Review of Novel High-Entropy Protective Materials: Wear, Irradiation, and Erosion Resistance Properties
Abstract
1. Introduction
1.1. High-Entropy Concept
1.2. Development and Types of HEMs
1.2.1. Alloys
1.2.2. Ceramics
1.2.3. Other Types of HEMs
1.3. Thermodynamic Descriptors
1.3.1. Configurational Mixing Entropy, ΔSmix
1.3.2. Atomic Size Difference (δ) and Mixing Enthalpy (ΔHmix)
1.3.3. Valence Electron Concentration (VEC)
1.3.4. Mixing Parameter (Ω)
1.3.5. Gamma (γ)
1.3.6. Entropy-Forming Ability (EFA)
2. Irradiation Resistance of HEMs
2.1. Irradiation Resistance Mechanisms in HEMs
2.1.1. Local Atomic Configuration
2.1.2. Compositional Complexity
2.1.3. Atomic-Level Pressure
2.1.4. Vacancy-Interstitial Defects and Localized Melting
2.2. Radiation Damage in HEMs
2.2.1. Radiation-Induced Defects in HEMs
2.2.2. Radiation-Induced Cavities in HEMs
2.3. Effect of Radiation on the Mechanical Properties of HEMs
2.4. Future Scope
3. Wear Resistance
3.1. Wear Performance of HEAs
3.1.1. Wear Performance of Bulk HEAs
3.1.2. Wear Performance of HEA Coatings and Films
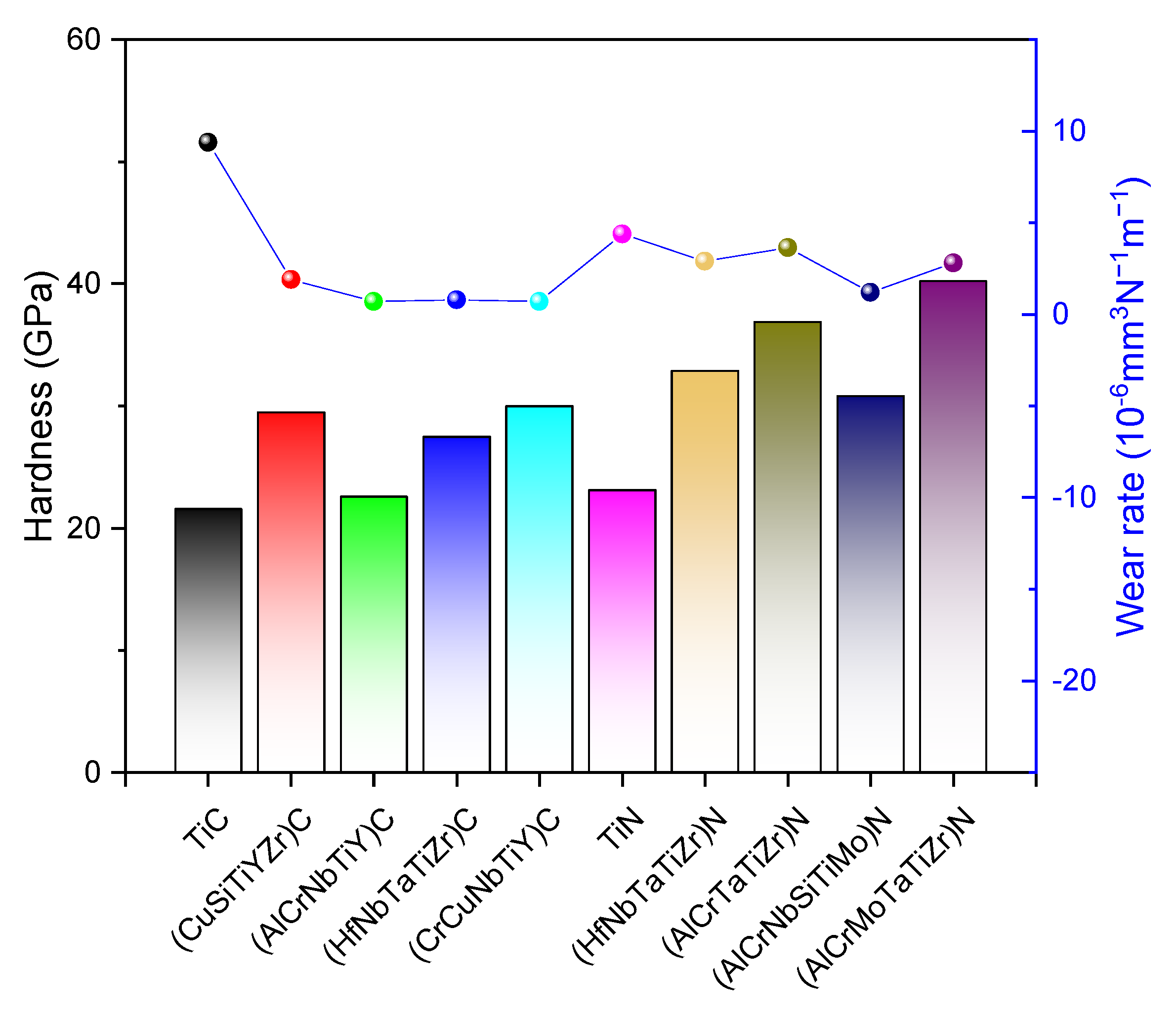
3.2. Wear Performance of HECs
3.3. Wear Performance of High-Entropy Composites
3.4. Future Scope
4. Erosion Resistance
4.1. Erosion Resistance of HEMs
4.1.1. Erosion Resistance of Bulk HEMs
4.1.2. Erosion Resistance of High-Entropy Coatings and Films
4.2. Improving Erosion Resistance of HEMs
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural Development in Equiatomic Multicomponent Alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Chen, R.; Cai, Z.; Pu, J.; Lu, Z.; Chen, S.; Zheng, S.; Zeng, C. Effects of Nitriding on the Microstructure and Properties of VAlTiCrMo High-Entropy Alloy Coatings by Sputtering Technique. J. Alloy. Compd. 2020, 827, 153836. [Google Scholar] [CrossRef]
- Manzoni, A.M.; Glatzel, U. New Multiphase Compositionally Complex Alloys Driven by the High Entropy Alloy Approach. Mater. Charact. 2019, 147, 512–532. [Google Scholar] [CrossRef]
- Wright, A.J.; Luo, J. A Step Forward from High-Entropy Ceramics to Compositionally Complex Ceramics: A New Perspective. J. Mater. Sci. 2020, 55, 9812–9827. [Google Scholar] [CrossRef]
- Castle, E.; Csanádi, T.; Grasso, S.; Dusza, J.; Reece, M. Processing and Properties of High-Entropy Ultra-High Temperature Carbides. Sci. Rep. 2018, 8, 8609. [Google Scholar] [CrossRef]
- Siddiqui, A.A.; Dubey, A.K.; Paul, C.P. A Study of Metallurgy and Erosion in Laser Surface Alloying of Al x Cu 0.5 FeNiTi High Entropy Alloy. Surf. Coat. Technol. 2019, 361, 27–34. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, L.; Gan, G.Y.; Wang, W.; Tang, B.Y. Investigation of Thermodynamic Properties of High Entropy (TaNbHfTiZr)C and (TaNbHfTiZr)N. J. Alloy. Compd. 2019, 788, 1076–1083. [Google Scholar] [CrossRef]
- Chen, T.K.; Shun, T.T.; Yeh, J.W.; Wong, M.S. Nanostructured Nitride Films of Multi-Element High-Entropy Alloys by Reactive DC Sputtering. Surf. Coat. Technol. 2004, 188–189, 193–200. [Google Scholar] [CrossRef]
- Zhang, H.; Hedman, D.; Feng, P.; Han, G.; Akhtar, F. A High-Entropy B4(HfMo2TaTi)C and SiC Ceramic Composite. Dalton Trans. 2019, 48, 5161–5167. [Google Scholar] [CrossRef]
- Wu, C.F.; Arifin, D.E.S.; Wang, C.A.; Ruan, J. Coalescence and Split of High-Entropy Polymer Lamellar Cocrystals. Polymer 2018, 138, 188–202. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, X.; Wang, H.; Wu, Y.; Jiang, S.; Lu, Z. Development of Advanced Materials via Entropy Engineering. Scr. Mater. 2019, 165, 164–169. [Google Scholar] [CrossRef]
- Ye, B.; Wen, T.; Nguyen, M.C.; Hao, L.; Wang, C.Z.; Chu, Y. First-Principles Study, Fabrication and Characterization of (Zr 0.25 Nb 0.25 Ti 0.25 V 0.25)C High-Entropy Ceramics. Acta Mater. 2019, 170, 15–23. [Google Scholar] [CrossRef]
- Sarker, P.; Harrington, T.; Toher, C.; Oses, C.; Samiee, M.; Maria, J.P.; Brenner, D.W.; Vecchio, K.S.; Curtarolo, S. High-Entropy High-Hardness Metal Carbides Discovered by Entropy Descriptors. Nat. Commun. 2018, 9, 4980. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, W.M.; Jiang, Z.-B.; Zhu, Q.Q.; Sun, S.K.; You, Y.; Plucknett, K.; Lin, H.T. Dense High-Entropy Boride Ceramics with Ultra-High Hardness. Scr. Mater. 2019, 164, 135–139. [Google Scholar] [CrossRef]
- Ye, B.; Wen, T.; Liu, D.; Chu, Y. Oxidation Behavior of (Hf 0.2 Zr 0.2 Ta 0.2 Nb 0.2 Ti 0.2)C High-Entropy Ceramics at 1073-1473 K in Air. Corros. Sci. 2019, 153, 327–332. [Google Scholar] [CrossRef]
- Nair, R.B.; Arora, H.S.; Mukherjee, S.; Singh, S.; Singh, H.; Grewal, H.S. Exceptionally High Cavitation Erosion and Corrosion Resistance of a High Entropy Alloy. Ultrason. Sonochemistry 2018, 41, 252–260. [Google Scholar] [CrossRef]
- Xia, S.-q.; Wang, Z.; Yang, T.-f.; Zhang, Y. Irradiation Behavior in High Entropy Alloys. J. Iron Steel Res. Int. 2015, 22, 879–884. [Google Scholar] [CrossRef]
- Alvi, S.; Akhtar, F. High Temperature Tribology of CuMoTaWV High Entropy Alloy. Wear 2019, 426–427, 412–419. [Google Scholar] [CrossRef]
- Xing, Q.; Feltrin, A.C.; Akhtar, F. Processing, Microstructure and High Temperature Dry Sliding Wear of a Cr-Fe-Hf-Mn-Ti-Ta-V High-Entropy Alloy Based Composite. Mater. Today Commun. 2021, 28, 102657. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, S.K.; Zhang, W.; You, Y.; Guo, W.M.; Chen, Z.W.; Yuan, J.H.; Lin, H.T. Improved Densification and Hardness of High-Entropy Diboride Ceramics from Fine Powders Synthesized via Borothermal Reduction Process. Ceram. Int. 2020, 46, 14299–14303. [Google Scholar] [CrossRef]
- Sarkar, A.; Wang, Q.; Schiele, A.; Chellali, M.R.; Bhattacharya, S.S.; Wang, D.; Brezesinski, T.; Hahn, H.; Velasco, L.; Breitung, B. High-Entropy Oxides: Fundamental Aspects and Electrochemical Properties. Adv. Mater. 2019, 31, e1806236. [Google Scholar] [CrossRef]
- Braic, V.; Balaceanu, M.; Braic, M.; Vladescu, A.; Panseri, S.; Russo, A. Characterization of Multi-Principal-Element (TiZrNbHfTa)N and (TiZrNbHfTa)C Coatings for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2012, 10, 197–205. [Google Scholar] [CrossRef]
- Nair, R.B.; Selvam, K.; Arora, H.S.; Mukherjee, S.; Singh, H.; Grewal, H.S. Slurry Erosion Behavior of High Entropy Alloys. Wear 2017, 386–387, 230–238. [Google Scholar] [CrossRef]
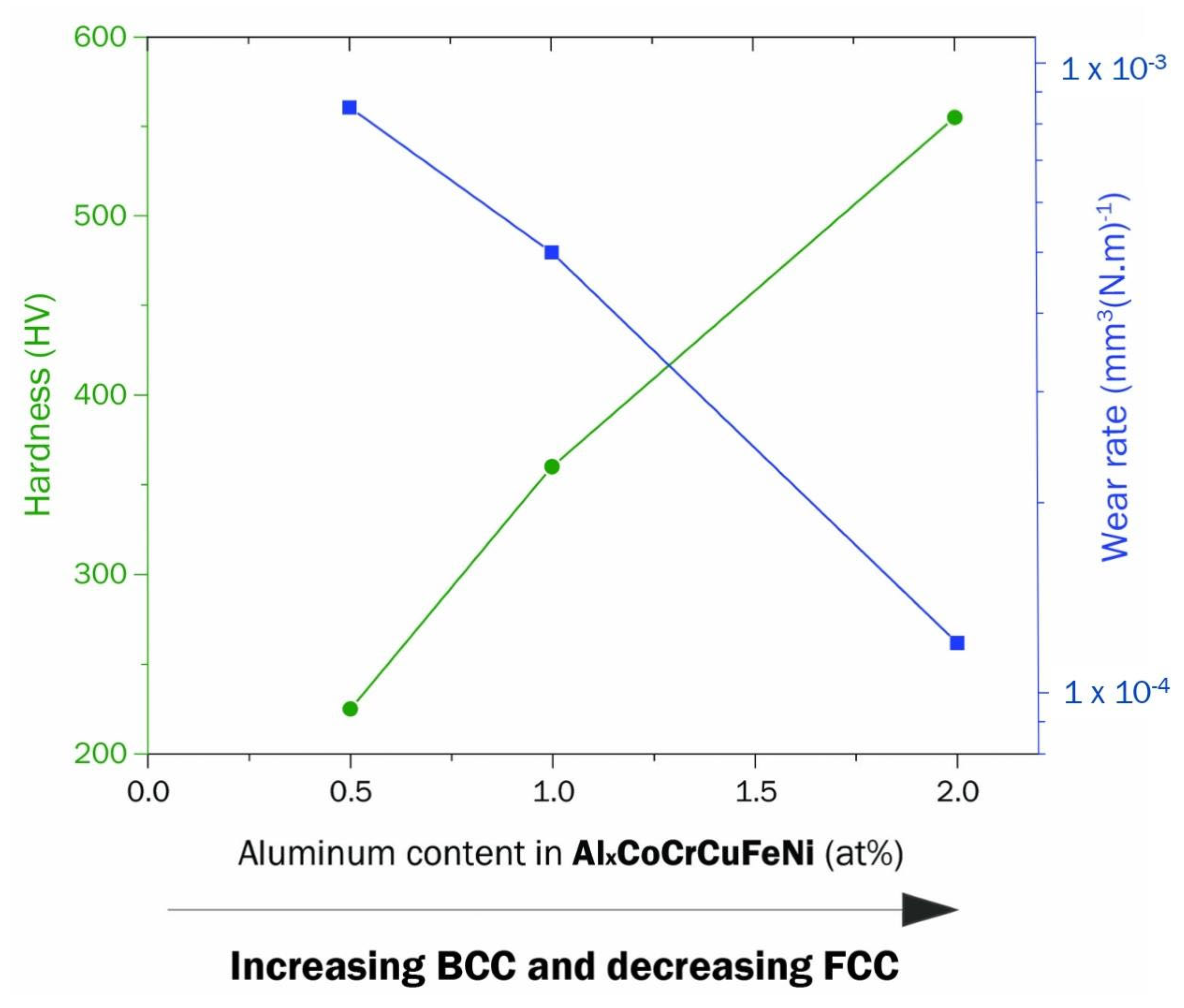
- Wu, J.M.; Lin, S.J.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Chen, H.C. Adhesive Wear Behavior of AlxCoCrCuFeNi High-Entropy Alloys as a Function of Aluminum Content. Wear 2006, 261, 513–519. [Google Scholar] [CrossRef]
- Gild, J.; Zhang, Y.; Harrington, T.; Jiang, S.; Hu, T.; Quinn, M.C.; Mellor, W.M.; Zhou, N.; Vecchio, K.; Luo, J. High-Entropy Metal Diborides: A New Class of High-Entropy Materials and a New Type of Ultrahigh Temperature Ceramics. Sci. Rep. 2016, 6, 37946. [Google Scholar] [CrossRef]
- Fujieda, T.; Shiratori, H.; Kuwabara, K.; Kato, T.; Yamanaka, K.; Koizumi, Y.; Chiba, A. First Demonstration of Promising Selective Electron Beam Melting Method for Utilizing High-Entropy Alloys as Engineering Materials. Mater. Lett. 2015, 159, 12–15. [Google Scholar] [CrossRef]
- Torralba, J.M.; Alvaredo, P.; García-Junceda, A. High-Entropy Alloys Fabricated via Powder Metallurgy. A Critical Review. Powder Metall. 2019, 62, 84–114. [Google Scholar] [CrossRef]
- Ostovari Moghaddam, A.; Shaburova, N.A.; Samodurova, M.N.; Abdollahzadeh, A.; Trofimov, E.A. Additive Manufacturing of High Entropy Alloys: A Practical Review. J. Mater. Sci. Technol. 2021, 77, 131–162. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-Resistant High-Entropy Alloys: A Review. Metals 2017, 7, 43. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Meng, X.; Xie, Y. A Review on High Entropy Alloys Coatings: Fabrication Processes and Property Assessment. Adv. Eng. Mater. 2019, 21, 1900343. [Google Scholar] [CrossRef]
- An, Z.; Jia, H.; Wu, Y.; Rack, P.D.; Patchen, A.D.; Liu, Y.; Ren, Y.; Li, N.; Liaw, P.K. Solid-Solution CrCoCuFeNi High-Entropy Alloy Thin Films Synthesized by Sputter Deposition. Mater. Res. Lett. 2015, 3, 203–209. [Google Scholar] [CrossRef]
- Feng, X.; Tang, G.; Sun, M.; Ma, X.; Wang, L.; Yukimura, K. Structure and Properties of Multi-Targets Magnetron Sputtered ZrNbTaTiW Multi-Elements Alloy Thin Films. Surf. Coat. Technol. 2013, 228, S424–S427. [Google Scholar] [CrossRef]
- Zhao, D.; Yamaguchi, T.; Wang, W. Fabrication and Wear Performance of Al0.8FeCrCoNi High Entropy Alloy Coating on Magnesium Alloy by Resistance Seam Welding. Mater. Lett. 2020, 265, 127250. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, W.; He, Y.; Li, M.; Guo, S. Formation of Core-Shell Structure in High Entropy Alloy Coating by Laser Cladding. Appl. Surf. Sci. 2016, 363, 543–547. [Google Scholar] [CrossRef]
- Yao, C.Z.; Zhang, P.; Liu, M.; Li, G.R.; Ye, J.Q.; Liu, P.; Tong, Y.X. Electrochemical Preparation and Magnetic Study of Bi-Fe-Co-Ni-Mn High Entropy Alloy. Electrochim. Acta 2008, 53, 8359–8365. [Google Scholar] [CrossRef]
- Tsau, C.H.; Yang, Y.C.; Lee, C.C.; Wu, L.Y.; Huang, H.J. The Low Electrical Resistivity of the High-Entropy Alloy Oxide Thin Films. Procedia Eng. 2012, 36, 246–252. [Google Scholar] [CrossRef]
- Shen, W.J.; Tsai, M.H.; Tsai, K.Y.; Juan, C.C.; Tsai, C.W.; Yeh, J.W.; Chang, Y.S. Superior Oxidation Resistance of (Al 0.34 Cr 0.22 Nb 0.11 Si 0.11 Ti 0.22) 50 N 50 High-Entropy Nitride. J. Electrochem. Soc. 2013, 160, C531–C535. [Google Scholar] [CrossRef]
- Dusza, J.; Švec, P.; Girman, V.; Sedlák, R.; Castle, E.G.; Csanádi, T.; Kovalčíková, A.; Reece, M.J. Microstructure of (Hf-Ta-Zr-Nb)C High-Entropy Carbide at Micro and Nano/Atomic Level. J. Eur. Ceram. Soc. 2018, 38, 4303–4307. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.P. Entropy-Stabilized Oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef] [PubMed]
- Mayrhofer, P.H.; Kirnbauer, A.; Ertelthaler, P.; Koller, C.M. High-Entropy Ceramic Thin Films; A Case Study on Transition Metal Diborides. Scr. Mater. 2018, 149, 93–97. [Google Scholar] [CrossRef]
- Gild, J.; Braun, J.; Kaufmann, K.; Marin, E.; Harrington, T.; Hopkins, P.; Vecchio, K.; Luo, J. A High-Entropy Silicide: (Mo0.2Nb0.2Ta0.2Ti0.2W0.2)Si2. J. Mater. 2019, 5, 337–343. [Google Scholar] [CrossRef]
- Zhang, H.; Akhtar, F. Processing and Characterization of Refractory Quaternary and Quinary High-Entropy Carbide Composite. Entropy 2019, 21, 474. [Google Scholar] [CrossRef]
- Wang, Q.; Sarkar, A.; Wang, D.; Velasco, L.; Azmi, R.; Bhattacharya, S.S.; Bergfeldt, T.; Düvel, A.; Heitjans, P.; Brezesinski, T.; et al. Multi-Anionic and -Cationic Compounds: New High Entropy Materials for Advanced Li-Ion Batteries. Energy Environ. Sci. 2019, 12, 2433–2442. [Google Scholar] [CrossRef]
- Jansson, U.; Lewin, E. Carbon-Containing Multi-Component Thin Films. Thin Solid Films 2019, 688, 137411. [Google Scholar] [CrossRef]
- Lewin, E. Multi-Component and High-Entropy Nitride Coatings—A Promising Field in Need of a Novel Approach. J. Appl. Phys. 2020, 127, 160901. [Google Scholar] [CrossRef]
- Yan, X.; Constantin, L.; Lu, Y.; Silvain, J.F.; Nastasi, M.; Cui, B. (Hf0.2Zr0.2Ta0.2Nb0.2Ti0.2)C High-Entropy Ceramics with Low Thermal Conductivity. J. Am. Ceram. Soc. 2018, 101, 4486–4491. [Google Scholar] [CrossRef]
- Qin, M.; Gild, J.; Hu, C.; Wang, H.; Bin Hoque, M.S.; Braun, J.L.; Harrington, T.J.; Hopkins, P.E.; Vecchio, K.S.; Luo, J. Dual-Phase High-Entropy Ultra-High Temperature Ceramics. J. Eur. Ceram. Soc. 2020, 40, 5037–5050. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, Y.; Lou, H.; Zeng, Z.; Prakapenka, V.B.; Greenberg, E.; Ren, Y.; Yan, J.; Okasinski, J.S.; Liu, X.; et al. Polymorphism in a High-Entropy Alloy. Nat. Commun. 2017, 8, 15687. [Google Scholar] [CrossRef]
- Yang, L.; Ge, H.; Zhang, J.; Xiong, T.; Jin, Q.; Zhou, Y.; Shao, X.; Zhang, B.; Zhu, Z.; Zheng, S.; et al. High He-Ion Irradiation Resistance of CrMnFeCoNi High-Entropy Alloy Revealed by Comparison Study with Ni and 304SS. J. Mater. Sci. Technol. 2019, 35, 300–305. [Google Scholar] [CrossRef]
- Braic, V.; Vladescu, A.; Balaceanu, M.; Luculescu, C.R.; Braic, M. Nanostructured Multi-Element (TiZrNbHfTa)N and (TiZrNbHfTa)C Hard Coatings. Surf. Coat. Technol. 2012, 211, 117–121. [Google Scholar] [CrossRef]
- Feng, L.; Fahrenholtz, W.G.; Hilmas, G.E. Low-Temperature Sintering of Single-Phase, High-Entropy Carbide Ceramics. J. Am. Ceram. Soc. 2019, 102, 7217–7224. [Google Scholar] [CrossRef]
- Wang, H.; Cao, Y.; Liu, W.; Wang, Y. Oxidation Behavior of (Hf0.2Ta0.2Zr0.2Ti0.2Nb0.2)C-XSiC Ceramics at High Temperature. Ceram. Int. 2020, 46, 11160–11168. [Google Scholar] [CrossRef]
- Lu, K.; Liu, J.X.; Wei, X.F.; Bao, W.; Wu, Y.; Li, F.; Xu, F.; Zhang, G.J. Microstructures and Mechanical Properties of High-Entropy (Ti0.2Zr0.2Hf0.2Nb0.2Ta0.2)C Ceramics with the Addition of SiC Secondary Phase. J. Eur. Ceram. Soc. 2020, 40, 1839–1847. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, J.; Liu, B.; Yan, J.; Lu, L.; Li, Y.; Gao, H.; Li, X. Three-Dimensional High-Entropy Alloy-Polymer Composite Nanolattices That Overcome the Strength-Recoverability Trade-Off. Nano Lett. 2018, 18, 4247–4256. [Google Scholar] [CrossRef]
- Huang, Y.J.; Yeh, J.W.; Chang-Mou Yang, A. “High-Entropy Polymers”: A New Route of Polymer Mixing with Suppressed Phase Separation. Materialia 2021, 15, 100978. [Google Scholar] [CrossRef]
- Ma, L.; Wang, L.; Zhang, T.; Inoue, A. Bulk Glass Formation of Ti-Zr-Hf-Cu-M (M = Fe, Co, Ni) Alloys. Mater. Trans. 2002, 43, 277–280. [Google Scholar] [CrossRef]
- Wang, W.H. High-Entropy Metallic Glasses. JOM 2014, 66, 2067–2077. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.J.; Ruan, H.H.; Wu, Y.; Wang, H.; Lu, Z.P. High Thermal Stability and Sluggish Crystallization Kinetics of High-Entropy Bulk Metallic Glasses. J. Appl. Phys. 2016, 119, 245112. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, K.; Feng, Y.; Li, Y.; Tang, W.; Zhang, Y.; Wei, B.; Hu, Z. Excellent Irradiation Tolerance and Mechanical Behaviors in High-Entropy Metallic Glasses. J. Nucl. Mater. 2019, 527, 151785. [Google Scholar] [CrossRef]
- Waseem, O.A. Can High-Entropy Interlayers Develop Intermetallic-Free Welded Joints of Dissimilar Metals? Eng 2020, 1, 183–187. [Google Scholar] [CrossRef]
- Ding, W.; Liu, N.; Fan, J.; Cao, J.; Wang, X. Diffusion Bonding of Copper to Titanium Using CoCrFeMnNi High-Entropy Alloy Interlayer. Intermetallics 2021, 129, 107027. [Google Scholar] [CrossRef]
- Reichardt, A.; Shapiro, A.A.; Otis, R.; Dillon, R.P.; Borgonia, J.P.; McEnerney, B.W.; Hosemann, P.; Beese, A.M. Advances in Additive Manufacturing of Metal-Based Functionally Graded Materials. Int. Mater. Rev. 2021, 66, 1–29. [Google Scholar] [CrossRef]
- Gwalani, B.; Gangireddy, S.; Shukla, S.; Yannetta, C.J.; Valentin, S.G.; Mishra, R.S.; Banerjee, R. Compositionally Graded High Entropy Alloy with a Strong Front and Ductile Back. Mater. Today Commun. 2019, 20, 100602. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and Properties of High-Entropy Alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Vishwanadh, B.; Sarkar, N.; Gangil, S.; Singh, S.; Tewari, R.; Dey, G.K.; Banerjee, S. Synthesis and Microstructural Characterization of a Novel Multicomponent Equiatomic ZrNbAlTiV High Entropy Alloy. Scr. Mater. 2016, 124, 146–150. [Google Scholar] [CrossRef]
- George, E.P.; Raabe, D.; Ritchie, R.O. High-Entropy Alloys. Nat. Rev. Mater. 2019, 4, 515–534. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-Solution Phase Formation Rules for Multi-Component Alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of Valence Electron Concentration on Stability of Fcc or Bcc Phase in High Entropy Alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.; Yang, Y.; Wang, J.; Liu, C.T. Atomic-Size Effect and Solid Solubility of Multicomponent Alloys. Scr. Mater. 2015, 94, 28–31. [Google Scholar] [CrossRef]
- Otto, F.; Yang, Y.; Bei, H.; George, E.P. Relative Effects of Enthalpy and Entropy on the Phase Stability of Equiatomic High-Entropy Alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar] [CrossRef]
- Zhu, Z.G.; Ma, K.H.; Wang, Q.; Shek, C.H. Compositional Dependence of Phase Formation and Mechanical Properties in Three CoCrFeNi-(Mn/Al/Cu) High Entropy Alloys. Intermetallics 2016, 79, 1–11. [Google Scholar] [CrossRef]
- Guo, S. Phase Selection Rules for Cast High Entropy Alloys: An Overview. Mater. Sci. Technol. 2015, 31, 1223–1230. [Google Scholar] [CrossRef]
- Guo, S.; Hu, Q.; Ng, C.; Liu, C.T. More than Entropy in High-Entropy Alloys: Forming Solid Solutions or Amorphous Phase. Intermetallics 2013, 41, 96–103. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, Y.L.; Lin, S.J.; Chen, S.K. High-Entropy Alloys—A New Era of Exploitation. Mater. Sci. Forum 2007, 560, 1–9. [Google Scholar] [CrossRef]
- Anand, G.; Goodall, R.; Freeman, C.L. Role of Configurational Entropy in Body-Centred Cubic or Face-Centred Cubic Phase Formation in High Entropy Alloys. Scr. Mater. 2016, 124, 90–94. [Google Scholar] [CrossRef]
- Cantor, B. Multicomponent High-Entropy Cantor Alloys. Prog. Mater. Sci. 2020, 120, 100754. [Google Scholar] [CrossRef]
- Ma, D.; Grabowski, B.; Körmann, F.; Neugebauer, J.; Raabe, D. Ab Initio Thermodynamics of the CoCrFeMnNi High Entropy Alloy: Importance of Entropy Contributions beyond the Configurational One. Acta Mater. 2015, 100, 90–97. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase Stability in High Entropy Alloys: Formation of Solid-Solution Phase or Amorphous Phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Takeuchi, A.; Wada, T.; Kato, H. Solid Solutions with Bcc, Hcp, and Fcc Structures Formed in a Composition Line in Multicomponent Ir–Rh–Ru–W–Mo System. Mater. Trans. 2019, 60, 2267–2276. [Google Scholar] [CrossRef]
- Takeuchi, A.; Wada, T.; Kato, H. High-Entropy Alloys with Hexagonal Close-Packed Structure in Ir26Mo20Rh22.5Ru20W11.5 and Ir25.5Mo20Rh20Ru25W9.5 Alloys Designed by Sandwich Strategy for the Valence Electron Concentration of Constituent Elements in the Periodic Chart. Mater. Trans. 2019, 60, 1666–1673. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of High-Entropy Stabilized Solid-Solution in Multi-Component Alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Harrington, T.J.; Gild, J.; Sarker, P.; Toher, C.; Rost, C.M.; Dippo, O.F.; McElfresh, C.; Kaufmann, K.; Marin, E.; Borowski, L.; et al. Phase Stability and Mechanical Properties of Novel High Entropy Transition Metal Carbides. Acta Mater. 2019, 166, 271–280. [Google Scholar] [CrossRef]
- Nagase, T.; Rack, P.D.; Noh, J.H.; Egami, T. In-Situ TEM Observation of Structural Changes in Nano-Crystalline CoCrCuFeNi Multicomponent High-Entropy Alloy (HEA) under Fast Electron Irradiation by High Voltage Electron Microscopy (HVEM). Intermetallics 2015, 59, 32–42. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, R.; Yang, Z.B.; Liu, C.H.; Chang, H.; Yang, J.J.; Liao, J.L.; Yang, Y.Y.; Liu, N. Preparation, Structure, and Properties of an AlCrMoNbZr High-Entropy Alloy Coating for Accident-Tolerant Fuel Cladding. Surf. Coat. Technol. 2018, 347, 13–19. [Google Scholar] [CrossRef]
- Tunes, M.A.; Vishnyakov, V.M.; Donnelly, S.E. Synthesis and Characterisation of High-Entropy Alloy Thin Films as Candidates for Coating Nuclear Fuel Cladding Alloys. Thin Solid Films 2018, 649, 115–120. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, R.; Yang, Z.B.; Liu, C.H.; Chang, H.; Yang, J.J.; Liao, J.L.; Yang, Y.Y.; Liu, N. Preparation, Structure, and Properties of High-Entropy Alloy Multilayer Coatings for Nuclear Fuel Cladding: A Case Study of AlCrMoNbZr/(AlCrMoNbZr)N. J. Nucl. Mater. 2018, 512, 15–24. [Google Scholar] [CrossRef]
- Pogrebnjak, A.D.; Yakushchenko, I.V.; Bondar, O.V.; Beresnev, V.M.; Oyoshi, K.; Ivasishin, O.M.; Amekura, H.; Takeda, Y.; Opielak, M.; Kozak, C. Irradiation Resistance, Microstructure and Mechanical Properties of Nanostructured (TiZrHfVNbTa)N Coatings. J. Alloy. Compd. 2016, 679, 155–163. [Google Scholar] [CrossRef]
- Yeh, J.W. Physical Metallurgy of High-Entropy Alloys. JOM 2015, 67, 2254–2261. [Google Scholar] [CrossRef]
- Lu, C.; Niu, L.; Chen, N.; Jin, K.; Yang, T.; Xiu, P.; Zhang, Y.; Gao, F.; Bei, H.; Shi, S.; et al. Enhancing Radiation Tolerance by Controlling Defect Mobility and Migration Pathways in Multicomponent Single-Phase Alloys. Nat. Commun. 2016, 7, 13564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Stocks, G.M.; Jin, K.; Lu, C.; Bei, H.; Sales, B.C.; Wang, L.; Béland, L.K.; Stoller, R.E.; Samolyuk, G.D.; et al. Influence of Chemical Disorder on Energy Dissipation and Defect Evolution in Concentrated Solid Solution Alloys. Nat. Commun. 2015, 6, 8736. [Google Scholar] [CrossRef] [PubMed]
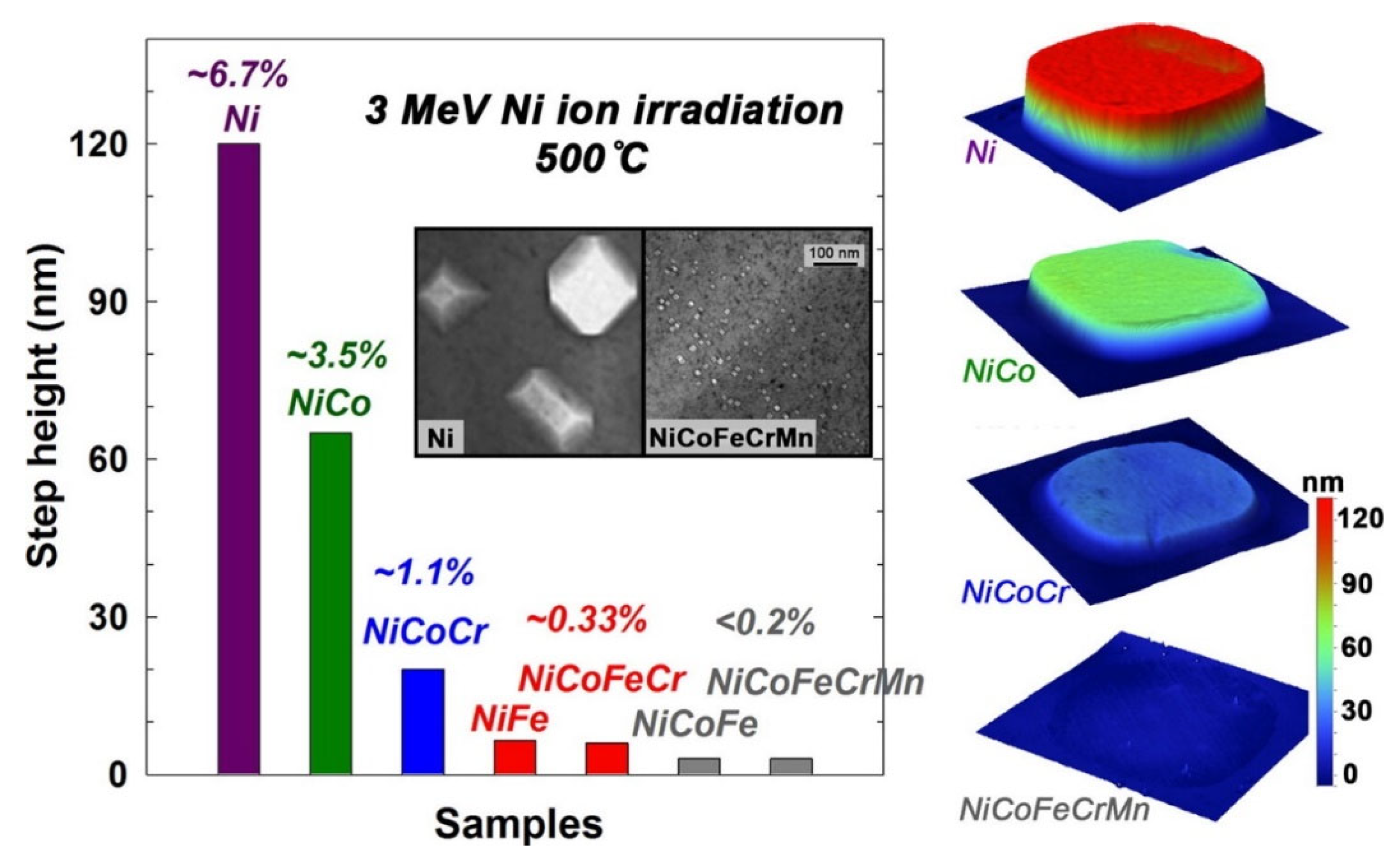
- Jin, K.; Lu, C.; Wang, L.M.; Qu, J.; Weber, W.J.; Zhang, Y.; Bei, H. Effects of Compositional Complexity on the Ion-Irradiation Induced Swelling and Hardening in Ni-Containing Equiatomic Alloys. Scr. Mater. 2016, 119, 65–70. [Google Scholar] [CrossRef]
- Egami, T.; Ojha, M.; Khorgolkhuu, O.; Nicholson, D.M.; Stocks, G.M. Local Electronic Effects and Irradiation Resistance in High-Entropy Alloys. JOM 2015, 67, 2345–2349. [Google Scholar] [CrossRef]
- Nagase, T.; Anada, S.; Rack, P.D.; Noh, J.H.; Yasuda, H.; Mori, H.; Egami, T. Electron-Irradiation-Induced Structural Change in Zr-Hf-Nb Alloy. Intermetallics 2012, 26, 122–130. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.T.; Dou, P. Thermodynamics of Vacancies and Clusters in High-Entropy Alloys. Phys. Rev. Mater. 2017, 1, 043601. [Google Scholar] [CrossRef]
- Nagase, T.; Anada, S.; Rack, P.D.; Noh, J.H.; Yasuda, H.; Mori, H.; Egami, T. MeV Electron-Irradiation-Induced Structural Change in the Bcc Phase of Zr–Hf–Nb Alloy with an Approximately Equiatomic Ratio. Intermetallics 2013, 38, 70–79. [Google Scholar] [CrossRef]
- Xia, S.Q.; Yang, X.; Yang, T.F.; Liu, S.; Zhang, Y. Irradiation Resistance in AlxCoCrFeNi High Entropy Alloys. JOM 2015, 67, 2340–2344. [Google Scholar] [CrossRef]
- Kiran Kumar, N.A.P.; Li, C.; Leonard, K.J.; Bei, H.; Zinkle, S.J. Microstructural Stability and Mechanical Behavior of FeNiMnCr High Entropy Alloy under Ion Irradiation. Acta Mater. 2016, 113, 230–244. [Google Scholar] [CrossRef]
- Zhao, S. Defect Properties in a VTaCrW Equiatomic High Entropy Alloy (HEA) with the Body Centered Cubic (Bcc) Structure. J. Mater. Sci. Technol. 2020, 44, 133–139. [Google Scholar] [CrossRef]
- Xia, S.; Gao, M.C.; Yang, T.; Liaw, P.K.; Zhang, Y. Phase Stability and Microstructures of High Entropy Alloys Ion Irradiated to High Doses. J. Nucl. Mater. 2016, 480, 100–108. [Google Scholar] [CrossRef]
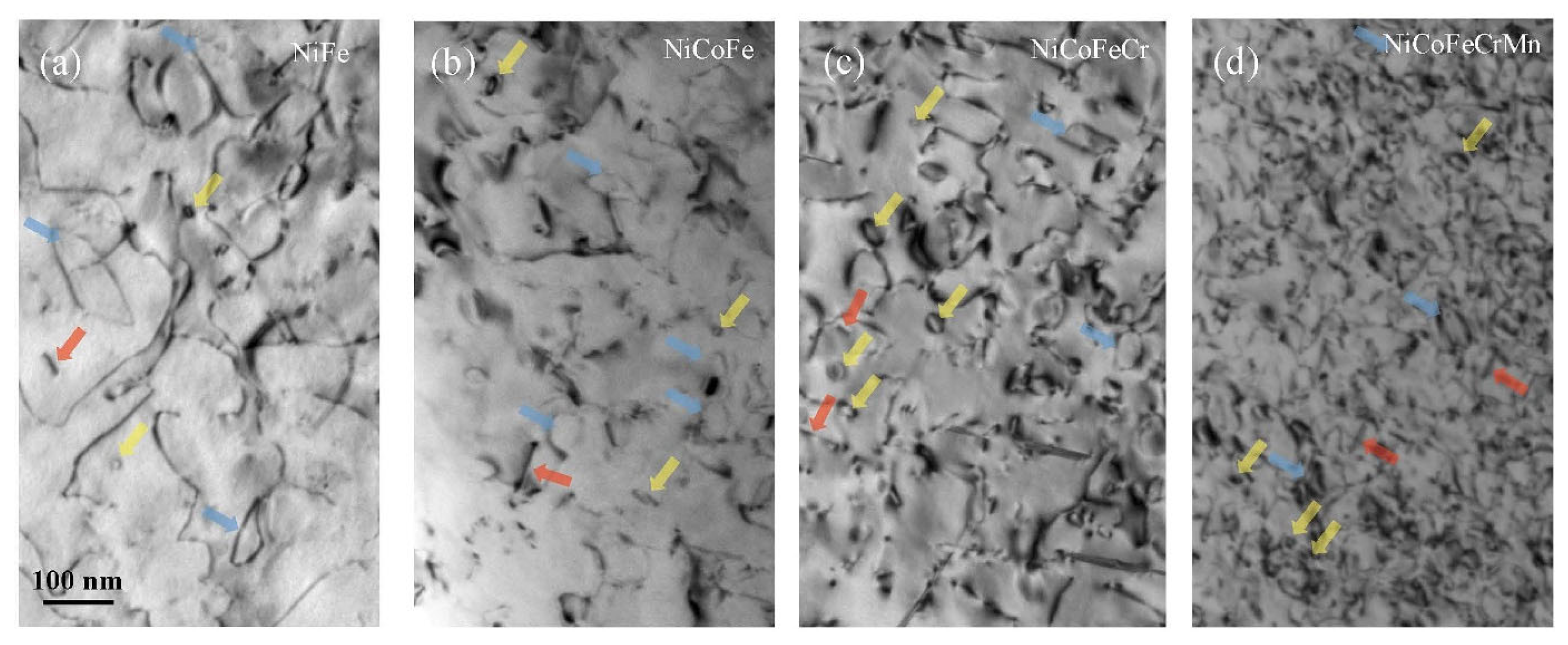
- Lu, C.; Yang, T.; Jin, K.; Gao, N.; Xiu, P.; Zhang, Y.; Gao, F.; Bei, H.; Weber, W.J.; Sun, K.; et al. Radiation-Induced Segregation on Defect Clusters in Single-Phase Concentrated Solid-Solution Alloys. Acta Mater. 2017, 127, 98–107. [Google Scholar] [CrossRef]
- Yang, T.-n.; Lu, C.; Velisa, G.; Jin, K.; Xiu, P.; Zhang, Y.; Bei, H.; Wang, L. Influence of Irradiation Temperature on Void Swelling in NiCoFeCrMn and NiCoFeCrPd. Scr. Mater. 2019, 158, 57–61. [Google Scholar] [CrossRef]
- Chen, D.; Tong, Y.; Wang, J.; Han, B.; Zhao, Y.L.; He, F.; Kai, J.J. Microstructural Response of He+ Irradiated FeCoNiCrTi0.2 High-Entropy Alloy. J. Nucl. Mater. 2018, 510, 187–192. [Google Scholar] [CrossRef]
- Chen, W.Y.; Kirk, M.A.; Hashimoto, N.; Yeh, J.W.; Liu, X.; Chen, Y. Irradiation Effects on Al0.3CoCrFeNi and CoCrMnFeNi High-Entropy Alloys, and 316H Stainless Steel at 500 °C. J. Nucl. Mater. 2020, 539, 152324. [Google Scholar] [CrossRef]
- He, M.R.; Wang, S.; Shi, S.; Jin, K.; Bei, H.; Yasuda, K.; Matsumura, S.; Higashida, K.; Robertson, I.M. Mechanisms of Radiation-Induced Segregation in CrFeCoNi-Based Single-Phase Concentrated Solid Solution Alloys. Acta Mater. 2017, 126, 182–193. [Google Scholar] [CrossRef]
- Wang, F.; Yan, X.; Wang, T.; Wu, Y.; Shao, L.; Nastasi, M.; Lu, Y.; Cui, B. Irradiation Damage in (Zr0.25Ta0.25Nb0.25Ti0.25)C High-Entropy Carbide Ceramics. Acta Mater. 2020, 195, 739–749. [Google Scholar] [CrossRef]
- Chang, S.; Tseng, K.-K.; Yang, T.-Y.; Chao, D.-S.; Yeh, J.-W.; Liang, J.-H. Irradiation-Induced Swelling and Hardening in HfNbTaTiZr Refractory High-Entropy Alloy. Mater. Lett. 2020, 272, 127832. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, H.; Gao, X.; Ren, C.; Gao, J.; Zhang, H.; Zheng, S.; Jin, Q.; Zhao, Y.; Lu, C.; et al. A Promising New Class of Irradiation Tolerant Materials: Ti2ZrHfV0.5Mo0.2 High-Entropy Alloy. J. Mater. Sci. Technol. 2019, 35, 369–373. [Google Scholar] [CrossRef]
- Tunes, M.A.; Le, H.; Greaves, G.; Schön, C.G.; Bei, H.; Zhang, Y.; Edmondson, P.D.; Donnelly, S.E. Investigating Sluggish Diffusion in a Concentrated Solid Solution Alloy Using Ion Irradiation with in Situ TEM. Intermetallics 2019, 110, 106461. [Google Scholar] [CrossRef]
- Chen, D.; Tong, Y.; Li, H.; Wang, J.; Zhao, Y.L.; Hu, A.; Kai, J.J. Helium Accumulation and Bubble Formation in FeCoNiCr Alloy under High Fluence He+ Implantation. J. Nucl. Mater. 2018, 501, 208–216. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, S.; Sun, J.; Tai, P.; Sheng, Y.; Zhao, Y.; Yeli, G.; Lin, W.; Liu, S.; Kai, W.; et al. Diffusion Controlled Helium Bubble Formation Resistance of FeCoNiCr High-Entropy Alloy in the Half-Melting Temperature Regime. J. Nucl. Mater. 2019, 526, 151747. [Google Scholar] [CrossRef]
- Pu, G.; Lin, L.; Ang, R.; Zhang, K.; Liu, B.; Liu, B.; Peng, T.; Liu, S.; Li, Q. Outstanding Radiation Tolerance and Mechanical Behavior in Ultra-Fine Nanocrystalline Al1.5CoCrFeNi High Entropy Alloy Films under He Ion Irradiation. Appl. Surf. Sci. 2020, 516, 146129. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, M.; Wang, L.; Liu, C.H.; Chang, H.; Yang, J.J.; Liao, J.L.; Yang, Y.Y.; Liu, N. Interface Stability, Mechanical and Corrosion Properties of AlCrMoNbZr/(AlCrMoNbZr)N High-Entropy Alloy Multilayer Coatings under Helium Ion Irradiation. Appl. Surf. Sci. 2019, 485, 108–118. [Google Scholar] [CrossRef]
- Komarov, F.F.; Pogrebnyak, A.D.; Konstantinov, S.V. Radiation Resistance of High-Entropy Nanostructured (Ti, Hf, Zr, V, Nb)N Coatings. Tech. Phys. 2015, 60, 1519–1524. [Google Scholar] [CrossRef][Green Version]
- Pickering, E.J.; Carruthers, A.W.; Barron, P.J.; Middleburgh, S.C.; Armstrong, D.E.J.; Gandy, A.S. High-Entropy Alloys for Advanced Nuclear Applications. Entropy 2021, 23, 98. [Google Scholar] [CrossRef]
- El-Atwani, O.; Li, N.; Li, M.; Devaraj, A.; Baldwin, J.K.S.; Schneider, M.M.; Sobieraj, D.; Wróbel, J.S.; Nguyen-Manh, D.; Maloy, S.A.; et al. Outstanding Radiation Resistance of Tungsten-Based High-Entropy Alloys. Sci. Adv. 2019, 5, eaav2002. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Richardson, M.D.; Jim, B.; Akhmadaliev, S.; Goodall, R.; Gandy, A.S. Radiation Damage Tolerance of a Novel Metastable Refractory High Entropy Alloy V2.5Cr1.2WMoCo0.04. J. Nucl. Mater. 2020, 531, 152005. [Google Scholar] [CrossRef]
- Li, C.; Hu, X.; Yang, T.; Kiran Kumar, N.A.P.; Wirth, B.D.; Zinkle, S.J. Neutron Irradiation Response of a Co-Free High Entropy Alloy. J. Nucl. Mater. 2019, 527, 151838. [Google Scholar] [CrossRef]
- Chen, W.Y.; Liu, X.; Chen, Y.; Yeh, J.W.; Tseng, K.K.; Natesan, K. Irradiation Effects in High Entropy Alloys and 316H Stainless Steel at 300 °C. J. Nucl. Mater. 2018, 510, 421–430. [Google Scholar] [CrossRef]
- Zhang, Z.; Armstrong, D.E.J.; Grant, P.S. The Effects of Irradiation on CrMnFeCoNi High-Entropy Alloy and Its Derivatives. Prog. Mater. Sci. 2021, 123, 100807. [Google Scholar] [CrossRef]
- Evans, D.; Chen, J.; Bokas, G.; Chen, W.; Hautier, G.; Sun, W. Visualizing Temperature-Dependent Phase Stability in High Entropy Alloys. npj Comput. Mater. 2021, 7, 151. [Google Scholar] [CrossRef]
- Rao, J.C.; Diao, H.Y.; Ocelík, V.; Vainchtein, D.; Zhang, C.; Kuo, C.; Tang, Z.; Guo, W.; Poplawsky, J.D.; Zhou, Y.; et al. Secondary Phases in AlxCoCrFeNi High-Entropy Alloys: An in-Situ TEM Heating Study and Thermodynamic Appraisal. Acta Mater. 2017, 131, 206–220. [Google Scholar] [CrossRef]
- Joseph, J.; Haghdadi, N.; Shamlaye, K.; Hodgson, P.; Barnett, M.; Fabijanic, D. The Sliding Wear Behaviour of CoCrFeMnNi and AlxCoCrFeNi High Entropy Alloys at Elevated Temperatures. Wear 2019, 428–429, 32–44. [Google Scholar] [CrossRef]
- Haghdadi, N.; Guo, T.; Ghaderi, A.; Hodgson, P.D.; Barnett, M.R.; Fabijanic, D.M. The Scratch Behaviour of AlXCoCrFeNi (X = 0.3 and 1.0) High Entropy Alloys. Wear 2019, 428–429, 293–301. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, H.; Wang, M.; Zhao, Y.; Zhang, X.; Wang, C. The Microstructures and Properties Changes Induced by Al:Co Ratios of the AlXCrCo2-XFeNi High Entropy Alloys. Mater. Sci. Eng. A 2018, 733, 153–163. [Google Scholar] [CrossRef]
- Cheng, H.; Fang, Y.; Xu, J.; Zhu, C.; Dai, P.; Xue, S. Tribological Properties of Nano/Ultrafine-Grained FeCoCrNiMnAlx High-Entropy Alloys over a Wide Range of Temperatures. J. Alloy. Compd. 2020, 817, 153305. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and Wear Behavior of AlxCo1.5CrFeNi1.5Tiy High-Entropy Alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Verma, A.; Tarate, P.; Abhyankar, A.C.; Mohape, M.R.; Gowtam, D.S.; Deshmukh, V.P.; Shanmugasundaram, T. High Temperature Wear in CoCrFeNiCux High Entropy Alloys: The Role of Cu. Scr. Mater. 2019, 161, 28–31. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, L.; Qiao, D.; Lu, Y.; Wang, T.; Cao, Z.; Li, T. Effect of Niobium on Microstructure and Properties of the CoCrFeNb x Ni High Entropy Alloys. J. Mater. Sci. Technol. 2017, 33, 712–717. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, M.; Yang, H.; Qiao, J.; Wu, Y. Surface Strengthening in Al0.25CoCrFeNi High-Entropy Alloy by Boronizing. Mater. Lett. 2019, 238, 258–260. [Google Scholar] [CrossRef]
- Lindner, T.; Löbel, M.; Sattler, B.; Lampke, T. Surface Hardening of FCC Phase High-Entropy Alloy System by Powder-Pack Boriding. Surf. Coat. Technol. 2019, 371, 389–394. [Google Scholar] [CrossRef]
- Nishimoto, A.; Fukube, T.; Maruyama, T. Microstructural, Mechanical, and Corrosion Properties of Plasma-Nitrided CoCrFeMnNi High-Entropy Alloys. Surf. Coat. Technol. 2019, 376, 52–58. [Google Scholar] [CrossRef]
- Poletti, M.G.; Fiore, G.; Gili, F.; Mangherini, D.; Battezzati, L. Development of a New High Entropy Alloy for Wear Resistance: FeCoCrNiW0.3 and FeCoCrNiW0.3 + 5 at.% of C. Mater. Des. 2017, 115, 247–254. [Google Scholar] [CrossRef]
- Kumar, A.; Swarnakar, A.K.; Basu, A.; Chopkar, M. Effects of Processing Route on Phase Evolution and Mechanical Properties of CoCrCuFeNiSix High Entropy Alloys. J. Alloy. Compd. 2018, 748, 889–897. [Google Scholar] [CrossRef]
- Jin, G.; Cai, Z.; Guan, Y.; Cui, X.; Liu, Z.; Li, Y.; Dong, M.; Zhang, D. High Temperature Wear Performance of Laser-Cladded FeNiCoAlCu High-Entropy Alloy Coating. Appl. Surf. Sci. 2018, 445, 113–122. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.L.; Yi, J.Z.; Zhang, C.H. Synthesis and Characterization of FeCoCrAlCu High-Entropy Alloy Coating by Laser Surface Alloying. Surf. Coat. Technol. 2015, 262, 64–69. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Q. MoFeCrTiWAlNb Refractory High-Entropy Alloy Coating Fabricated by Rectangular-Spot Laser Cladding. Intermetallics 2018, 102, 78–87. [Google Scholar] [CrossRef]
- Shu, F.; Yang, B.; Dong, S.; Zhao, H.; Xu, B.; Xu, F.; Liu, B.; He, P.; Feng, J. Effects of Fe-to-Co Ratio on Microstructure and Mechanical Properties of Laser Cladded FeCoCrBNiSi High-Entropy Alloy Coatings. Appl. Surf. Sci. 2018, 450, 538–544. [Google Scholar] [CrossRef]
- Shu, F.Y.; Wu, L.; Zhao, H.Y.; Sui, S.H.; Zhou, L.; Zhang, J.; He, W.X.; He, P.; Xu, B.S. Microstructure and High-Temperature Wear Mechanism of Laser Cladded CoCrBFeNiSi High-Entropy Alloy Amorphous Coating. Mater. Lett. 2018, 211, 235–238. [Google Scholar] [CrossRef]
- Juan, Y.F.; Li, J.; Jiang, Y.Q.; Jia, W.L.; Lu, Z.J. Modified Criterions for Phase Prediction in the Multi-Component Laser-Clad Coatings and Investigations into Microstructural Evolution/Wear Resistance of FeCrCoNiAlMox Laser-Clad Coatings. Appl. Surf. Sci. 2019, 465, 700–714. [Google Scholar] [CrossRef]
- Tüten, N.; Canadinc, D.; Motallebzadeh, A.; Bal, B. Microstructure and Tribological Properties of TiTaHfNbZr High Entropy Alloy Coatings Deposited on Ti–6Al–4V Substrates. Intermetallics 2019, 105, 99–106. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Vilar, R.; Shen, J. Dry Sliding Wear Behavior of Laser Clad TiVCrAlSi High Entropy Alloy Coatings on Ti-6Al-4V Substrate. Mater. Des. 2012, 41, 338–343. [Google Scholar] [CrossRef]
- Wang, X.R.; Wang, Z.Q.; He, P.; Lin, T.S.; Shi, Y. Microstructure and Wear Properties of CuNiSiTiZr High-Entropy Alloy Coatings on TC11 Titanium Alloy Produced by Electrospark—Computer Numerical Control Deposition Process. Surf. Coat. Technol. 2015, 283, 156–161. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, B.; Yu, Y.; Zhang, Z.; Zhu, S.; Lou, X.; Wang, Z. Study of High Temperature Friction and Wear Performance of (CoCrFeMnNi)85Ti15 High-Entropy Alloy Coating Prepared by Plasma Cladding. Surf. Coat. Technol. 2020, 384, 125337. [Google Scholar] [CrossRef]
- Wu, C.L.; Zhang, S.; Zhang, C.H.; Zhang, H.; Dong, S.Y. Phase Evolution and Properties in Laser Surface Alloying of FeCoCrAlCuNix High-Entropy Alloy on Copper Substrate. Surf. Coat. Technol. 2017, 315, 368–376. [Google Scholar] [CrossRef]
- Jin, B.; Zhang, N.; Guan, S.; Zhang, Y.; Li, D. Microstructure and Properties of Laser Re-Melting FeCoCrNiAl0.5Six High-Entropy Alloy Coatings. Surf. Coat. Technol. 2018, 349, 867–873. [Google Scholar] [CrossRef]
- Cheng, J.B.; Liang, X.B.; Xu, B.S. Effect of Nb Addition on the Structure and Mechanical Behaviors of CoCrCuFeNi High-Entropy Alloy Coatings. Surf. Coat. Technol. 2014, 240, 184–190. [Google Scholar] [CrossRef]
- Sha, C.; Zhou, Z.; Xie, Z.; Munroe, P. FeMnNiCoCr-Based High Entropy Alloy Coatings: Effect of Nitrogen Additions on Microstructural Development, Mechanical Properties and Tribological Performance. Appl. Surf. Sci. 2020, 507, 145101. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Sobol, O.V.; Beresnev, V.M.; Serdyuk, I.V.; Pogrebnyak, A.D.; Kolesnikov, D.A.; Nemchenko, U.S. Tribological Characteristics of (TiZrHfVNbTa)N Coatings Applied Using the Vacuum Arc Deposition Method. J. Frict. Wear 2014, 35, 359–364. [Google Scholar] [CrossRef]
- Ren, B.; Shen, Z.; Liu, Z. Structure and Mechanical Properties of Multi-Element (AlCrMnMoNiZr)N x Coatings by Reactive Magnetron Sputtering. J. Alloy. Compd. 2013, 560, 171–176. [Google Scholar] [CrossRef]
- Huang, P.K.; Yeh, J.W. Effects of Nitrogen Content on Structure and Mechanical Properties of Multi-Element (AlCrNbSiTiV)N Coating. Surf. Coat. Technol. 2009, 203, 1891–1896. [Google Scholar] [CrossRef]
- Kirnbauer, A.; Kretschmer, A.; Koller, C.M.; Wojcik, T.; Paneta, V.; Hans, M.; Schneider, J.M.; Polcik, P.; Mayrhofer, P.H. Mechanical Properties and Thermal Stability of Reactively Sputtered Multi-Principal-Metal Hf-Ta-Ti-V-Zr Nitrides. Surf. Coat. Technol. 2020, 389, 125674. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, X.H.; Liao, W.B.; Zhao, K. Effects of Nitrogen Content on the Structure and Mechanical Properties of (Al0.5CrFeNiTi0.25)Nx High-Entropy Films by Reactive Sputtering. Entropy 2018, 20, 624. [Google Scholar] [CrossRef] [PubMed]
- Tsai, D.C.; Huang, Y.L.; Lin, S.R.; Liang, S.C.; Shieu, F.S. Effect of Nitrogen Flow Ratios on the Structure and Mechanical Properties of (TiVCrZrY)N Coatings Prepared by Reactive Magnetron Sputtering. Appl. Surf. Sci. 2010, 257, 1361–1367. [Google Scholar] [CrossRef]
- Braic, V.; Parau, A.C.; Pana, I.; Braic, M.; Balaceanu, M. Effects of Substrate Temperature and Carbon Content on the Structure and Properties of (CrCuNbTiY)C Multicomponent Coatings. Surf. Coat. Technol. 2014, 258, 996–1005. [Google Scholar] [CrossRef]
- Braic, M.; Braic, V.; Balaceanu, M.; Zoita, C.N.; Vladescu, A.; Grigore, E. Characteristics of (TiAlCrNbY)C Films Deposited by Reactive Magnetron Sputtering. Surf. Coatings Technol. 2010, 204, 2010–2014. [Google Scholar] [CrossRef]
- Braic, M.; Balaceanu, M.; Vladescu, A.; Zoita, C.N.; Braic, V. Deposition and Characterization of Multi-Principal-Element (CuSiTiYZr)C Coatings. Appl. Surf. Sci. 2013, 284, 671–678. [Google Scholar] [CrossRef]
- Cheng, K.H.; Lai, C.H.; Lin, S.J.; Yeh, J.W. Structural and Mechanical Properties of Multi-Element (AlCrMoTaTiZr)N x Coatings by Reactive Magnetron Sputtering. Thin Solid Films 2011, 519, 3185–3190. [Google Scholar] [CrossRef]
- Lai, C.H.; Cheng, K.H.; Lin, S.J.; Yeh, J.W. Mechanical and Tribological Properties of Multi-Element (AlCrTaTiZr)N Coatings. Surf. Coatings Technol. 2008, 202, 3732–3738. [Google Scholar] [CrossRef]
- Liu, X.; Yin, H.; Xu, Y. Microstructure, Mechanical and Tribological Properties of Oxide Dispersion Strengthened High-Entropy Alloys. Materials 2017, 10, 1312. [Google Scholar] [CrossRef]
- Xu, H.; Zang, J.; Yuan, Y.; Zhou, Y.; Tian, P.; Wang, Y. In-Situ Assembly from Graphene Encapsulated CoCrFeMnNi High-Entropy Alloy Nanoparticles for Improvement Corrosion Resistance and Mechanical Properties in Metal Matrix Composites. J. Alloy. Compd. 2019, 811, 152082. [Google Scholar] [CrossRef]
- Meng, G.; Lin, X.; Xie, H.; Wang, C.; Wang, S.; Ding, X. Reinforcement and Substrate Interaction in Laser Surface Forming of AlCoCrCuFeNi Particle Reinforced AZ91D Matrix Composites. J. Alloy. Compd. 2016, 672, 660–667. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, N.; Du, G.; Zhang, M.; Zhao, X.; Cheng, H.; Wu, J. High-Temperature Oxidation Resistance, Mechanical and Wear Resistance Properties of Ti(C,N)-Based Cermets with Al0.3CoCrFeNi High-Entropy Alloy as a Metal Binder. J. Alloy. Compd. 2020, 815, 152486. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, J.; Guo, Z.; Yang, T.; Liu, J.; Chai, B. The Microstructure and Properties of Novel Ti(C,N)-Based Cermets with Multi-Component CoCrFeNiCu High-Entropy Alloy Binders. Mater. Sci. Eng. A 2019, 766, 138345. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, S.; Xu, Y. Microstructure and Tribological Performance of Fe50Mn30Co10Cr10 High-Entropy Alloy Based Self-Lubricating Composites. Mater. Lett. 2018, 233, 142–145. [Google Scholar] [CrossRef]
- Zhang, A.; Han, J.; Su, B.; Li, P.; Meng, J. Microstructure, Mechanical Properties and Tribological Performance of CoCrFeNi High Entropy Alloy Matrix Self-Lubricating Composite. Mater. Des. 2017, 114, 253–263. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, W.; Li, T.; Zhang, M.; Liu, B.; Liu, Y.; Wang, L.; Hu, S. Effect of WC Content on Microstructures and Mechanical Properties of FeCoCrNi High-Entropy Alloy/WC Composite Coatings by Plasma Cladding. Surf. Coat. Technol. 2020, 385, 125326. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Liu, B.; Yi, D.; Yang, X.; Zhang, W.; Chen, G.; Liu, Y.; Bai, P. Influence of NbC Particles on Microstructure and Mechanical Properties of AlCoCrFeNi High-Entropy Alloy Coatings Prepared by Laser Cladding. J. Alloy. Compd. 2019, 788, 485–494. [Google Scholar] [CrossRef]
- Guo, Y.; Shang, X.; Liu, Q. Microstructure and Properties of In-Situ TiN Reinforced Laser Cladding CoCr2FeNiTix High-Entropy Alloy Composite Coatings. Surf. Coat. Technol. 2018, 344, 353–358. [Google Scholar] [CrossRef]
- Meng, G.H.; Lin, X.; Xie, H.; Yue, T.M.; Ding, X.; Sun, L.; Qi, M. The Effect of Cu Rejection in Laser Forming of AlCoCrCuFeNi/Mg Composite Coating. Mater. Des. 2016, 108, 157–167. [Google Scholar] [CrossRef]
- Finnie, I. Some Reflections on the Past and Future of Erosion. Wear 1995, 186–187, 1–10. [Google Scholar] [CrossRef]
- Ji, X.; Ji, C.; Cheng, J.; Shan, Y.; Tian, S. Erosive Wear Resistance Evaluation with the Hardness after Strain-Hardening and Its Application for a High-Entropy Alloy. Wear 2018, 398–399, 178–182. [Google Scholar] [CrossRef]
- Rajahram, S.S.; Harvey, T.J.; Wood, R.J.K. Evaluation of a Semi-Empirical Model in Predicting Erosion-Corrosion. Wear 2009, 267, 1883–1893. [Google Scholar] [CrossRef]
- Hong, S.; Wu, Y.; Zhang, J.; Zheng, Y.; Zheng, Y.; Lin, J. Synergistic Effect of Ultrasonic Cavitation Erosion and Corrosion of WC-CoCr and FeCrSiBMn Coatings Prepared by HVOF Spraying. Ultrason. Sonochemistry 2016, 31, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, B. Tribology—Friction and Wear of Engineering Materials. Tribol. Int. 1992, 25, 357. [Google Scholar] [CrossRef]
- Siddhartha; Bisht, R. A Modified Approach for Better Prediction of Erosion Wear of Materials: Redefining the Paradigms. Mater. Des. 2013, 47, 395–407. [Google Scholar] [CrossRef]
- Karlsdottir, S.N.; Csaki, I.; Antoniac, I.V.; Manea, C.A.; Stefanoiu, R.; Magnus, F.; Miculescu, F. Corrosion Behavior of AlCrFeNiMn High Entropy Alloy in a Geothermal Environment. Geothermics 2019, 81, 32–38. [Google Scholar] [CrossRef]
- Moore, M.A. The Relationship between the Abrasive Wear Resistance, Hardness and Microstructure of Ferritic Materials. Wear 1974, 28, 59–68. [Google Scholar] [CrossRef]
- Nair, R.B.; Arora, H.S.; Ayyagari, A.; Mukherjee, S.; Grewal, H.S. High Entropy Alloys: Prospective Materials for Tribo-Corrosion Applications. Adv. Eng. Mater. 2018, 20, 1700946. [Google Scholar] [CrossRef]
- Zhao, J.H.; Ji, X.L.; Shan, Y.P.; Fu, Y.; Yao, Z. On the Microstructure and Erosion–Corrosion Resistance of AlCrFeCoNiCu High-Entropy Alloy via Annealing Treatment. Mater. Sci. Technol. 2016, 32, 1271–1275. [Google Scholar] [CrossRef]
- Lee, C.P.; Chang, C.C.; Chen, Y.Y.; Yeh, J.W.; Shih, H.C. Effect of the Aluminium Content of AlxCrFe1.5MnNi0.5 High-Entropy Alloys on the Corrosion Behaviour in Aqueous Environments. Corros. Sci. 2008, 50, 2053–2060. [Google Scholar] [CrossRef]
- Tung, C.C.; Yeh, J.W.; Shun, T.t.; Chen, S.K.; Huang, Y.S.; Chen, H.C. On the Elemental Effect of AlCoCrCuFeNi High-Entropy Alloy System. Mater. Lett. 2007, 61, 1–5. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, D.; Maulik, O.; Pradhan, A.K.; Kumar, V.; Patniak, A. Synthesis and Air Jet Erosion Study of AlXFe1.5CrMnNi0.5 (x = 0.3, 0.5) High-Entropy Alloys. Metall. Mater. Trans. A 2018, 49, 5607–5618. [Google Scholar] [CrossRef]
- Srivastava, M.; Jadhav, M.; Chethan; Chakradhar, R.P.S.; Muniprakash, M.; Singh, S. Synthesis and Properties of High Velocity Oxy-Fuel Sprayed FeCoCrNi2Al High Entropy Alloy Coating. Surf. Coat. Technol. 2019, 378, 124950. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, A.; Ji, X.; Jiang, J.; Bao, Y. Slurry Erosion Behavior of AlxCoCrFeNiTi0.5 High-Entropy Alloy Coatings Fabricated by Laser Cladding. Metals 2018, 8, 126. [Google Scholar] [CrossRef]
- Lee, C.P.; Chen, Y.Y.; Hsu, C.Y.; Yeh, J.W.; Shih, H.C. Enhancing Pitting Corrosion Resistance of AlxCrFe1.5MnNi0.5 High-Entropy Alloys by Anodic Treatment in Sulfuric Acid. Thin Solid Films 2008, 517, 1301–1305. [Google Scholar] [CrossRef]
- Ji, X.; Duan, H.; Zhang, H.; Ma, J. Slurry Erosion Resistance of Laser Clad NiCoCrFeAl3 High-Entropy Alloy Coatings. Tribol. Trans. 2015, 58, 1119–1123. [Google Scholar] [CrossRef]
- Fan, A.; Long, J.; Tao, Z. An Investigation of the Corrosive Wear of Stainless Steels in Aqueous Slurries. Wear 1996, 193, 73–77. [Google Scholar] [CrossRef]
- Matsumura, M.; Oka, Y.; Hiura, H.; Yano, M. The Role of Passivating Film in Preventing Slurry Erosion-Corrosion of Austenitic Stainless Steel. ISIJ Int. 1991, 31, 168–176. [Google Scholar] [CrossRef]
- Cheng, H.; Pan, Z.; Fu, Y.; Wang, X.; Wei, Y.; Luo, H.; Li, X. Review—Corrosion-Resistant High-Entropy Alloy Coatings: A Review. J. Electrochem. Soc. 2021, 168, 111502. [Google Scholar] [CrossRef]

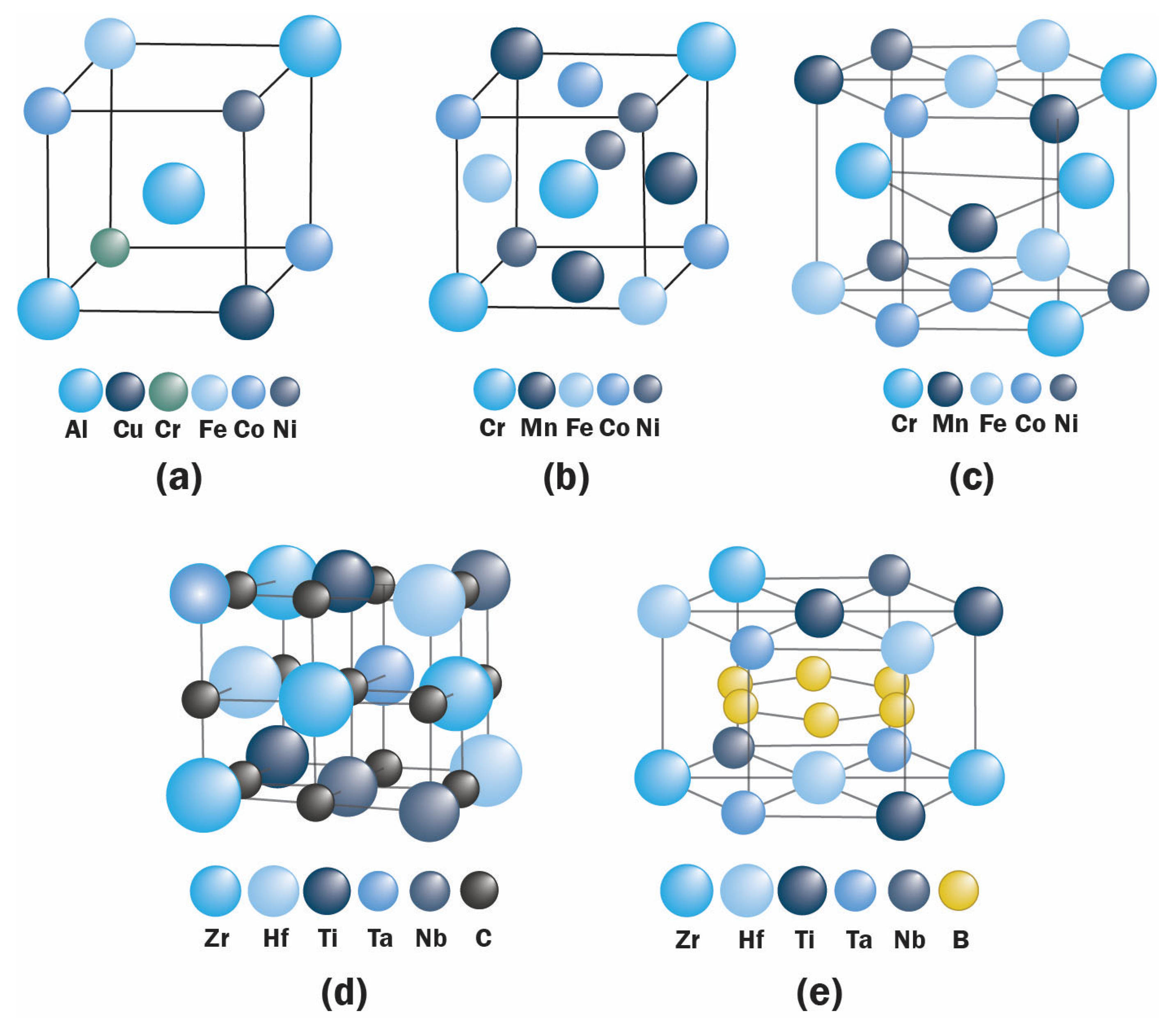






| Preparation Method | Coating Composition | Coating Microstructure | Substrate | Temperature | COF | Ref. |
|---|---|---|---|---|---|---|
| Laser cladding | FeNiCoAlCu | BCC | AISI 1045 | RT | 0.8–0.9 | [136] |
| 600 °C | 0.3 | |||||
| Laser cladding | FeCoCrAlCu | BCC | Q235 | RT | 0.87 | [137] |
| Laser cladding | MoFeCrTiWAlNb | BCC + (Nb,Ti)C | M2 steel | RT | 0.5–0.6 | [138] |
| Laser cladding | TiVCrAlSi | BCC + (Ti,V)5Si3 | Ti-6Al-4V | RT | ~0.3 | [143] |
| Magnetron sputtering | TiTaHfNbZr | Amorphous | Ti-6Al-4V | RT | 0.1–0.2 | [142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feltrin, A.C.; Xing, Q.; Akinwekomi, A.D.; Waseem, O.A.; Akhtar, F. Review of Novel High-Entropy Protective Materials: Wear, Irradiation, and Erosion Resistance Properties. Entropy 2023, 25, 73. https://doi.org/10.3390/e25010073
Feltrin AC, Xing Q, Akinwekomi AD, Waseem OA, Akhtar F. Review of Novel High-Entropy Protective Materials: Wear, Irradiation, and Erosion Resistance Properties. Entropy. 2023; 25(1):73. https://doi.org/10.3390/e25010073
Chicago/Turabian StyleFeltrin, Ana C., Qiuwei Xing, Akeem Damilola Akinwekomi, Owais Ahmed Waseem, and Farid Akhtar. 2023. "Review of Novel High-Entropy Protective Materials: Wear, Irradiation, and Erosion Resistance Properties" Entropy 25, no. 1: 73. https://doi.org/10.3390/e25010073
APA StyleFeltrin, A. C., Xing, Q., Akinwekomi, A. D., Waseem, O. A., & Akhtar, F. (2023). Review of Novel High-Entropy Protective Materials: Wear, Irradiation, and Erosion Resistance Properties. Entropy, 25(1), 73. https://doi.org/10.3390/e25010073







