Entropy Production and Filling Time in Hydrogen Refueling Stations: An Economic Assessment
Abstract
:1. Introduction
- To include entropy production in a high-fidelity thermodynamic model of a hydrogen refueling station.
- To minimize the filling time of the HRS system under entropy production using optimal control and to compare it with SAE J2601 in a multi-objective approach.
- To analyze the operating cost of the HRS for different operating conditions.
2. Materials and Methods
2.1. Station Layout
2.2. Mathematical Model
2.3. Entropy Production
2.4. Thermophysical Properties
2.5. Optimization Problem
2.6. Economic Assessment
2.7. Optimization Implementation and Solution Method
3. Results
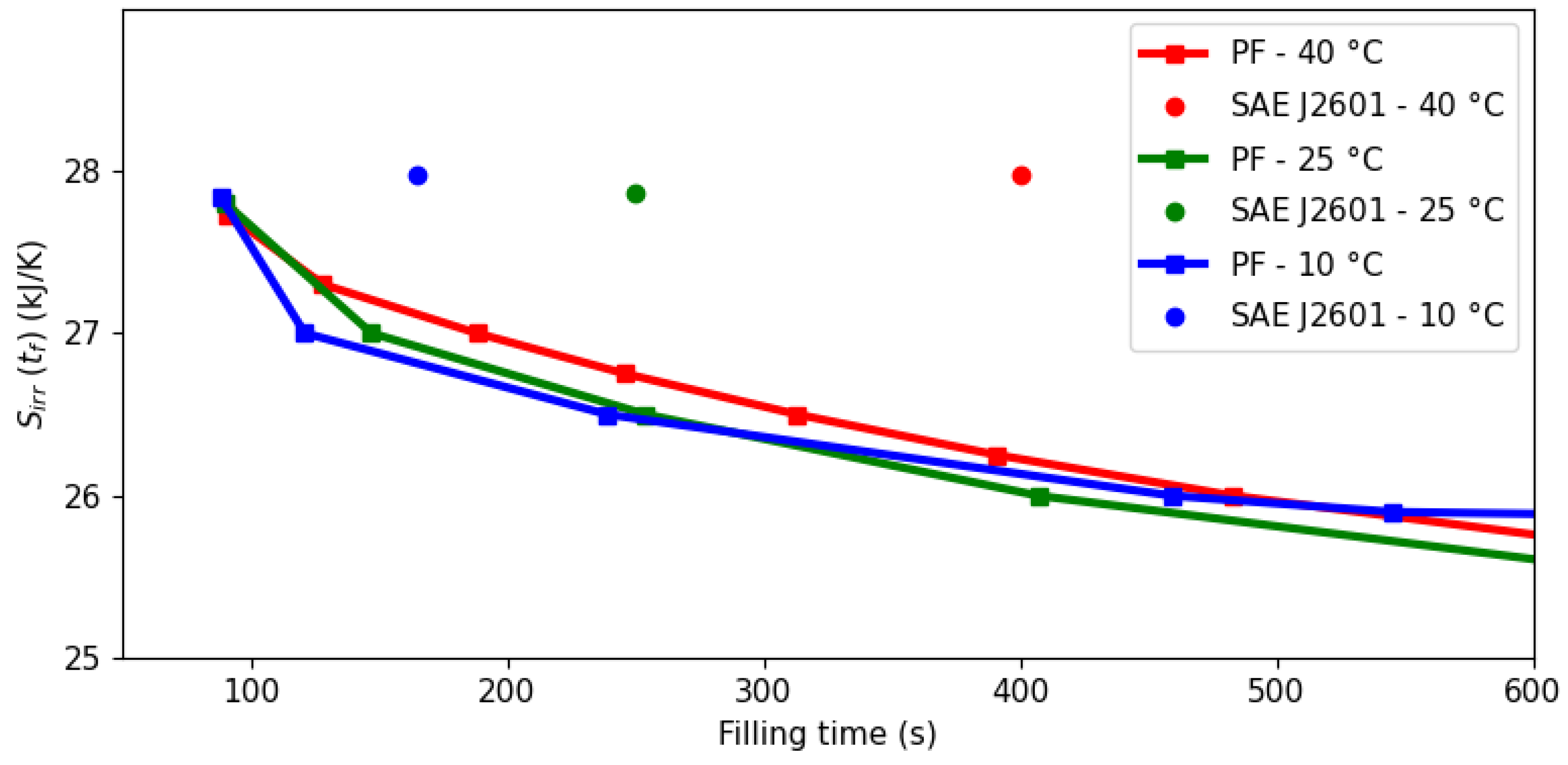
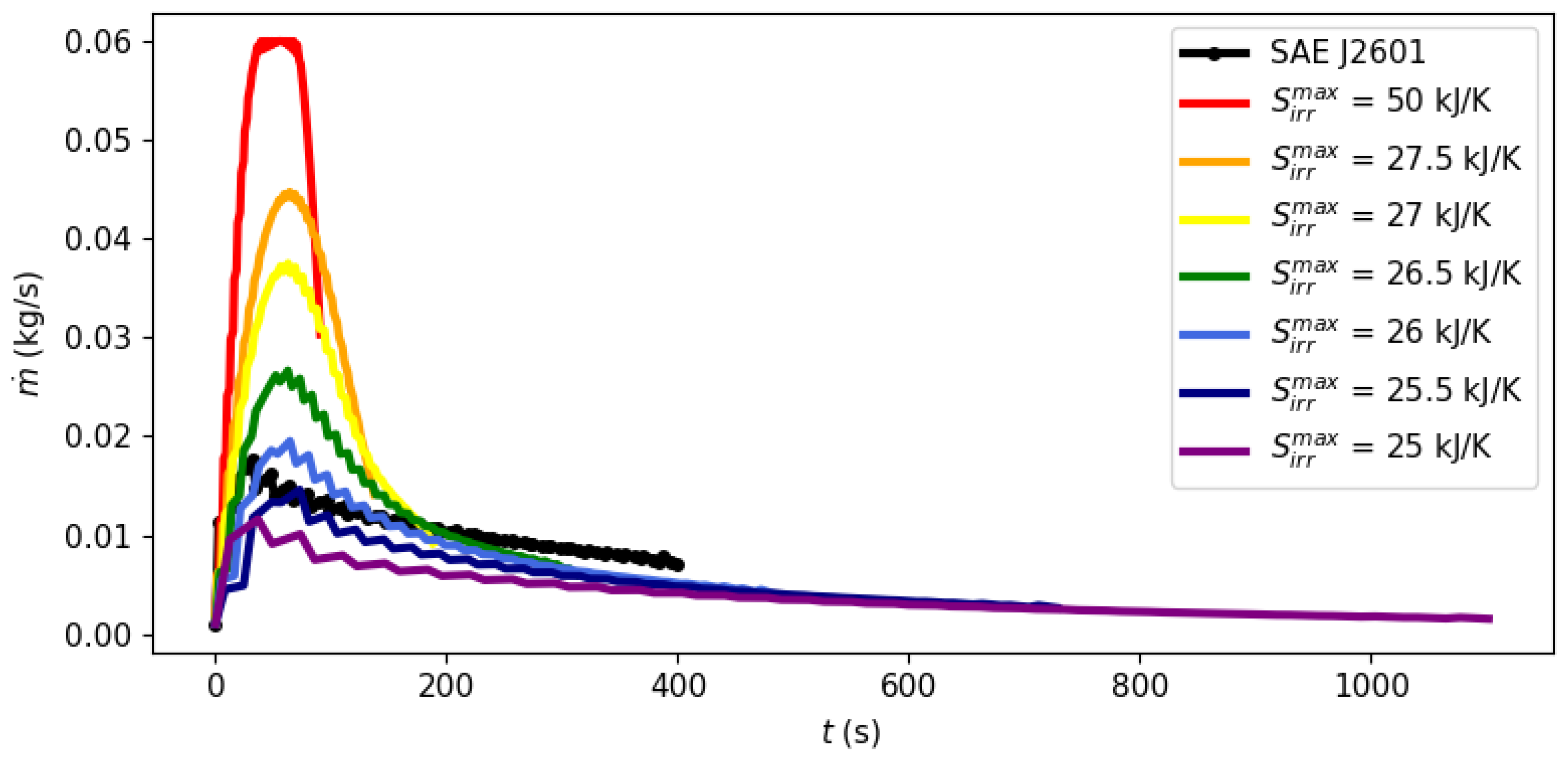
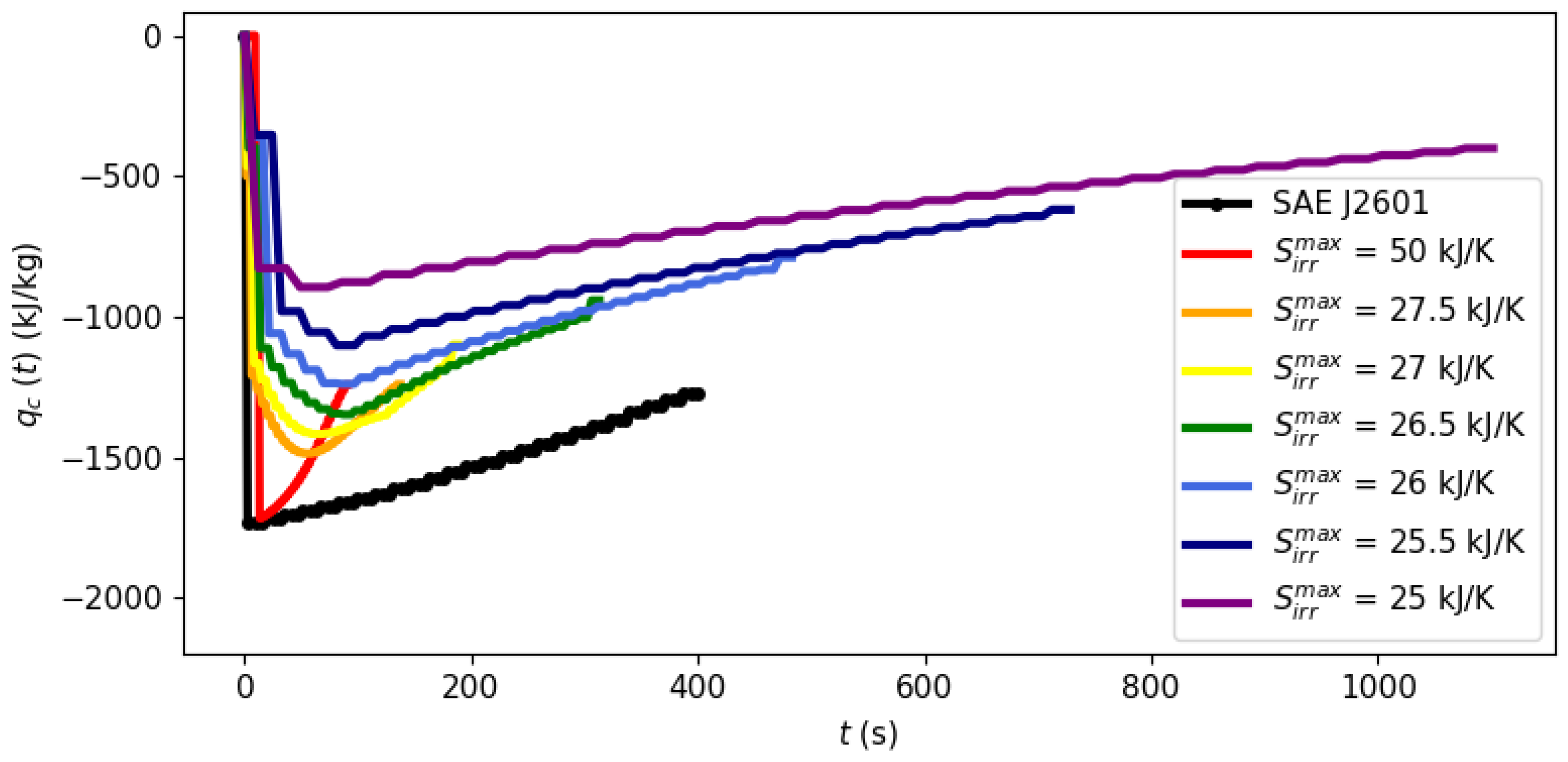
3.1. Filling Time and Entropy Production
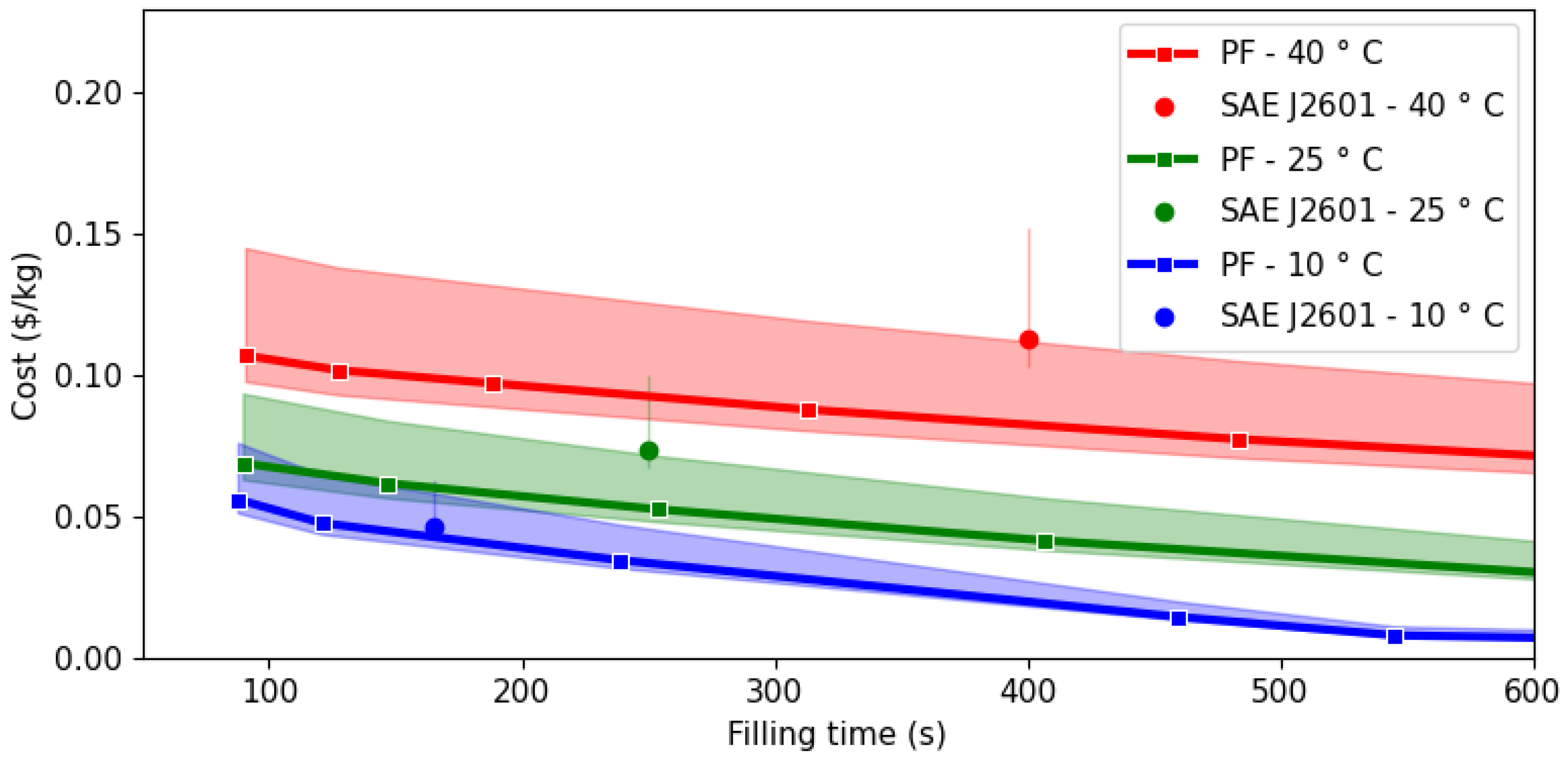
3.2. Economic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Latin | |
| A | Area, m2 |
| a | Helmholtz equation parameter, - |
| b | Helmholtz equation parameter, - |
| D | Helmholtz equation parameter, - |
| DAE | Differential algebraic equations |
| Index | |
| k | Valve flow capacity coefficient, m3 h−1 |
| h | Heat transfer coefficient, kW m−2 K−1 |
| HRS | Hydrogen refueling station |
| Mass flow rate, kg s−1 | |
| Pressure, kPa | |
| Critical pressure, kPa | |
| PF | Pareto frontier |
| Pipe segment, - | |
| Specific heat flow, kJ kg−1 | |
| R | Ideal gas constant, kJ kg−1 K−1 |
| Entropy, kJ K−1 | |
| Total entropy production, kJ K−1 | |
| Specific entropy, kJ kg−1 K−1 | |
| SOC | State of charge |
| Temperature, K | |
| t | Time, s |
| Helmholtz equation parameter, - | |
| Critical temperature, K | |
| Specific internal energy, kJ kg−1 | |
| V | Vehicle tank volume, m3 |
| Subscripts | |
| Greek | |
| Helmholtz equation parameter, - | |
| Helmholtz equation parameter, - | |
| Helmholtz equation parameter, - | |
| Helmholtz equation parameter, - | |
| Density, kg m−3 | |
| Density at normal conditions (273.15 K, 101.325 kPa), kg m−3 | |
| Critical density, kg m−3 | |
| Maximum admissible density in the vehicle tank kg m−3 | |
| Helmholtz equation parameter, - | |
| f | Final |
| Flow control valve | |
| i | Index |
| Inlet valve | |
| Irreversible | |
| k | Index |
| w | Wall |
| 0 | Tank |
| 32 | Pipe segment 32 |
| 54 | Pipe segment 54 |
| ∞ | Ambient |
| 0 | Ideal gas |
| r | Residual |
| Reference state | |
| k | Index |
Appendix A. Thermodynamic Properties of Hydrogen
| k | ||
|---|---|---|
| 1 | −1.4579856475 | - |
| 2 | 1.888076782 | - |
| 3 | 1.616 | −16.0205159149 |
| 4 | −0.4117 | −22.6580178006 |
| 5 | −0.792 | −60.0090511389 |
| 6 | 0.758 | −74.9434303817 |
| 7 | 1.217 | −206.9392065168 |
| i | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | −6.93643 | 0.6844 | 1 | - | - | - | - | - |
| 2 | 0.01 | 1 | 4 | - | - | - | - | - |
| 3 | 2.1101 | 0.989 | 1 | - | - | - | - | - |
| 4 | 4.52059 | 0.489 | 1 | - | - | - | - | - |
| 5 | 0.732564 | 0.803 | 2 | - | - | - | - | - |
| 6 | −1.34086 | 1.1444 | 2 | - | - | - | - | - |
| 7 | 0.130985 | 1.409 | 3 | - | - | - | - | - |
| 8 | −0.777414 | 1.754 | 1 | 1 | - | - | - | - |
| 9 | 0.351944 | 1.311 | 3 | 1 | - | - | - | - |
| 10 | −0.0211716 | 4.187 | 2 | - | −1.685 | −0.1710 | 0.7164 | 1.506 |
| 11 | 0.0226312 | 5.646 | 1 | - | −0.489 | −0.2245 | 1.3444 | 0.156 |
| 12 | 0.032187 | 0.791 | 3 | - | −0.103 | −0.1304 | 1.4517 | 1.736 |
| 13 | −0.0231752 | 7.249 | 1 | - | −2.506 | −0.2785 | 0.7204 | 0.67 |
| 14 | 0.0557346 | 2.986 | 1 | - | −1.607 | −0.3967 | 1.5445 | 1.662 |
References
- Genovese, M.; Blekhman, D.; Dray, M.; Fragiacomo, P. Hydrogen station in situ back-to-back fueling data for design and modeling. J. Clean. Prod. 2021, 329, 129737. [Google Scholar] [CrossRef]
- Shell and USP Pioneer World’s First Ethanol-Based Hydrogen Fuel Station. Available online: https://www.fuelsandlubes.com/shell-and-usp-pioneer-worlds-first-ethanol-based-hydrogen-fuel-station/ (accessed on 30 June 2024).
- Hynion. Hynion and Toyota Norway Enter into a Cooperation Agreement to Drive the Infrastructure for Hydrogen in Norway. Available online: https://www.hynion.com/news/hynion-and-toyota-norway-enter-into-a-cooperation-agreement-to-drivethe-infrastructure-for-hydrogen-in-norway#:~:text=Currently%2C%20there%20is%20only%20one,station%20at%20HÃÿvik%20outside%20Oslo (accessed on 29 June 2024).
- Reddi, K.; Elgowainy, A.; Rustagi, N.; Gupta, E. Impact of hydrogen SAE J2601 fueling methods on fueling time of light-duty fuel cell electric vehicles. Int. J. Hydrogen Energy 2017, 42, 16675–16685. [Google Scholar] [CrossRef]
- Olmos, F.; Manousiouthakis, V.I. Hydrogen car fill-up process modeling and simulation. Int. J. Hydrogen Energy 2013, 38, 3401–3418. [Google Scholar] [CrossRef]
- Olmos, F.; Manousiouthakis, V.I. Gas tank fill-up in globally minimum time: Theory and application to hydrogen. Int. J. Hydrogen Energy 2014, 39, 12138–12157. [Google Scholar] [CrossRef]
- Mendoza, D.; Rincon, D.; Santoro, B. Increasing energy efficiency of hydrogen refueling stations via optimal thermodynamic paths. Int. J. Hydrogen Energy 2024, 50, 1138–1151. [Google Scholar] [CrossRef]
- Kizilova, N.; Shankar, A.; Kjelstrup, S. A Minimum Entropy Production Approach to Optimization of Tubular Chemical Reactors with Nature-Inspired Design. Energies 2024, 17, 432. [Google Scholar] [CrossRef]
- Kingston, D.; Wilhelmsen, Ø.; Kjelstrup, S. Minimum entropy production in a distillation column for air separation described by a continuous non-equilibrium model. Chem. Eng. Sci. 2020, 218, 115539. [Google Scholar] [CrossRef]
- Magnanelli, E.; Johannessen, E.; Kjelstrup, S. Entropy production minimization as design principle for membrane systems: Comparing equipartition results to numerical optima. Ind. Eng. Chem. Res. 2017, 56, 4856–4866. [Google Scholar] [CrossRef]
- Johannessen, E.; Nummedal, L.; Kjelstrup, S. Minimizing the entropy production in heat exchange. Int. J. Heat Mass Transf. 2002, 45, 2649–2654. [Google Scholar] [CrossRef]
- Johannessen, E.; Kjelstrup, S. Minimum entropy production rate in plug flow reactors: An optimal control problem solved for SO2 oxidation. Energy 2004, 29, 2403–2423. [Google Scholar] [CrossRef]
- Nummedal, L.; Kjelstrup, S.; Costea, M. Minimizing the entropy production rate of an exothermic reactor with a constant heat-transfer coefficient: The ammonia reaction. Ind. Eng. Chem. Res. 2003, 42, 1044–1056. [Google Scholar] [CrossRef]
- Kjelstrup, S.; Johannessen, E.; Rosjorde, A.; Nummedal, L.; Bedeaux, D. Minimizing the entropy production of the methanol producing reaction in a methanol reactor. Int. J. Thermodyn. 2000, 3, 147–153. [Google Scholar]
- Røsjorde, A.; Kjelstrup, S.; Johannessen, E.; Hansen, R. Minimizing the entropy production in a chemical process for dehydrogenation of propane. Energy 2007, 4, 335–343. [Google Scholar] [CrossRef]
- Elgowainy, A.; Reddi, K.; Lee, D.-Y.; Rustagi, N.; Gupta, E. Techno-economic and thermodynamic analysis of pre-cooling systems at gaseous hydrogen refueling stations. Int. J. Hydrogen Energy 2017, 42, 29067–29079. [Google Scholar] [CrossRef]
- Leachman, J.W.; Jacobsen, R.T.; Penoncello, S.G.; Lemmon, E.W. Fundamental Equations of State for Parahydrogen, Normal Hydrogen, and Orthohydrogen. J. Phys. Chem. Ref. Data 2009, 38, 721–748. [Google Scholar] [CrossRef]
- Orange County, California Electricity Rates & Statistics. Available online: https://findenergy.com/ca/orange-county-electricity/ (accessed on 9 July 2024).
- Bynum, M.L.; Hackebeil, G.A.; Hart, W.E.; Laird, C.D.; Nicholson, B.L.; Siirola, J.D.; Watson, J.P.; Woodruff, D.L. Pyomo-Optimization Modeling in Python, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Nicholson, B.; Siirola, J.D.; Watson, J.P.; Zavala, V.M.; Biegler, L.T. pyomo.dae: A modeling and automatic discretization framework for optimization with differential and algebraic equations. Math. Program. Comput. 2018, 10, 187e223. [Google Scholar] [CrossRef]
- Detailed Cost Analysis of Hydrogen Refueling Costs for Fleets. Chem. Ing. Tech. 2024, 96, 86–99. [CrossRef]
- Simunović, J.; Pivac, I.; Barbir, F. Techno-economic assessment of hydrogen refueling station: A case study in Croatia. Int. J. Hydrogen Energy 2022, 47, 24155–24168. [Google Scholar] [CrossRef]


| Thermophysical | Geometry | ||
|---|---|---|---|
| (kW m−2 K−1) | 0.06 | (m3) | 0.12 |
| (kW m−2 K−1) | 0.006 | (m3) | 0.0576 |
| (kg m−3) | 1550 | (m2) | 2.34 |
| (kJ kg−1 K−1) | 1.374 | (m2) | 2.88 |
| Ambient Temperature (°C) | Compression Work (kJ) | ||
|---|---|---|---|
| SAE J2601 | Min. time (= 50 kJ/K) | Min. time (= 26 kJ/K) | |
| 10 °C | 3572 kJ | 3225 kJ | 748 kJ |
| 25 °C | 5664 kJ | 5277 kJ | 3117 kJ |
| 40 °C | 8625 kJ | 8185 kJ | 5930 kJ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, B.F.; Rincón, D.; Mendoza, D.F. Entropy Production and Filling Time in Hydrogen Refueling Stations: An Economic Assessment. Entropy 2024, 26, 735. https://doi.org/10.3390/e26090735
Santoro BF, Rincón D, Mendoza DF. Entropy Production and Filling Time in Hydrogen Refueling Stations: An Economic Assessment. Entropy. 2024; 26(9):735. https://doi.org/10.3390/e26090735
Chicago/Turabian StyleSantoro, Bruno F., David Rincón, and Diego F. Mendoza. 2024. "Entropy Production and Filling Time in Hydrogen Refueling Stations: An Economic Assessment" Entropy 26, no. 9: 735. https://doi.org/10.3390/e26090735








