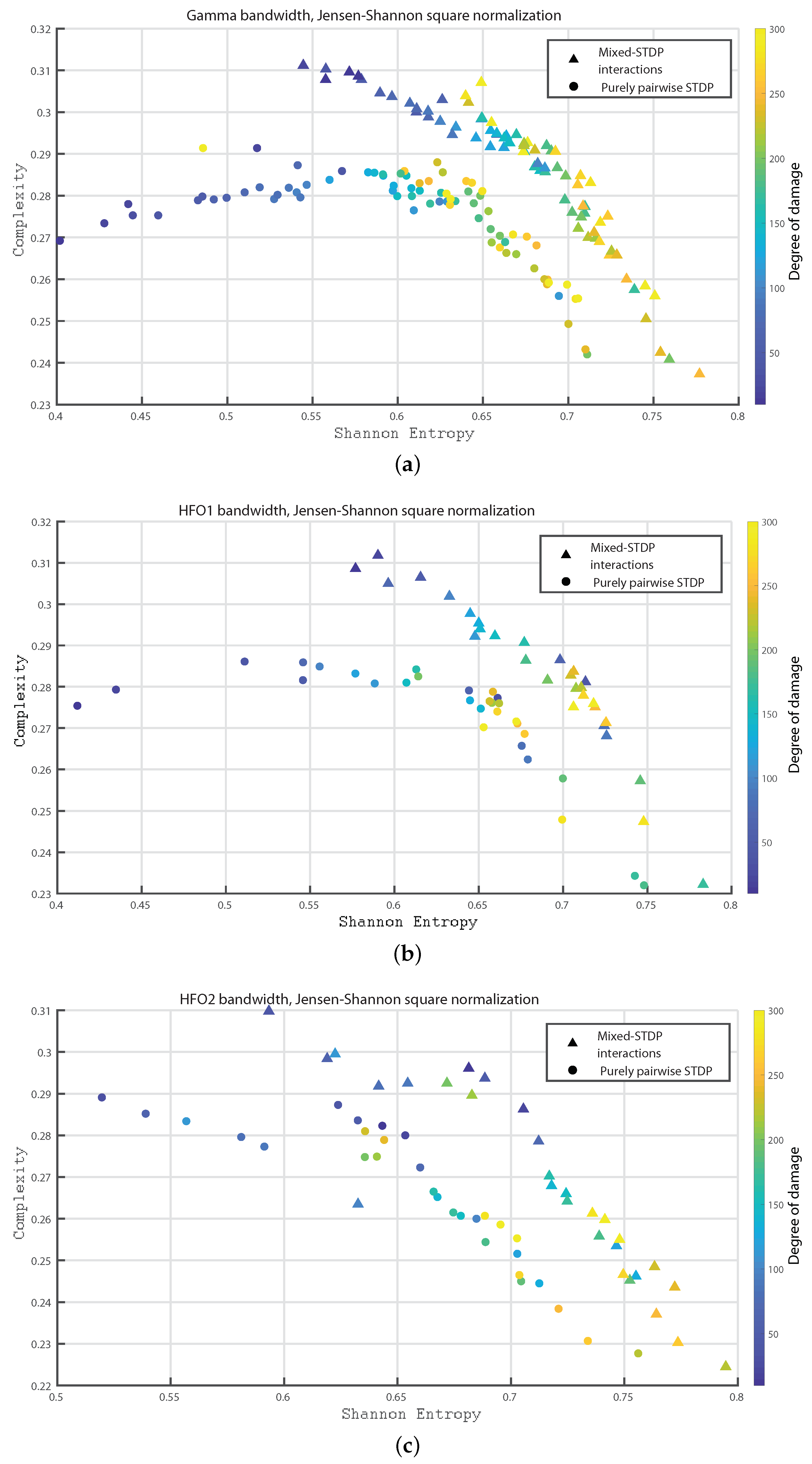
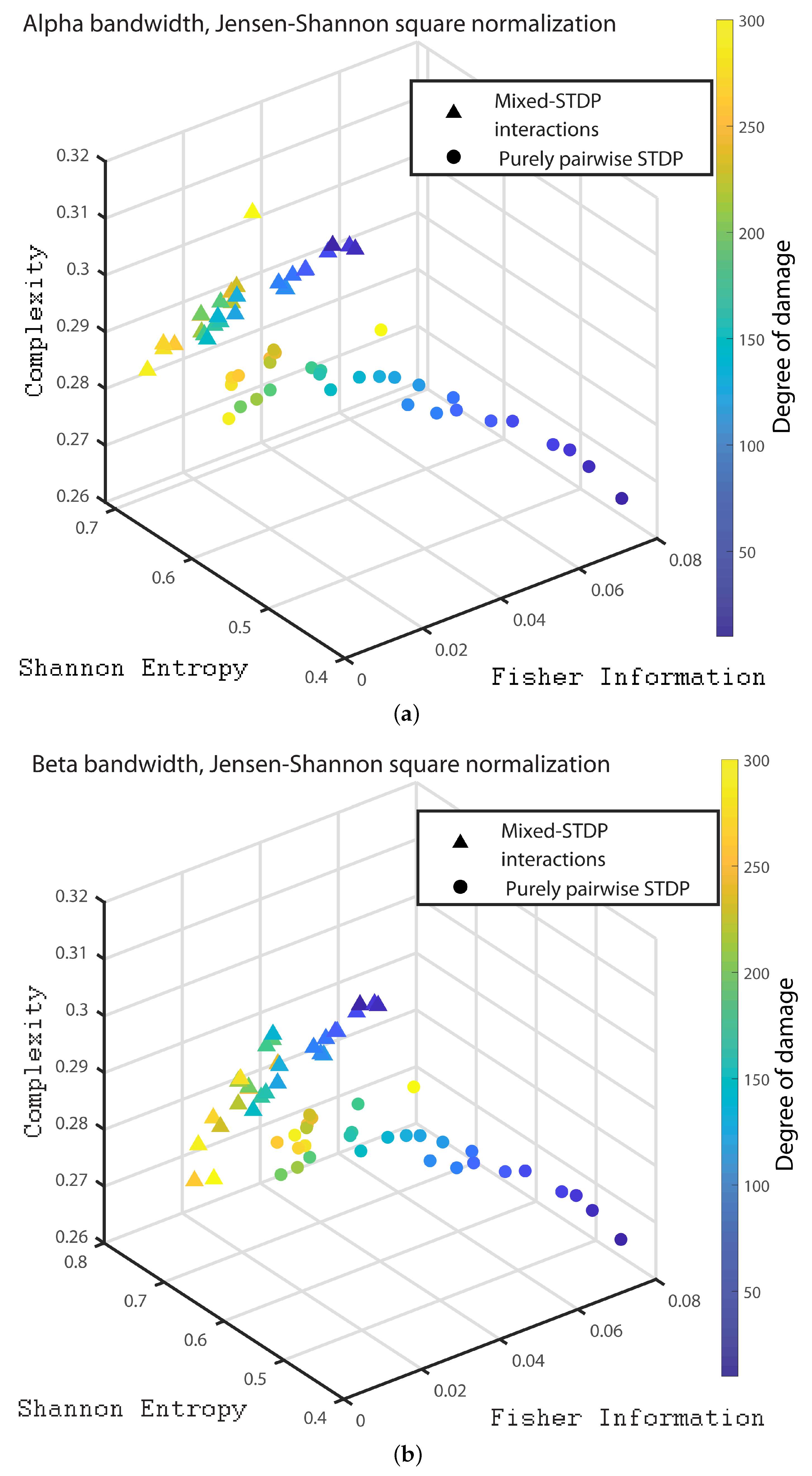
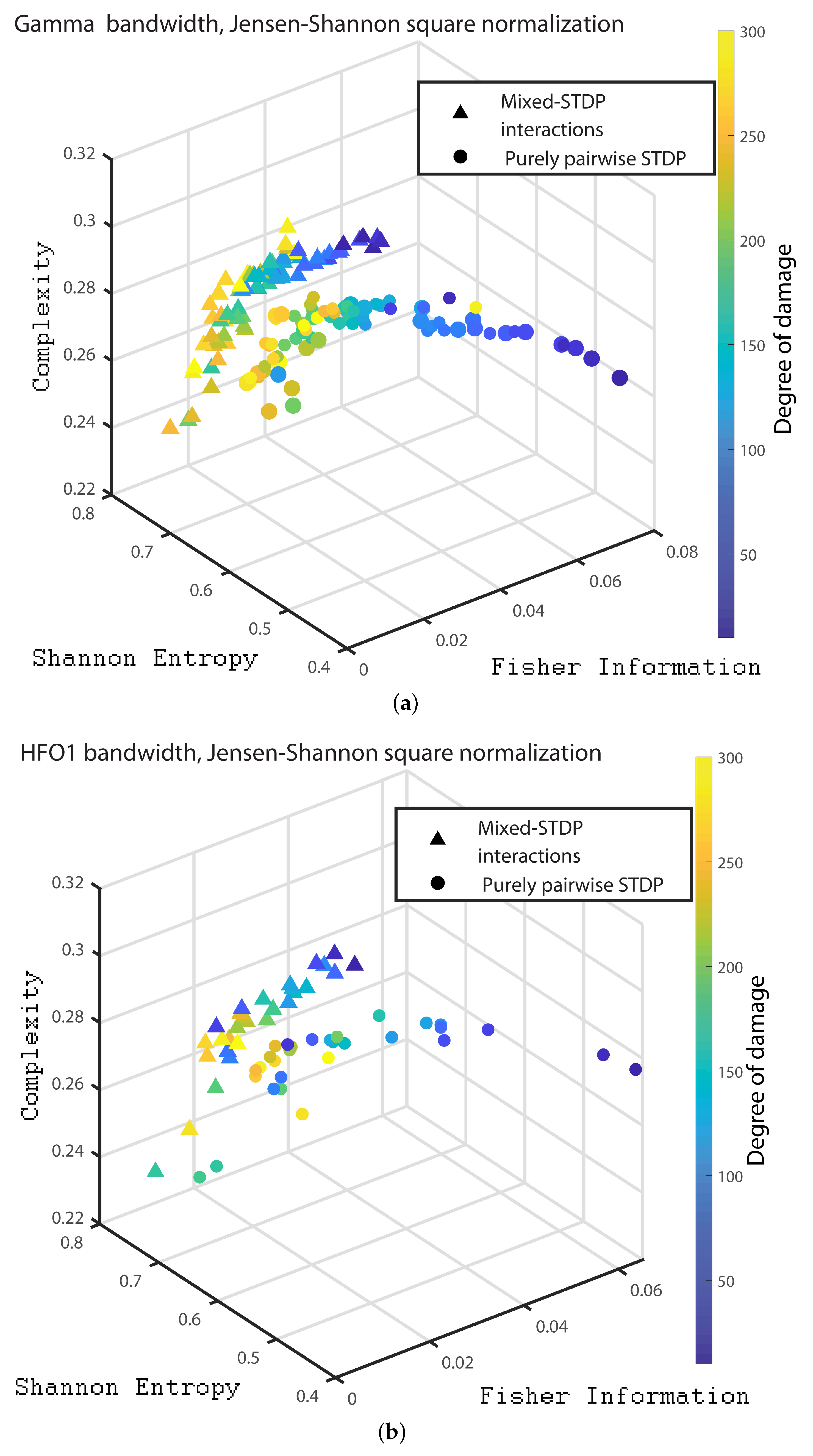
3. Results
- Delta bandwidth: 0.2 to 4 Hz
- Theta bandwidth: 4 to 8 Hz
- Alpha bandwidth: 8 to 12 Hz
- Beta bandwidth: 12 to 30 Hz
- Gamma bandwidth: 30 to 100 Hz
- HFO bandwidth 1: 100 to 150 Hz
- HFO bandwidth 2: 150 to 200 Hz
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buzsáki, G. Rhythms of the Brain; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Buzsáki, G.; Watson, B.O. Brain rhythms and neural syntax: Implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues Clin. Neurosci. 2012, 14, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Gruart, A.; Munoz, M.D.; Delgado-Garcia, J.M. Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. J. Neurosci. 2006, 26, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, J.R.; Heynen, A.J.; Shuler, M.G.; Bear, M.F. Learning induces long-term potentiation in the hippocampus. Science 2006, 313, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Citri, A.; Malenka, R. Synaptic Plasticity: Multiple Forms, Functions, and Mechanisms. Neuropsychopharmacology 2008, 33, 18–41. [Google Scholar] [CrossRef]
- Kullmann, D.; Asztely, F.; Walker, M. The role of mammalian ionotropic receptors in synaptic plasticity: LTP, LTD and epilepsy. Cell. Mol. Life Sci. 2000, 57, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.; Spruston, N. Postsynaptic depolarization requirements for LTP and LTD: A critique of spike timing-dependent plasticity. Nat. Neurosci. 2005, 8, 839–841. [Google Scholar] [CrossRef]
- Sharma, S.; Tiarks, G.; Haight, J.; Bassuk, A.G. Neuropathophysiological Mechanisms and Treatment Strategies for Post-traumatic Epilepsy. Front. Mol. Neurosci. 2021, 14, 612073. [Google Scholar] [CrossRef] [PubMed]
- Quintá, H.; Barrantes, F. Chapter Seven—Damage and repair of the axolemmal membrane: From neural development to axonal trauma and restoration. Curr. Top. Membr. 2019, 84, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J. Seizure-induced neuronal injury: Human data. Neurology 2002, 59, S15–S20. [Google Scholar] [CrossRef] [PubMed]
- Siman, R.; Noszek, J.; Kegerise, C. Calpain I Activation Is Specifically Related to Excitatory Amino Acid Induction of Hippocampal Damage. J. Neurosci. Off. J. Soc. Neurosci. 1989, 9, 1579–1590. [Google Scholar] [CrossRef]
- Ozkan, E.; Creson, T.; Kramár, E.; Rojas, C.; Seese, R.; Babyan, A.; Shi, Y.; Lucero, R.; Xu, X.; Noebels, J.; et al. Reduced Cognition in Syngap1 Mutants Is Caused by Isolated Damage within Developing Forebrain Excitatory Neurons. Neuron 2014, 82, 1317–1333. [Google Scholar] [CrossRef]
- Einarsdottir, H.; Montani, F.; Schultz, S.R. A mathematical model of receptive field reorganization following stroke. In Proceedings of the IEEE 6th International Conference on Development and Learning, ICDL’07, London, UK, 11–13 July 2007; IEEE: New York, NY, USA, 2007; pp. 211–216. [Google Scholar]
- Andrew, R.D.; Hartings, J.A.; Ayata, C.; Brennan, K.C.; Dawson-Scully, K.D.; Farkas, E.; Herreras, O.; Kirov, S.A.; Müller, M.; Ollen-Bittle, N.; et al. The Critical Role of Spreading Depolarizations in Early Brain Injury: Consensus and Contention. Neurocrit. Care 2022, 37 (Suppl. 1), 83–101. [Google Scholar] [CrossRef]
- Schumm, S.N.; Gabrieli, D.; Meaney, D.F. Neuronal Degeneration Impairs Rhythms Between Connected Microcircuits. Front. Comput. Neurosci. 2020, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Izhikevich, E. Polychronization: Computation with spikes. Neural Comput. 2006, 18, 245–282. [Google Scholar] [CrossRef]
- Koch, C.; Segev, I. Methods in Neuronal Modeling; Massachusetts Institute of Technology: Cambridge, MA, USA, 1998. [Google Scholar]
- Izhikevich, E.M. Dynamical Systems in Neuroscience: The Geometry of Excitability and Bursting; Computational Neuroscience Series; MIT Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Brette, R.; Gerstner, W. Adaptive exponential integrate-and-fire model as an effective description of neuronal activity. J. Neurophysiol. 2005, 94, 3637–3642. [Google Scholar] [CrossRef]
- Pfister, J.; Gerstner, W. Triplets of spikes in a model of spike timing-dependent plasticity. J. Neurosci. 2006, 26, 9673–9682. [Google Scholar] [CrossRef]
- Rosso, O.A.; Masoller, C. Detecting and quantifying stochastic and coherence resonances via information-theory complexity measurements. Phys. Rev. E 2009, 79, 040106. [Google Scholar] [CrossRef]
- Rosso, O.A.; Masoller, C. Detecting and quantifying temporal correlations in stochastic resonance via information theory measures. Eur. Phys. J. B 2009, 69, 37–43. [Google Scholar] [CrossRef]
- Baravalle, R.; Rosso, O.A.; Montani, F. Rhythmic activities of the brain: Quantifying the high complexity of beta and gamma oscillations during visuomotor tasks. Chaos 2018, 28, 075513. [Google Scholar] [CrossRef]
- Baravalle, R.; Rosso, O.A.; Montani, F. Causal Shannon–Fisher Characterization of Motor/Imagery Movements in EEG. Entropy 2018, 20, 660. [Google Scholar] [CrossRef]
- Gerstner, W.; Kistler, W.; Naud, R.; Paninski, L. Neuronal Dynamics: From Single Neurons to Networks and Models of Cognition; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Song, S.; Sjöström, P.J.; Reigl, M.; Nelson, S.; Chklovskii, D.B. Highly Nonrandom Features of Synaptic Connectivity in Local Cortical Circuits. PLoS Biol. 2005, 3, e68. [Google Scholar] [CrossRef]
- Soriano, J.; Martínez, M.; Tlusty, T.; Moses, E. Development of input connections in neural cultures. Proc. Natl. Acad. Sci. USA 2008, 105, 13758–13763. [Google Scholar] [CrossRef]
- De Luise, R.; Baravalle, R.; Rosso, O.A.; Montani, F. Network configurations of pain: An efficiency characterization of information transmission. Eur. Phys. J. B 2021, 94, 34. [Google Scholar] [CrossRef]
- Bandt, C.; Pompe, B. Permutation Entropy: A Natural Complexity Measure for Time Series. Phys. Rev. Lett. 2002, 88, 174102. [Google Scholar] [CrossRef]
- Olivares, F.; Plastino, A.; Rosso, O.A. Ambiguities in the Bandt-Pompe’s methodology for local entropic quantifiers. Physica A 2012, 391, 2518–2526. [Google Scholar] [CrossRef]
- Olivares, F.; Plastino, A.; Rosso, O.A. Contrasting chaos with noise via local versus global information quantifiers. Phys. Lett. A 2012, 376, 1577–1583. [Google Scholar] [CrossRef]
- Zunino, L.; Soriano, M.C.; Fischer, I.; Rosso, O.A.; Mirasso, C.R. Permutation-information-theory approach to unveil delay dynamics from time-series analysis. Phys. Rev. E 2010, 82, 046212. [Google Scholar] [CrossRef]
- Lamberti, P.W.; Majtey, A.P.; Borras, A.; Casas, M.; Plastino, A. Metric character of the quantum Jensen-Shannon divergence. Phys. Rev. A 2008, 77, 052311. [Google Scholar] [CrossRef]
- Mateos, D.M.; Riveaud, L.; Lamberti, P.W. Detecting dynamical changes in time series by using the Jensen Shannon divergence. Chaos 2017, 27, 083118. [Google Scholar] [CrossRef]
- Frauscher, B.; Ellenrieder, N.; Zelmann, R.; Doležalová, I.; Minotti, L.; Olivier, A.; Hall, J.; Hoffmann, D.; Nguyen, D.; Kahane, P.; et al. Atlas of the normal intracranial electroencephalogram: Neurophysiological awake activity in different cortical areas. Brain 2018, 141, 1130–1144. [Google Scholar] [CrossRef]
- Frauscher, B.; Ellenrieder, N.; Zelmann, R.; Rogers, C.; Nguyen, D.; Kahane, P.; Dubeau, F.; Gotman, J. High-Frequency Oscillations in the Normal Human Brain. Ann. Neurol. 2018, 84, 374–385. [Google Scholar] [CrossRef]
- Ellenrieder, N.; Gotman, J.; Zelmann, R.; Rogers, C.; Nguyen, D.; Kahane, P.; Dubeau, F.; Frauscher, B. How the Human Brain Sleeps: Direct Cortical Recordings of Normal Brain Activity. Ann. Neurol. 2020, 87, 289–301. [Google Scholar] [CrossRef]
- Stoelzel, C.; Bereshpolova, Y.; Swadlow, H. Stability of thalamocortical synaptic transmission across awake brain states. J. Neurosci. 2009, 29, 6851–6859. [Google Scholar] [CrossRef]
- Djebari, S.; Iborra-Lázaro, G.; Temprano-Carazo, S.; Sánchez-Rodríguez, I.; Nava-Mesa, M.O.; Múnera, A.; Gruart, A.; Delgado-García, J.M.; Jiménez-Díaz, L.; Navarro-López, J.D. G-Protein-Gated Inwardly Rectifying Potassium (Kir3/GIRK) Channels Govern Synaptic Plasticity That Supports Hippocampal-Dependent Cognitive Functions in Male Mice. J. Neurosci. 2021, 41, 7086–7102. [Google Scholar] [CrossRef]
- Wiegert, J.; Oertner, T. Long-term depression triggers the selective elimination of weakly integrated synapses. Proc. Natl. Acad. Sci. USA 2013, 110, E4510–E4519. [Google Scholar] [CrossRef]
- Gjorgjieva, J.; Clopath, C.; Audet, J.; Pfister, J.-P. A triplet spike-timing-dependent plasticity model generalizes the Bienenstock-Cooper-Munro rule to higher-order spatiotemporal correlations. Proc. Natl. Acad. Sci. USA 2011, 108, 19383–19388. [Google Scholar] [CrossRef]
- Zanin, M.; Olivares, F. Ordinal patterns-based methodologies for distinguishing chaos from noise in discrete time series. Commun. Phys. 2021, 4, 190. [Google Scholar] [CrossRef]
- Lee, C.; Panda, P.; Srinivasan, G.; Roy, K. Training Deep Spiking Convolutional Neural Networks with STDP-Based Unsupervised Pre-training Followed by Supervised Fine-Tuning. Front. Neurosci. 2018, 12, 435. [Google Scholar] [CrossRef]
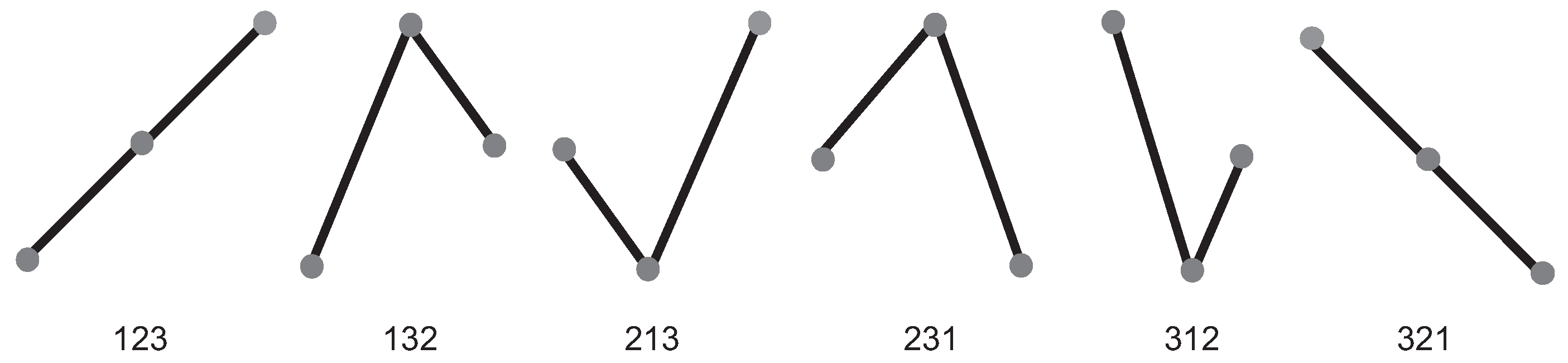




| Type of Neuron | a | b | c | d |
|---|---|---|---|---|
| IB | 0.02 | 0.2 | −55 | 4 |
| LTS | 0.02 | 0.25 | −65 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallares Di Nunzio, M.; Montani, F. Spike Timing-Dependent Plasticity with Enhanced Long-Term Depression Leads to an Increase of Statistical Complexity. Entropy 2022, 24, 1384. https://doi.org/10.3390/e24101384
Pallares Di Nunzio M, Montani F. Spike Timing-Dependent Plasticity with Enhanced Long-Term Depression Leads to an Increase of Statistical Complexity. Entropy. 2022; 24(10):1384. https://doi.org/10.3390/e24101384
Chicago/Turabian StylePallares Di Nunzio, Monserrat, and Fernando Montani. 2022. "Spike Timing-Dependent Plasticity with Enhanced Long-Term Depression Leads to an Increase of Statistical Complexity" Entropy 24, no. 10: 1384. https://doi.org/10.3390/e24101384
APA StylePallares Di Nunzio, M., & Montani, F. (2022). Spike Timing-Dependent Plasticity with Enhanced Long-Term Depression Leads to an Increase of Statistical Complexity. Entropy, 24(10), 1384. https://doi.org/10.3390/e24101384






