Entropy Could Quantify Brain Activation Induced by Mechanical Impedance-Restrained Active Arm Motion: A Functional NIRS Study
Abstract
:1. Introduction
2. Methods
2.1. Overall Configuration
2.2. Stimuli Generation
2.2.1. Robot System
2.2.2. Control Method
2.2.3. Visual Guidance Feedback System
2.3. Measurement
2.3.1. Functional Near-Infrared Spectroscopy(fNIRS) System
2.3.2. Subject Information
2.3.3. Experiment Protocol
2.4. Data Analysis
2.4.1. Data Processing
2.4.2. Entropy Calculation
2.4.3. Signal Amplitude Calculation
2.4.4. Beta Value Calculation
2.4.5. Statistical Analysis
2.4.6. Brain Activation Map
3. Results
3.1. Control Verification
3.1.1. Validation of Impedance Control
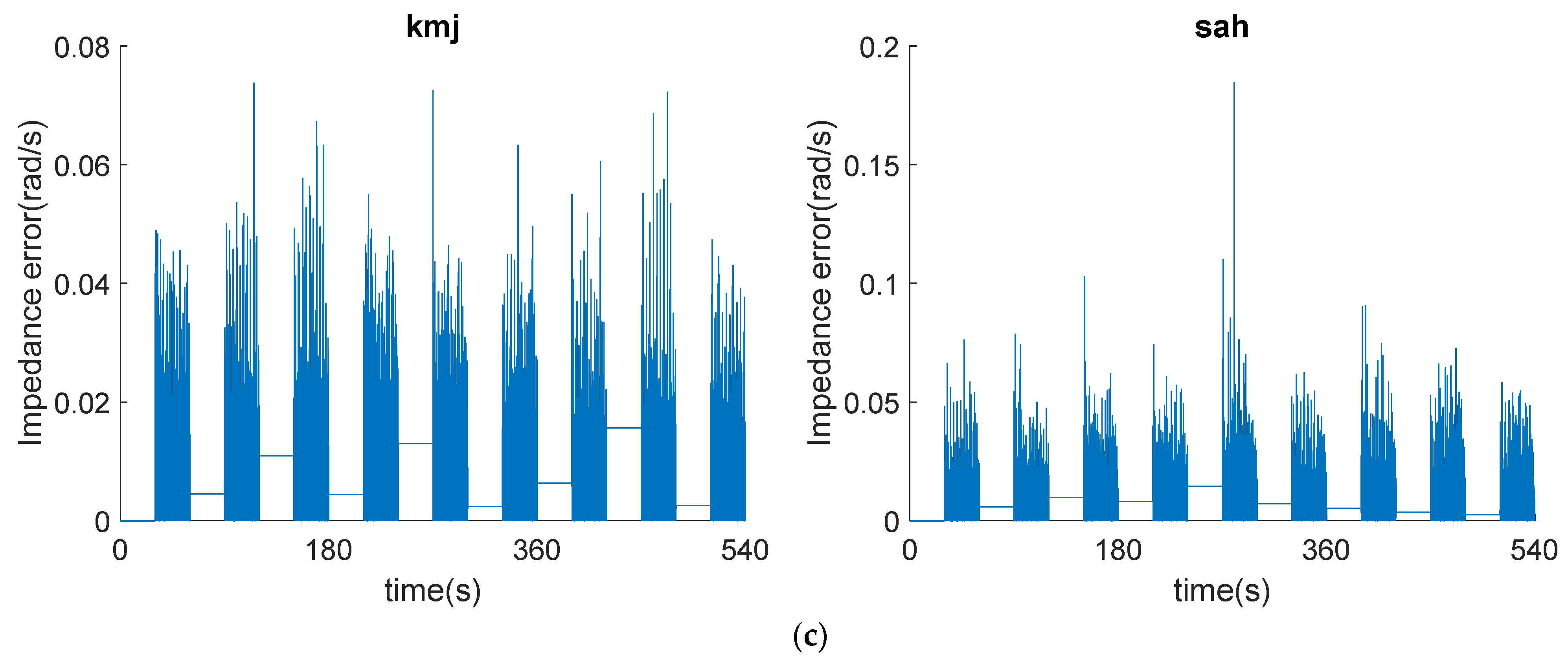
Contact Test
Impedance Error Measurement
3.2. Entropy Changes Owing to Physical Stimuli
3.2.1. Entropy Difference between Rest and Task
3.2.2. Entropy Change over Rest Duration
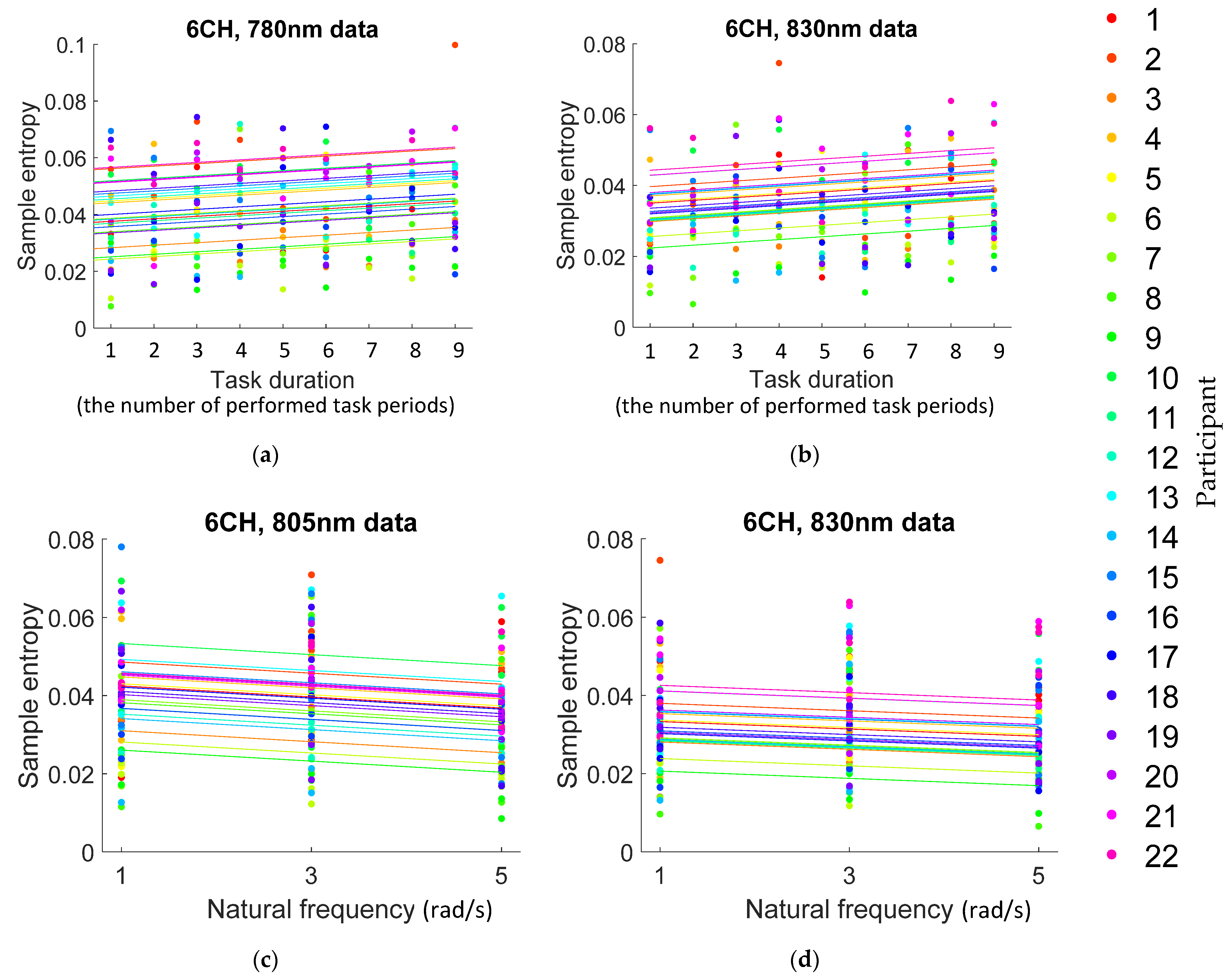
3.2.3. Relationship between Task Duration and Entropy
3.2.4. Relationship between Task Strength and Entropy
3.3. Comparision of Entropy Change with Signal Amplitude and Beta Value
3.4. Comparision of Entropy Change with Brain Activation Map
4. Discussion
4.1. Desired Impedance Is Achieved
4.2. Brain Activation Could Be Evaluated by the Entropy
4.2.1. Rest State and Task State
4.2.2. Task Duration
4.2.3. Task Strength
4.3. Comparison with Signal Amplitude and Beta Value
4.4. Physiological Interpretations
4.5. Reason for Not Considering Damping Ratio
4.6. Consideration of Entropy Calculation
4.7. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friston, K.J.; Jezzard, P.; Turner, R. Analysis of functional MRI time-series. Hum. Brain Mapp. 1994, 1, 153–171. [Google Scholar] [CrossRef]
- Worsley, K.J.; Friston, K.J. Analysis of fMRI time-series revisited—Again. Neuroimage 1995, 2, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friston, K.J.; Fletcher, P.; Josephs, O.; Holmes, A.; Rugg, M.; Turner, R. Event-related fMRI: Characterizing differential responses. Neuroimage 1998, 7, 30–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoshi, Y.; Tamura, M. Detection of dynamic changes in cerebral oxygenation coupled to neuronal function during mental work in man. Neurosci. Lett. 1993, 150, 5–8. [Google Scholar] [CrossRef]
- Hoshi, Y.; Tamura, M. Dynamic multichannel near-infrared optical imaging of human brain activity. J. Appl. Physiol. 1993, 75, 1842–1846. [Google Scholar] [CrossRef] [Green Version]
- Stelzer, J.; Lohmann, G.; Mueller, K.; Buschmann, T.; Turner, R. Deficient approaches to human neuroimaging. Front. Hum. Neurosci. 2014, 8, 462. [Google Scholar] [CrossRef] [Green Version]
- Reddan, M.C.; Lindquist, M.A.; Wager, T.D. Effect size estimation in neuroimaging. JAMA Psychiatry 2017, 74, 207–208. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004, 23, S208–S219. [Google Scholar] [CrossRef] [Green Version]
- Cox, R.W. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996, 29, 162–173. [Google Scholar] [CrossRef]
- Penny, W.D.; Friston, K.J.; Ashburner, J.T.; Kiebel, S.J.; Nichols, T.E. Statistical Parametric Mapping: The Analysis of Functional Brain Images; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Ye, J.C.; Tak, S.; Jang, K.E.; Jung, J.; Jang, J. NIRS-SPM: Statistical parametric mapping for near-infrared spectroscopy. Neuroimage 2009, 44, 428–447. [Google Scholar] [CrossRef]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, D280–D298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernet, C.R. Misconceptions in the use of the General Linear Model applied to functional MRI: A tutorial for junior neuro-imagers. Front. Neurosci. 2014, 8, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dueñas, J.; Sulzer, J.; Stämpfli, P.; Hepp-Reymond, M.-C.; Kollias, S.; Seifritz, E.; Gassert, R. BOLD signal in sensorimotor regions reveals differential encoding of passive forefinger velocity and displacement amplitude. NeuroImage 2018, 173, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Kronbichler, M.; Richlan, F.; Hawelka, S.; Hutzler, F.; Jacobs, A.M. A model-guided dissociation between subcortical and cortical contributions to word recognition. Sci. Rep. 2019, 9, 4506. [Google Scholar] [CrossRef] [PubMed]
- Jahani, S.; Fantana, A.L.; Harper, D.; Ellison, J.M.; Boas, D.A.; Forester, B.P.; Yücel, M.A. fNIRS can robustly measure brain activity during memory encoding and retrieval in healthy subjects. Sci. Rep. 2017, 7, 9533. [Google Scholar] [CrossRef] [PubMed]
- Freund, P.; Weiskopf, N.; Ward, N.S.; Hutton, C.; Gall, A.; Ciccarelli, O.; Craggs, M.; Friston, K.; Thompson, A.J. Disability, atrophy and cortical reorganization following spinal cord injury. Brain 2011, 134, 1610–1622. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Wixted, J.T.; Smith, C.N.; Squire, L.R. Different nonlinear functions in hippocampus and perirhinal cortex relating functional MRI activity to memory strength. Proc. Natl. Acad. Sci. USA 2011, 108, 5783–5788. [Google Scholar] [CrossRef] [Green Version]
- Logothetis, N.K.; Pauls, J.; Augath, M.; Trinath, T.; Oeltermann, A. Neurophysiological investigation of the basis of the fMRI signal. Nature 2001, 412, 150–157. [Google Scholar] [CrossRef]
- Xu, J. Implications of cortical balanced excitation and inhibition, functional heterogeneity, and sparseness of neuronal activity in fMRI. Neurosci. Biobehav. Rev. 2015, 57, 264–270. [Google Scholar] [CrossRef] [Green Version]
- Nakata, H.; Domoto, R.; Mizuguchi, N.; Sakamoto, K.; Kanosue, K. Negative BOLD responses during hand and foot movements: An fMRI study. PLoS ONE 2019, 14, e0215736. [Google Scholar] [CrossRef]
- Stevens, C.F.; Zador, A.M. Input synchrony and the irregular firing of cortical neurons. Nat. Neurosci. 1998, 1, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Chawla, D.; Lumer, E.D.; Friston, K.J. The relationship between synchronization among neuronal populations and their mean activity levels. Neural Comput. 1999, 11, 1389–1411. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G.; Kaila, K.; Raichle, M. Inhibition and brain work. Neuron 2007, 56, 771–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, S.; Cuthill, I.C. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol. Rev. 2007, 82, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Josephs, O.; Rees, G.; Turner, R. Nonlinear event-related responses in fMRI. Magn. Reson. Med. 1998, 39, 41–52. [Google Scholar] [CrossRef]
- Henson, R.N.; Price, C.J.; Rugg, M.D.; Turner, R.; Friston, K.J. Detecting latency differences in event-related BOLD responses: Application to words versus nonwords and initial versus repeated face presentations. Neuroimage 2002, 15, 83–97. [Google Scholar] [CrossRef] [Green Version]
- Lindquist, M.A.; Loh, J.M.; Atlas, L.Y.; Wager, T.D. Modeling the hemodynamic response function in fMRI: Efficiency, bias and mis-modeling. Neuroimage 2009, 45, S187–S198. [Google Scholar] [CrossRef] [Green Version]
- Handwerker, D.A.; Ollinger, J.M.; D’Esposito, M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage 2004, 21, 1639–1651. [Google Scholar] [CrossRef]
- Wager, T.D.; Vazquez, A.; Hernandez, L.; Noll, D.C. Accounting for nonlinear BOLD effects in fMRI: Parameter estimates and a model for prediction in rapid event-related studies. NeuroImage 2005, 25, 206–218. [Google Scholar] [CrossRef]
- Vazquez, A.L.; Noll, D.C. Nonlinear aspects of the BOLD response in functional MRI. Neuroimage 1998, 7, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Friston, K.J.; Mechelli, A.; Turner, R.; Price, C.J. Nonlinear responses in fMRI: The Balloon model, Volterra kernels, and other hemodynamics. NeuroImage 2000, 12, 466–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleki-Balajoo, S.; Hossein-Zadeh, G.-A.; Soltanian-Zadeh, H.; Ekhtiari, H. Locally estimated hemodynamic response function and activation detection sensitivity in heroin-cue reactivity study. Basic Clin. Neurosci. 2016, 7, 299. [Google Scholar] [CrossRef] [PubMed]
- Marrelec, G.; Benali, H.; Ciuciu, P.; Pélégrini-Issac, M.; Poline, J.B. Robust Bayesian estimation of the hemodynamic response function in event-related BOLD fMRI using basic physiological information. Hum. Brain Mapp. 2003, 19, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carp, J. The secret lives of experiments: Methods reporting in the fMRI literature. Neuroimage 2012, 63, 289–300. [Google Scholar] [CrossRef]
- Ashburner, J.; Barnes, G.; Chen, C.-C.; Daunizeau, J.; Flandin, G.; Friston, K.; Kiebel, S.; Kilner, J.; Litvak, V.; Moran, R. SPM12 Manual; Wellcome Trust Centre for Neuroimaging: London, UK, 2014; Volume 2464. [Google Scholar]
- Chen, G.; Taylor, P.A.; Cox, R.W. Is the statistic value all we should care about in neuroimaging? Neuroimage 2017, 147, 952–959. [Google Scholar] [CrossRef]
- Collell, G.; Fauquet, J. Brain activity and cognition: A connection from thermodynamics and information theory. Front. Psychol. 2015, 6, 818. [Google Scholar] [CrossRef] [Green Version]
- De Araujo, D.; Tedeschi, W.; Santos, A.C.d.; Elias, J., Jr.; Neves, U.P.d.C.; Baffa, O. Shannon entropy applied to the analysis of event-related fMRI time series. NeuroImage 2003, 20, 311–317. [Google Scholar] [CrossRef]
- Porta, A.; Faes, L. Assessing causality in brain dynamics and cardiovascular control. Philos. Trans. R. Soc. A 2013, 371, 20120517. [Google Scholar] [CrossRef]
- Faes, L.; Marinazzo, D.; Nollo, G.; Porta, A. An information-theoretic framework to map the spatiotemporal dynamics of the scalp electroencephalogram. IEEE Trans. Biomed. Eng. 2016, 63, 2488–2496. [Google Scholar] [CrossRef]
- Mateos, D.; Guevara Erra, R.; Wennberg, R.; Perez Velazquez, J. Measures of entropy and complexity in altered states of consciousness. Cogn. Neurodyn. 2018, 12, 73–84. [Google Scholar] [CrossRef]
- Andres, D.S.; Cerquetti, D.; Merello, M.; Stoop, R. Neuronal entropy depends on the level of alertness in the parkinsonian globus pallidus in vivo. Front. Neurol. 2014, 5, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarjam, P.; Epps, J.; Chen, F.; Lovell, N.H. Estimating cognitive workload using wavelet entropy-based features during an arithmetic task. Comput. Biol. Med. 2013, 43, 2186–2195. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.J.; Jang, S.H.; Seo, J.P.; Chang, P.H. The Optimal Speed for Cortical Activation of Passive Wrist Movements Performed by a Rehabilitation Robot: A Functional NIRS Study. Front. Hum. Neurosci. 2017, 11, 194. [Google Scholar] [CrossRef] [Green Version]
- Sulzer, J.S.; Chib, V.S.; Hepp-Reymond, M.-C.; Kollias, S.; Gassert, R. BOLD correlations to force in precision grip: An event-related study. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 2342–2346. [Google Scholar]
- Burdet, E.; Osu, R.; Franklin, D.W.; Milner, T.E.; Kawato, M. The central nervous system stabilizes unstable dynamics by learning optimal impedance. Nature 2001, 414, 446. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Bae, S.J.; Jang, S.H.; Chang, P.H. Design of a clinically relevant upper-limb exoskeleton robot for stroke patients with spasticity. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; pp. 622–627. [Google Scholar]
- Günzkofer, F.; Bubb, H.; Bengler, K. Maximum elbow joint torques for digital human models. Int. J. Hum. Factors Modell. Simul. 2012, 3, 109–132. [Google Scholar] [CrossRef]
- Mantegazza, P.; Dozio, E.; Papacharalambous, S. RTAI: Real time application interface. Linux J. 2000, 2000, 10. [Google Scholar]
- Jin, M.; Kang, S.H.; Chang, P.H. Robust compliant motion control of robot with nonlinear friction using time-delay estimation. IEEE Trans. Ind. Electron. 2008, 55, 258–269. [Google Scholar] [CrossRef]
- Hogan, N. An organizing principle for a class of voluntary movements. J. Neurosci. 1984, 4, 2745–2754. [Google Scholar] [CrossRef]
- Quaresima, V.; Ferrari, M. Functional near-infrared spectroscopy (fNIRS) for assessing cerebral cortex function during human behavior in natural/social situations: A concise review. Org. Res. Methods 2019, 22, 46–68. [Google Scholar] [CrossRef]
- Lee, G.; Jin, S.H.; An, J. Motion artifact correction of multi-measured functional near-infrared spectroscopy signals based on signal reconstruction using an artificial neural network. Sensors 2018, 18, 2957. [Google Scholar] [CrossRef] [Green Version]
- Banaji, M.; Mallet, A.; Elwell, C.E.; Nicholls, P.; Cooper, C.E. A model of brain circulation and metabolism: NIRS signal changes during physiological challenges. PLoS Comput. Biol. 2008, 4, e1000212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratton, G.; Chiarelli, A.M.; Fabiani, M. From brain to blood vessels and back: A noninvasive optical imaging approach. Neurophotonics 2017, 4, 031208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayhew, J.E.; Askew, S.; Zheng, Y.; Porrill, J.; Westby, G.M.; Redgrave, P.; Rector, D.M.; Harper, R.M. Cerebral vasomotion: A 0.1-Hz oscillation in reflected light imaging of neural activity. Neuroimage 1996, 4, 183–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obrig, H.; Neufang, M.; Wenzel, R.; Kohl, M.; Steinbrink, J.; Einhäupl, K.; Villringer, A. Spontaneous low frequency oscillations of cerebral hemodynamics and metabolism in human adults. Neuroimage 2000, 12, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.V.; Kainerstorfer, J.; Borisov, S.V.; Barbour, R.L.; VanMeter, J. Event-related fast optical signal in a rapid object recognition task: Improving detection by the independent component analysis. Brain Res. 2008, 1236, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Vanhatalo, S.; Voipio, J.; Kaila, K. Full-band EEG (fbEEG): A new standard for clinical electroencephalography. Clin. EEG Neurosci. 2005, 36, 311–317. [Google Scholar] [CrossRef]
- Siddiqui, K. Heuristics for sample size determination in multivariate statistical techniques. World Appl. Sci. J. 2013, 27, 285–287. [Google Scholar]
- Naseer, N.; Hong, K.-S. fNIRS-based brain-computer interfaces: A review. Front. Hum. Neurosci. 2015, 9, 3. [Google Scholar] [CrossRef] [Green Version]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol.-Heart Circul. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [Green Version]
- Chon, K.H.; Scully, C.G.; Lu, S. Approximate entropy for all signals. IEEE Eng. Med. Biol. Mag. 2009, 28, 18–23. [Google Scholar] [CrossRef]
- Richman, J.S.; Lake, D.E.; Moorman, J.R. Sample entropy. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2004; Volume 384, pp. 172–184. [Google Scholar]
- Lee, K. Sample Entropy. Available online: https://kr.mathworks.com/matlabcentral/fileexchange/35784-sample-entropy (accessed on 24 June 2019).
- Wilkinson, G.; Rogers, C. Symbolic description of factorial models for analysis of variance. J. R. Stat. Soc. Ser. C Appl. Stat. 1973, 22, 392–399. [Google Scholar] [CrossRef]
- Fox, M.D.; Snyder, A.Z.; Zacks, J.M.; Raichle, M.E. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat. Neurosci. 2006, 9, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.; Piccirelli, M.; Hepp-Reymond, M.-C.; Eng, K.; Michels, L. Brain Activation During Visually Guided Finger Movements. Front. Hum. Neurosci. 2020, 14, 309. [Google Scholar] [CrossRef] [PubMed]
- Barron, H.C.; Garvert, M.M.; Behrens, T.E. Repetition suppression: A means to index neural representations using BOLD? Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150355. [Google Scholar] [CrossRef]
- Henson, R.; Shallice, T.; Dolan, R. Neuroimaging evidence for dissociable forms of repetition priming. Science 2000, 287, 1269–1272. [Google Scholar] [CrossRef] [Green Version]
- Müller, N.G.; Strumpf, H.; Scholz, M.; Baier, B.; Melloni, L. Repetition suppression versus enhancement—It’s quantity that matters. Cereb. Cortex 2013, 23, 315–322. [Google Scholar] [CrossRef]
- Ponce-Alvarez, A.; He, B.J.; Hagmann, P.; Deco, G. Task-driven activity reduces the cortical activity space of the brain: Experiment and whole-brain modeling. PLoS Comput. Biol. 2015, 11, e1004445. [Google Scholar] [CrossRef] [Green Version]
- Cabel, D.W.; Cisek, P.; Scott, S.H. Neural activity in primary motor cortex related to mechanical loads applied to the shoulder and elbow during a postural task. J. Neurophysiol. 2001, 86, 2102–2108. [Google Scholar] [CrossRef] [Green Version]
- Grooms, D.R.; Criss, C.R.; Simon, J.E.; Haggerty, A.L.; Wohl, T.R. Neural Correlates of Knee Extension and Flexion Force Control: A Kinetically-Instrumented Neuroimaging Study. Front. Hum. Neurosci. 2020, 14, 622637. [Google Scholar] [CrossRef]
- Caramazza, A.; Coltheart, M. Cognitive neuropsychology twenty years on. Cogn. Neuropsychol. 2006, 23, 3–12. [Google Scholar] [CrossRef]
- McIntosh, A.R. Mapping cognition to the brain through neural interactions. Memory 1999, 7, 523–548. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.D.; Aflalo, T.N.; Kastner, S.; Graziano, M.S. Complex organization of human primary motor cortex: A high-resolution fMRI study. J. Neurophysiol. 2008, 100, 1800–1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzolatti, G.; Luppino, G. The cortical motor system. Neuron 2001, 31, 889–901. [Google Scholar] [CrossRef] [Green Version]
- Castiello, U. The neuroscience of grasping. Nat. Rev. Neurosci. 2005, 6, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.A.; Snyder, L.H.; Bradley, D.C.; Xing, J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu. Rev. Neurosci. 1997, 20, 303–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passingham, R.E. The Frontal Lobes and Voluntary Action; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Chabris, C.F.; Simons, D.J. The Invisible Gorilla: And Other Ways Our Intuitions Deceive Us; Harmony: New York, NY, USA, 2010. [Google Scholar]
- Handel, S. Listening: An Introduction to the Perception of Auditory Events; The MIT Press: Cambridge, MA, USA, 1993. [Google Scholar]
- Magagnin, V.; Bassani, T.; Bari, V.; Turiel, M.; Maestri, R.; Pinna, G.D.; Porta, A. Non-stationarities significantly distort short-term spectral, symbolic and entropy heart rate variability indices. Physiol. Measur. 2011, 32, 1775. [Google Scholar] [CrossRef]
- Lewis, G.F.; Furman, S.A.; McCool, M.F.; Porges, S.W. Statistical strategies to quantify respiratory sinus arrhythmia: Are commonly used metrics equivalent? Biol. Psychol. 2012, 89, 349–364. [Google Scholar] [CrossRef] [Green Version]
- Zhivomirov, H.; Nedelchev, I. A Method for Signal Stationarity Estimation. Rom. J. Acoust. Vib. 2020, 17, 149–155. [Google Scholar]
- Keller, K.; Mangold, T.; Stolz, I.; Werner, J. Permutation entropy: New ideas and challenges. Entropy 2017, 19, 134. [Google Scholar] [CrossRef] [Green Version]
- Porta, A.; Bari, V.; De Maria, B.; Cairo, B.; Vaini, E.; Malacarne, M.; Pagani, M.; Lucini, D. On the relevance of computing a local version of sample entropy in cardiovascular control analysis. IEEE Trans. Biomed. Eng. 2018, 66, 623–631. [Google Scholar] [CrossRef]



| Relationship | Entropy | Signal Amplitude | Beta Value |
|---|---|---|---|
| Rest vs. Task | Entropy (Rest) < Entropy (Task) | Signal amplitude (Rest) < Signal amplitude (Task) | Beta value (Rest) < Beta value (Task) |
| Rest duration | ns 1 | ns 1 | ns 1 |
| Task duration (TD) | TD ∝ Entropy | ns 1 | ns 1 |
| Natural frequency (NF) | NF ∝ 1/Entropy | NF ∝ Signal amplitude | NF ∝ Beta value |
| Damping ratio (DR) | ns 1 | ns 1 | ns 1 |
| Relationship | Entropy | Signal Amplitude | Beta Value |
|---|---|---|---|
| Rest vs. Task | CH6 | Whole CHs. except CH8, 12 | Whole CHs. except CH8, 12 |
| Rest duration | ns 1 | ns 1 | ns 1 |
| Task duration (TD) | CH4, 5, 6, 8, 9, 12 | ns 1 | ns 1 |
| Natural frequency (NF) | CH1, 6, 16 | Whole CHs. | Whole CHs. |
| Damping ratio (DR) | CH4 | ns 1 | ns 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, B.; Jang, S.-H.; Chang, P.-H. Entropy Could Quantify Brain Activation Induced by Mechanical Impedance-Restrained Active Arm Motion: A Functional NIRS Study. Entropy 2022, 24, 556. https://doi.org/10.3390/e24040556
Yu B, Jang S-H, Chang P-H. Entropy Could Quantify Brain Activation Induced by Mechanical Impedance-Restrained Active Arm Motion: A Functional NIRS Study. Entropy. 2022; 24(4):556. https://doi.org/10.3390/e24040556
Chicago/Turabian StyleYu, Byeonggi, Sung-Ho Jang, and Pyung-Hun Chang. 2022. "Entropy Could Quantify Brain Activation Induced by Mechanical Impedance-Restrained Active Arm Motion: A Functional NIRS Study" Entropy 24, no. 4: 556. https://doi.org/10.3390/e24040556
APA StyleYu, B., Jang, S.-H., & Chang, P.-H. (2022). Entropy Could Quantify Brain Activation Induced by Mechanical Impedance-Restrained Active Arm Motion: A Functional NIRS Study. Entropy, 24(4), 556. https://doi.org/10.3390/e24040556






