Kinetics of Precipitation Processes at Non-Zero Input Fluxes of Segregating Particles
Abstract
1. Introduction
2. Precipitation Kinetics at Non-Zero Input Fluxes of Segregating Particles: Numerical Computations
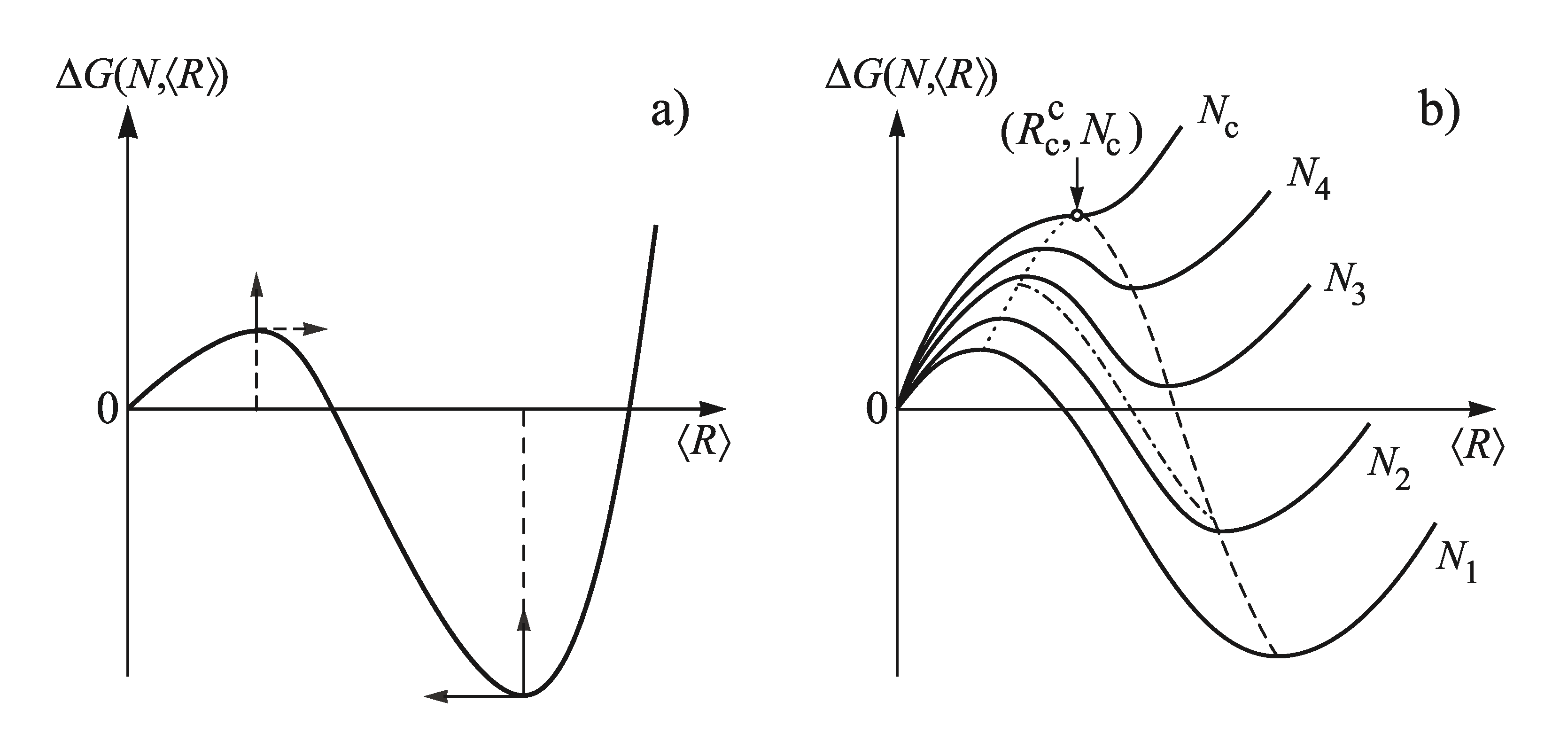
2.1. Some General Considerations
2.2. Basic Kinetic Equations
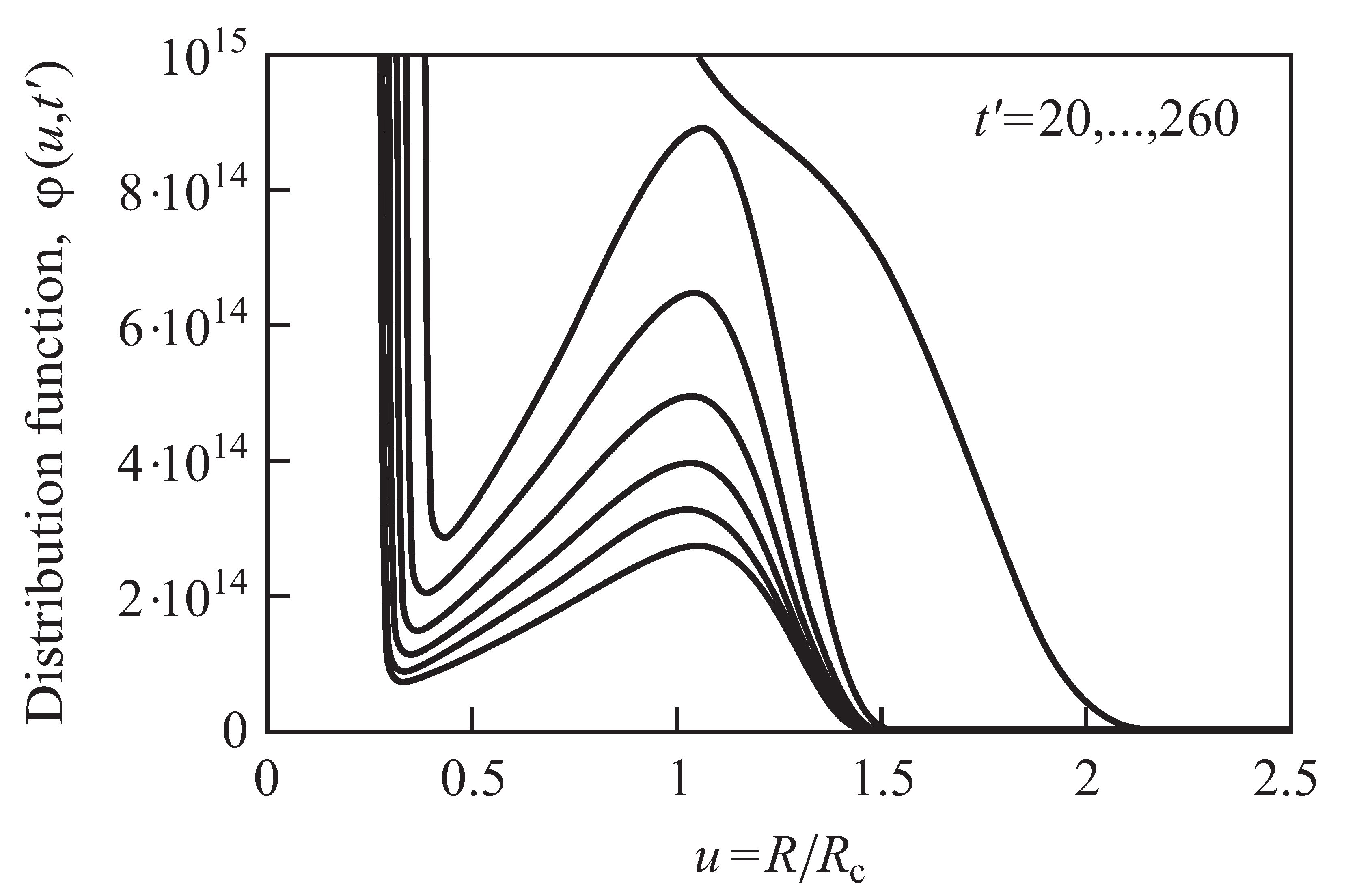
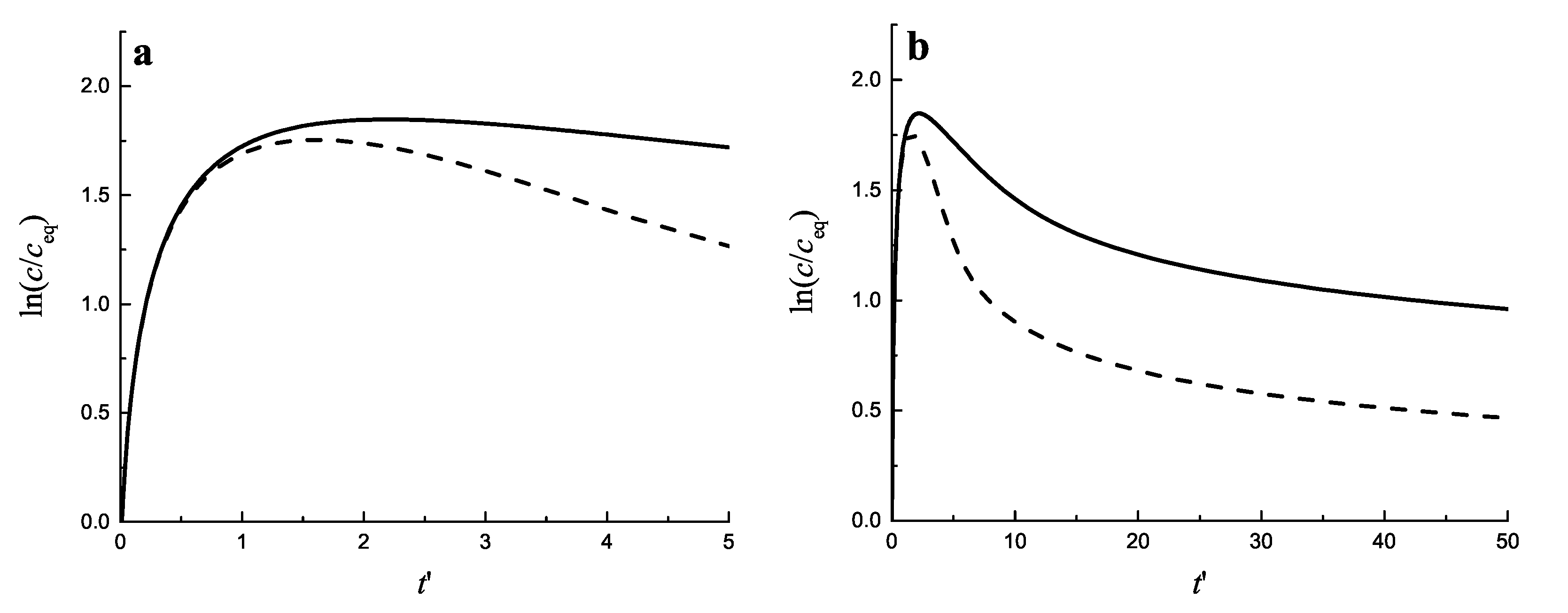
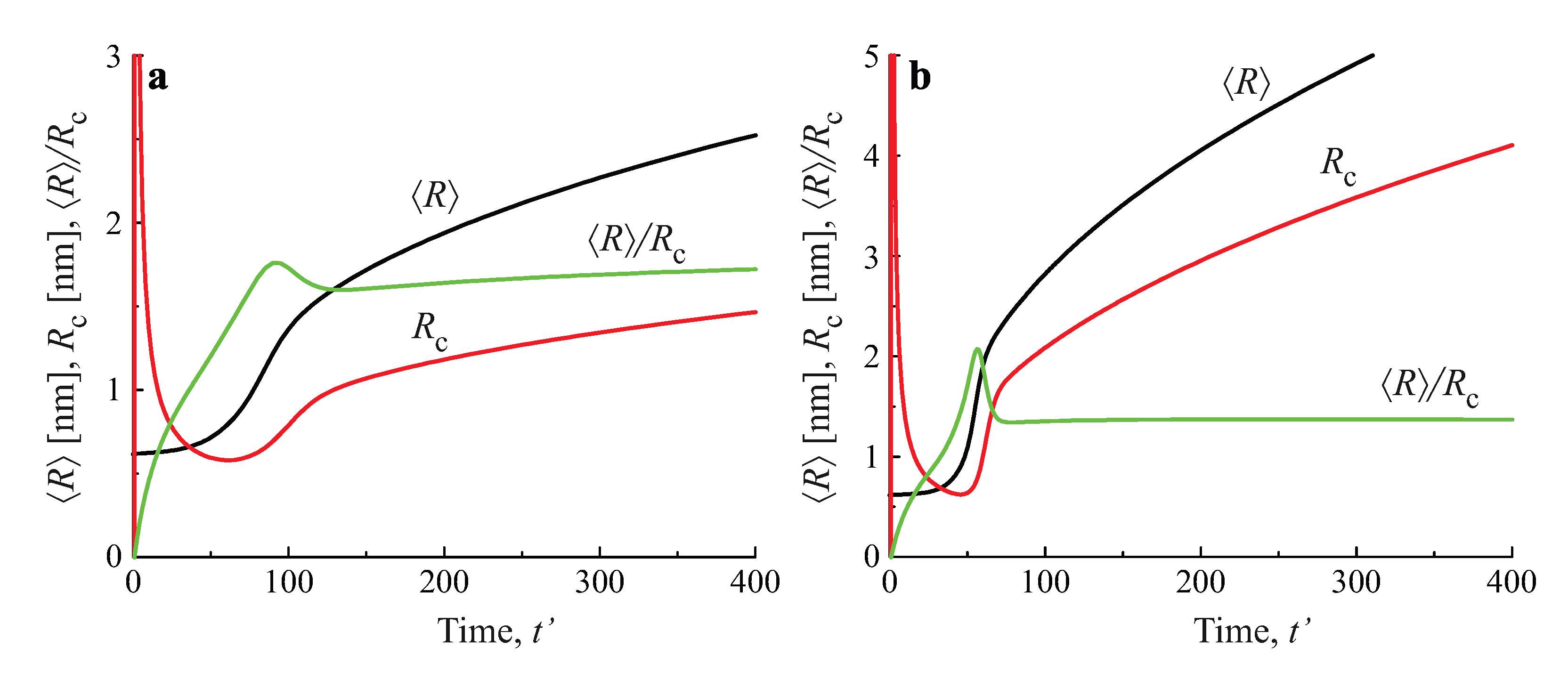
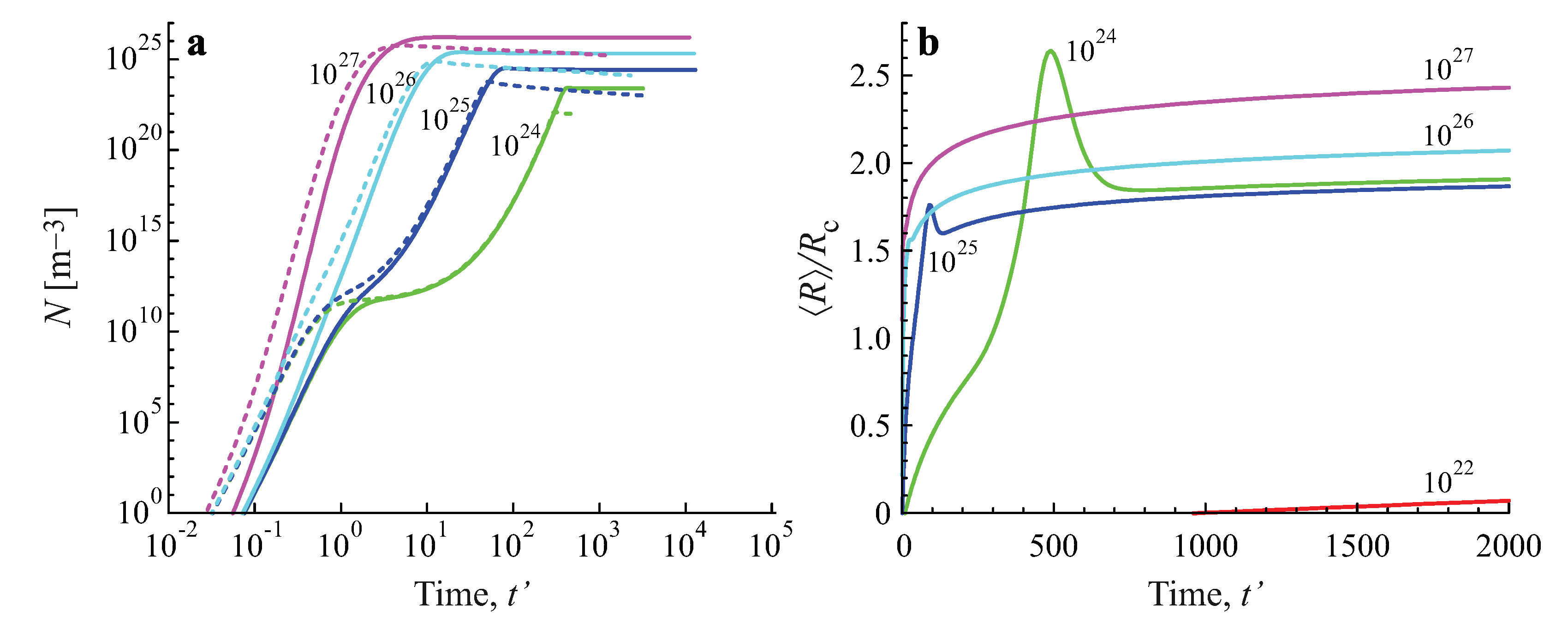
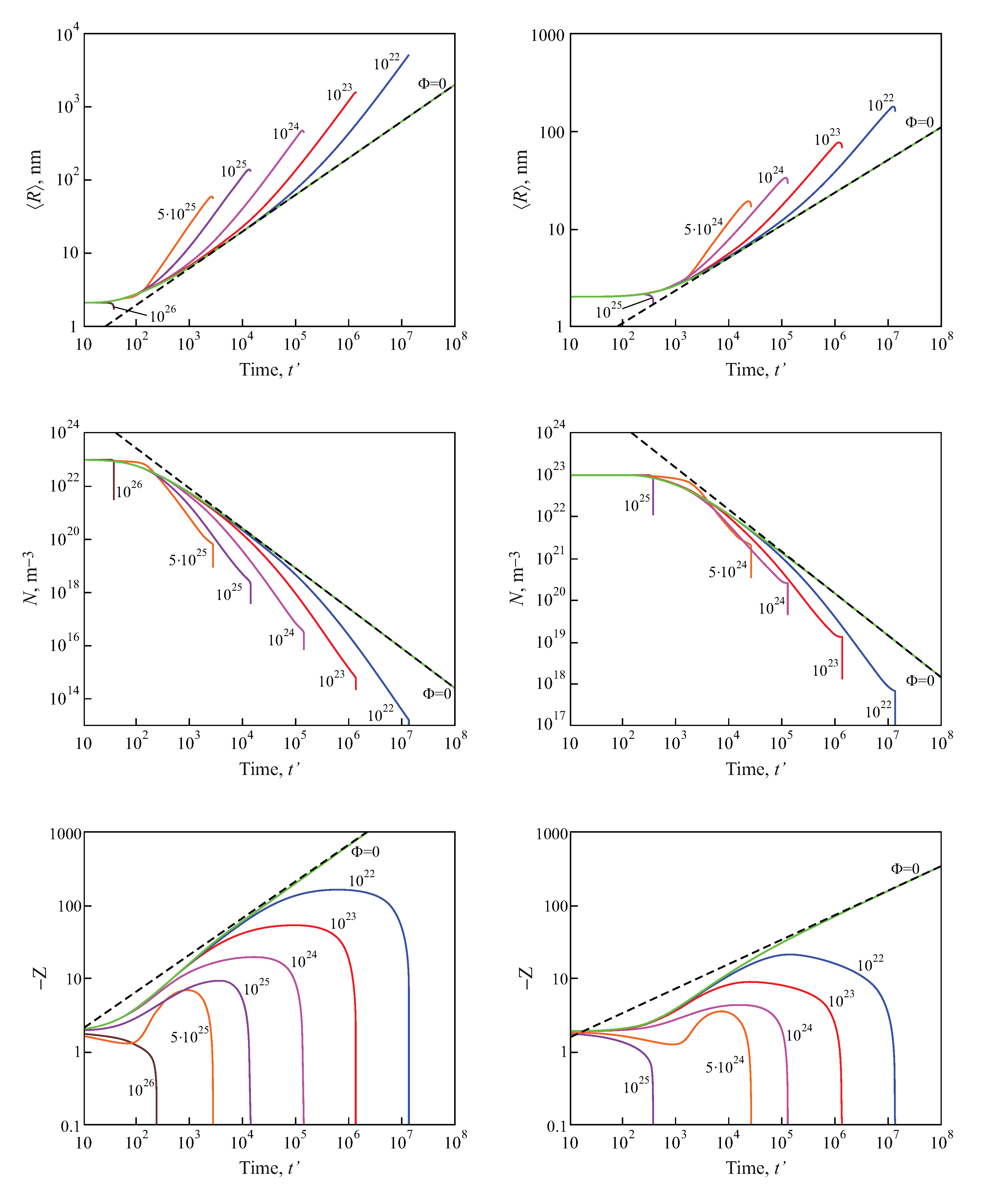
2.3. Results of the Numerical Solution of the Kinetic Equations
3. Theoretical Analysis
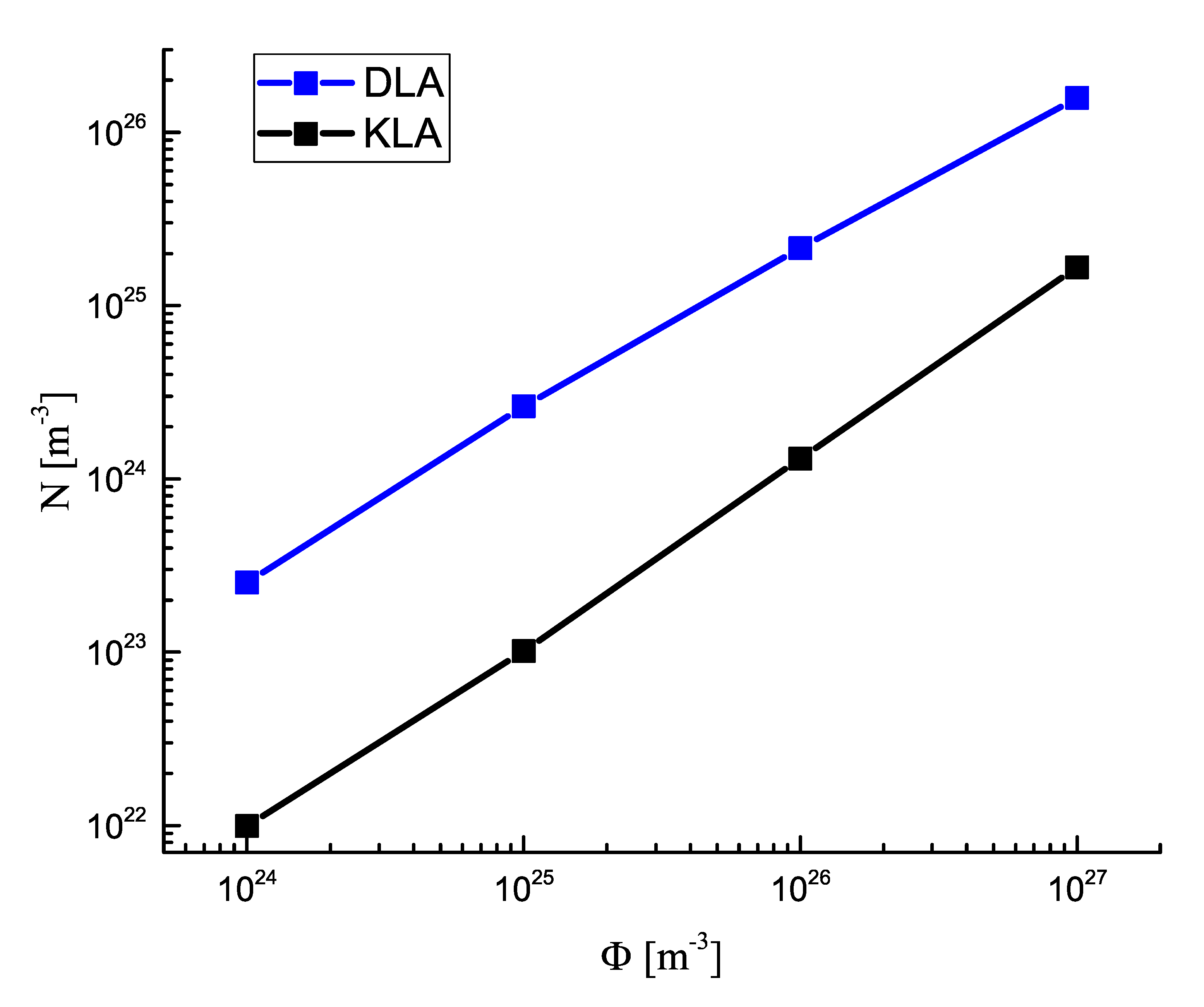
3.1. Number of Clusters in Dependence on the Rate of Input Fluxes of the Segregating Component
3.2. Coarsening in Closed Systems: Lifshitz-Slezov-Wagner−Approach
3.3. Coarsening at Constant Input Fluxes of the Segregating Component: Alternative Approach
3.3.1. Basic Ideas
3.3.2. Derivation of the Kinetic Equations Modeling Coarsening in a Closed System
3.3.3. Account of Additional Factors like Elastic Stresses on Coarsening
3.3.4. Application to Coarsening at Non-Zero Input Fluxes of Segregating Particles
3.3.5. Possible Further Applications of the Method: General Theoretical Approach to the Description of Coarsening in Open Systems and at Time-Dependent Boundary Conditions
4. Summary of Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schick, C.; Mathot, V. (Eds.) Fast Scanning Calorimetry; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Van Westen, T.; Groot, R.D. Effect of Temperature Cycling on Ostwald Ripening. Cryst. Growth Des. 2018, 18, 4952–4962. [Google Scholar] [CrossRef] [PubMed]
- Shirinyan, A.S.; Bilogorodskyy, Y.S.; Wilde, G.; Schmelzer, J.W.P. Size-Dependent Hysteresis and Phase Formation Kinetics during Temperature Cycling of Metal Nanopowders. J. Phys. Condens. Matter 2011, 23, 245301. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Groot, R.D.; McCartney, G.; Guo, E.; Bent, J.; van Dalen, G.; Schuetz, P.; Rockett, P.; Lee, P.D. Ice Crystal Coarsening in Ice Cream during Cooling: A Comparison of Theory and Experiment. Crystals 2019, 9, 321. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Röpke, G.; Ludwig, F.-P. Nuclear Multi-Fragmentation Processes and Nucleation Theory. Phys. Rev. 1997, C 55, 1917–1927. [Google Scholar]
- Schmelzer, J.W.P.; Labudde, D.; Röpke, G. Nucleation and Growth in Freely Expanding Gases. Physica 1998, A 254, 389–410. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P. (under participation of Slezov, V.V.; Gutzow, I.S.; Todorova, S.; Schmelzer, J., Jr.); Bubble Formation and Growth in Viscous Liquids; Project Report for BASF Ludwigshafen: Ludwigshafen, Germany, 1998; 203p. [Google Scholar]
- Ulbricht, H.; Schmelzer, J.; Mahnke, R.; Schweitzer, F. Thermodynamics of Finite Systems and the Kinetics of First-Order Phase Transitions; Teubner: Leipzig, Germany, 1988. [Google Scholar]
- Schmelzer, J.W.P.; Abyzov, A.S. Thermodynamic analysis of nucleation in confined space: Generalized Gibbs approach. J. Chem. Phys. 2011, 134, 054511. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Abyzov, A.S. Comments on the thermodynamic analysis of nucleation in confined space. J. Non-Cryst. Solids 2014, 384, 2–7. [Google Scholar] [CrossRef]
- Leubner, I.H. Crystal formation (nucleation) in the presence of growth restrainers. J. Crystal Growth 1987, 84, 496–502. [Google Scholar] [CrossRef]
- Leubner, I.H. Crystal formation (nucleation) under kinetically-controlled and diffusion-controlled growth conditions. J. Phys. Chem. 1987, 91, 6069–6073. [Google Scholar] [CrossRef]
- Ludwig, F.-P.; Schmelzer, J.W.P. Cluster Formation and Growth in Segregation Processes with Constant Rates of Supply of Monomers. Z. Phys. Chem. 1995, 192, 155–167. [Google Scholar] [CrossRef]
- Slezov, V.V. Kinetics of First-Order Phase Transitions; WILEY-VCH: Berlin, Germany, 2009. [Google Scholar]
- Beenakker, C.W.J.; Ross, J. Theory of Ostwald ripening for open systems. J. Chem. Phys. 1985, 83, 4710–4714. [Google Scholar] [CrossRef]
- Park, J.; Joo, J.; Kwon, S.G.; Jang, Y.; Hyeon, T. Synthesis of Monodisperse Spherical Nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 4630–4660. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Sugimoto, T. Underlying mechanisms in size control of uniform nanoparticles. J. Colloid Interface Sci. 2007, 309, 106–118. [Google Scholar] [CrossRef]
- Floris, E.; Piras, A.; Pezzicoli, F.S.; Zamparo, M.; Dall’Asta, L.; Gamba, A. Phase separation and critical size in molecular sorting. Phys. Rev. 2022, E 106, 044412. [Google Scholar] [CrossRef]
- Slezov, V.V.; Schmelzer, J.W.P. Kinetics of Nucleation-Growth Processes: The First Stages. In Nucleation Theory and Applications; Schmelzer, J.W.P., Röpke, G., Priezzhev, V.B., Eds.; Workshop Proceedings 1997–1999; Joint Institute for Nuclear Research Publishing Department: Dubna, Russia, 1999; pp. 6–81. [Google Scholar]
- Schmelzer, J.W.P.; Röpke, G.; Schmelzer, J., Jr.; Slezov, V.V. Shapes of Cluster Size Distributions Evolving in Nucleation-Growth Processes. In Nucleation Theory and Applications; Schmelzer, J.W.P., Röpke, G., Priezzhev, V.B., Eds.; Workshop Proceedings 1997–1999; Joint Institute for Nuclear Research Publishing Department: Dubna, Russia, 1999; pp. 82–129. [Google Scholar]
- Schmelzer, J., Jr.; Lembke, U.; Kranold, R. Nucleation and growth of AgCl clusters in a sodium borate glass: Numerical analysis and SAXS results. J. Chem. Phys. 2000, 113, 1268–1275. [Google Scholar] [CrossRef]
- Slezov, V.V.; Schmelzer, J.W.P. Kinetics of Formation of a New Phase with an Arbitrary Stoichiometric Composition in a Multi-Component Solid Solution. Phys. Rev. 2002, E 65, 031506. [Google Scholar]
- Lifshitz, I.M.; Slezov, V.V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Slezov, V.V.; Sagalovich, V.V. Diffusive decomposition of solid solutions. Usphekhi Fiz. Nauk (UFN) 1987, 151, 67–104. [Google Scholar] [CrossRef]
- Davydov, L.N.; Kukushkin, S.A. International Conference “Mechanisms and Nonlinear Problems of Nucleation and Growth of Crystals and Thin Films”, Dedicated to the Memory of Professor V. V. Slezov, an Outstanding Theoretical Physicist. Phys. Solid State 2019, 61, 2249–2253. [Google Scholar] [CrossRef]
- Gutzow, I.S.; Schmelzer, J.W.P. The Vitreous State: Thermodynamics, Structure, Rheology, and Crystallization; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Gutzow, I.S.; Schmelzer, J.W.P. The Vitreous State: Thermodynamics, Structure, Rheology, and Crystallization, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Wagner, C. Theorie der Alterung von Niederschlägen durch Umlösen (Ostwald-Reifung) (Engl.: Theory of coarsening of precipitates by redissolution (Ostwald-ripening)). Z. für Elektrochem. Berichte der Bunsenges. für Phys. Chem. 1961, 65, 581–591. [Google Scholar]
- Kelton, K.F.; Greer, A.L.; Thompson, C.V. Transient nucleation in condensed systems. J. Chem. Phys. 1983, 79, 6261–6276. [Google Scholar] [CrossRef]
- Bartels, J.; Lembke, U.; Pascova, R.; Schmelzer, J.W.P.; Gutzow, I. Evolution of Cluster Size Distributions in Nucleation and Growth Processes. J. Non-Cryst. Solids 1991, 136, 181–197. [Google Scholar] [CrossRef]
- Abyzov, A.S.; Schmelzer, J.W.P.; Fokin, V.M.; Zanotto, E.D. Crystallization of supercooled liquids: Self-consistency correction of the steady-state nucleation rate. Entropy 2020, 22, 558. [Google Scholar] [CrossRef] [PubMed]
- Slezov, V.V.; Schmelzer, J.W.P.; Abyzov, A.S. A New Method of Determination of the Coefficients of Emission in Nucleation Theory. In Nucleation Theory and Applications; Schmelzer, J.W.P., Ed.; WILEY-VCH Publishers: Berlin/Weinheim, Germany, 2005; pp. 39–73. [Google Scholar]
- Skripov, V.P.; Koverda, V.P. Spontaneous Crystallization of Undercooled Liquids; Nauka: Moscow, Russia, 1984. (In Russian) [Google Scholar]
- Schmelzer, J.W.P.; Schmelzer, J., Jr. Kinetics of Nucleation at Increasing Supersaturation. J. Colloid Interface Sci. 1999, 215, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Kashchiev, D.; Borissova, A.; Hammond, R.B.; Roberts, K.J. Effect of cooling rate on the critical undercooling for crystallization. J. Cryst. Growth 2010, 312, 698–704. [Google Scholar] [CrossRef]
- Yang, B.; Abyzov, A.S.; Zhuravlev, E.; Gao, Y.; Schmelzer, J.W.P.; Schick, C. Size and rate dependence of crystal nucleation in single tin drops by fast scanning calorimetry. J. Chem. Phys. 2013, 138, 054501. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Perepezko, J.H.; Schmelzer, J.W.P.; Gao, Y.; Schick, C. Dependence of crystal nucleation on prior liquid overheating by differential fast scanning calorimeter. J. Chem. Phys. 2014, 140, 104513. [Google Scholar] [CrossRef]
- Tanaka, K.K.; Kimura, Y. Theoretical Analysis of Crystallization by Homogeneous Nucleation of Water Droplets. Phys. Chem. Chem. Phys. 2019, 21, 2410–2418. [Google Scholar] [CrossRef]
- Deubener, J.; Schmelzer, J.W.P. Statistical Approach to Crystal Nucleation in Glass-forming Liquids. Entropy 2021, 23, 246. [Google Scholar] [CrossRef]
- Todorova, S.; Gutzow, I.; Schmelzer, J.W.P. Kinetics of nucleation at increasing or decreasing supersaturation. In Nucleation Theory and Applications; Schmelzer, J.W.P., Röpke, G., Priezzhev, V.B., Eds.; Workshop Proceedings 2000–2002; Joint Institute for Nuclear Research Publishing Department: Dubna, Russia, 2002; pp. 215–233. [Google Scholar]
- Schmelzer, J.W.P.; Abyzov, A.S.; Ferreira, E.B.; Fokin, V.M. Curvature dependence of the surface tension and crystal nucleation in liquids. Int. J. Appl. Glass Sci. 2019, 10, 57–60. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Abyzov, A.S.; Baidakov, V.G. Entropy and the Tolman Parameter in Nucleation Theory. Entropy 2019, 21, 670. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, J.W.P.; Tropin, T.V. Theory of Crystal Nucleation of Glass-forming Liquids: Some New Developments. Int. J. Appl. Glass Sci. 2022, 13, 171–198. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Abyzov, A.S.; Fokin, V.M.; Schick, C.; Zanotto, E.D. Crystallization in glass-forming liquids: Maxima of nucleation, growth, and overall crystallization rates. J. Non-Cryst. Solids 2015, 429, 24–32. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Abyzov, A.S. Pressure-induced crystallization of liquids: Maxima of nucleation, growth, and overall crystallization rates. Int. J. Appl. Glass Sci. 2018, 9, 198–207. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Abyzov, A.S.; Baidakov, V.G. Time of formation of the first supercritical nucleus, time-lag, and the steady-state nucleation rate. Int. J. Appl. Glass Sci. 2017, 8, 48–60. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P. Zur Kinetik des Keimwachstums in Lösungen (Engl.: On the kinetics of cluster growth in solutions). Z. Phys. Chem. (Leipzig) 1985, 266, 1057–1070. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P. Zur Kinetik des Wachstums von Tropfen in der Gasphase (Engl.: On the kinetics of growth of droplets in the vapor phase). Z. Phys. Chem. (Leipzig) 1985, 266, 1121–1134. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Gutzow, I. On the Kinetic Description of Ostwald Ripening in Elastic Media. Z. Phys. Chem. (Leipzig) 1988, 269, 753–767. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Pascova, R.; Gutzow, I. Cluster Growth and Ostwald Ripening in Viscoelastic Media. Phys. Status Solidi 1990, A 117, 363–375. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Pascova, R.; Gutzow, I. Kinetics of Phase Segregation in Elastic and Viscoelastic Media. J. Cryst. Growth 1990, 104, 505–520. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P. On the Kinetics of Decomposition of Dilute Al-(Zn, Mg) Alloys. Phys. Status Solidi 1990, B 161, 173–177. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Schweitzer, F. Ostwald Ripening of Bubbles in Liquid-Gas Solutions. J. Non-Equilib. Thermodyn. 1987, 12, 255–270. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Schweitzer, F. On the Kinetic Description of Condensation in Binary Vapours. Ann. der Phys. 1987, 44, 283–296. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Ulbricht, H. Thermodynamics of Finite Systems and the Kinetics of First-Order Phase Transitions. J. Colloid Interface Sci. 1987, 117, 325–338. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P. Formation and Growth of Bubbles in One-Component Closed Isochoric Systems. Z. Phys. Chem. (Leipzig) 1988, 269, 633–646. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Ulbricht, H. Kinetics of First-Order Phase Transitions in Adiabatic Systems. J. Colloid Interface Sci. 1989, 128, 104–114. [Google Scholar] [CrossRef]
- Kawasaki, K.; Enomoto, Y. Statistical theory of Ostwald ripening with elastic field interaction. Physica 1988, A 150, 463–498. [Google Scholar] [CrossRef]
- Enomoto, Y.; Kawasaki, K. Computer simulation of coarsening with elastic field interactions. Acta Metall. 1989, 37, 1399–1406. [Google Scholar] [CrossRef]
- Lifshitz, I.M.; Slezov, V.V. The kinetics of precipitation from solid solutions. Solid State Phys. (Fizika Tverd. Tela) 1959, 1, 1401–1407. [Google Scholar]
- Gibbs, J.W. On the Equilibrium of Heterogeneous Substances. Trans. Conn. Acad. Arts Sci. 1878, 3, 108–248, 343–524, reprinted in: Gibbs, J.W. The Collected Works; Volume 1, Thermodynamics; Longmans & Green: New York, NY, USA; London, UK; Toronto, ON, Canada, 1928. [Google Scholar]
- Rusanov, A.I. Phasengleichgewichte und Grenzflächenerscheinungen (English: Phase Equilibria and Surface Phenomena); Akademie-Verlag: Berlin, Germany, 1978. [Google Scholar]
- Rusanov, A.I. My Discoveries (A Review). Russ. J. Gen. Chem. 2022, 92, 539–583. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Boltachev, G.S.; Baidakov, V.G. Classical and Generalized Gibbs’ Approaches and the Work of Critical Cluster Formation in Nucleation Theory. J. Chem. Phys. 2006, 124, 194503. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, J.W.P.; Abyzov, A.S. Crystallization of glass-forming liquids: Thermodynamic driving force. J. Non-Cryst. Solids 2016, 449, 41–49. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Tropin, T.V.; Fokin, V.M.; Abyzov, A.S.; Zanotto, E.D. Effects of Glass Transition and Structural Relaxation on Crystal Nucleation: Theoretical Description and Model Analysis. Entropy 2020, 22, 1098. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Möller, J. Evolution of Cluster Size Distribution Function for Ostwald Ripening in Viscoelastic Media. J. Phase Transitions 1992, 38, 261–272. [Google Scholar] [CrossRef]
- Slezov, V.V.; Schmelzer, J.W.P.; Möller, J. Ostwald Ripening in Porous Materials. J. Cryst. Growth 1993, 132, 419–426. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Möller, J.; Slezov, V.V. Ostwald Ripening in Porous Materials: The Case of Arbitrary Pore Size Distributions. J. Phys. Chem. Solids 1995, 56, 1013–1022. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Möller, J.; Slezov, V.V.; Gutzow, I.; Pascova, R. Ostwald Ripening in Porous Materials. Quim. Nova 1998, 21, 529–533. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmelzer, J.W.P.; Tropin, T.V.; Abyzov, A.S. Kinetics of Precipitation Processes at Non-Zero Input Fluxes of Segregating Particles. Entropy 2023, 25, 329. https://doi.org/10.3390/e25020329
Schmelzer JWP, Tropin TV, Abyzov AS. Kinetics of Precipitation Processes at Non-Zero Input Fluxes of Segregating Particles. Entropy. 2023; 25(2):329. https://doi.org/10.3390/e25020329
Chicago/Turabian StyleSchmelzer, Jürn W. P., Timur V. Tropin, and Alexander S. Abyzov. 2023. "Kinetics of Precipitation Processes at Non-Zero Input Fluxes of Segregating Particles" Entropy 25, no. 2: 329. https://doi.org/10.3390/e25020329
APA StyleSchmelzer, J. W. P., Tropin, T. V., & Abyzov, A. S. (2023). Kinetics of Precipitation Processes at Non-Zero Input Fluxes of Segregating Particles. Entropy, 25(2), 329. https://doi.org/10.3390/e25020329









